Seasonal Dynamics of Litterfall in a Sub-Alpine Spruce-Fir Forest on the Eastern Tibetan Plateau: Allometric Scaling Relationships Based on One Year of Observations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Litter Collection
2.3. Litterfall Component Categories
2.4. Statistical Analyses
3. Results
3.1. Litter Production and Litter Components
3.2. Litterfall Dynamics
3.3. Evergreen Versus Deciduous
3.4. Tree Versus Shrub Litter Production
3.5. Allometric Scaling Relationships of Litterfall
4. Discussion
4.1. Annual Litter Production
4.2. Litterfall Dynamics
4.3. Evergreen Versus Deciduous, Tree Versus Shrub
4.4. Litter Components and Allometric Scaling Relationships
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Finzi, A.C.; Allen, A.S.; Delucia, E.H.; Ellsworth, D.S.; Schlesinger, W.H. Forest litter production, chemistry, and decomposition following two years of Free-Air CO2 enrichment. Ecology 2001, 82, 470–484. [Google Scholar]
- Paudel, E.; Dossa, G.G.O.; Xu, J.; Harrison, R.D. Litterfall and nutrient return along a disturbance gradient in a tropical montane forest. For. Ecol. Manag. 2015, 353, 97–106. [Google Scholar] [CrossRef]
- Wiegert, R.G.; Monk, C.D. Litter production and energy accumulation in three plantations of longleaf pine (Pinus palustriis Mill). Ecology 1972, 53, 949–953. [Google Scholar] [CrossRef]
- Meentemeyer, V.; Box, E.O.; Thompson, R. World patterns and amounts of terrestrial plant litter production. Bioscience 1982, 32, 125–128. [Google Scholar] [CrossRef]
- Ong, C.S.P.; Juan, J.C.; Yule, C.M. Litterfall production and chemistry of Koompassia malaccensis and Shorea uliginosa in a tropical peat swamp forest: Plant nutrient regulation and climate relationships. Trees 2015, 29, 527–537. [Google Scholar] [CrossRef]
- Vogt, K.A.; Grier, C.C.; Vogt, D.J. Production, turnover, and nutrient dynamics of above-and belowground detritus of world forests. Adv. Ecol. Res. 1986, 15, 303–377. [Google Scholar]
- Yang, W.Q.; Wang, K.Y.; Kellomäki, S.; Zhang, J. Annual and monthly variations in litter macronutrients of three subalpine forests in Western China. Pedosphere 2006, 15, 653–659. [Google Scholar] [CrossRef]
- Bray, J.R.; Gorham, E. Litter production in forests of the world. Adv. Ecol. Res. 1964, 2, 101–157. [Google Scholar]
- Bigelow, S.W.; Canham, C.D. Litterfall as a niche construction process in a northern hardwood forest. Ecosphere 2015, 6, 117. [Google Scholar] [CrossRef]
- Wang, J.N.; Xu, B.; Wu, Y.; Gao, J.; Shi, F.S. Flower litters of alpine plants affect soil nitrogen and phosphorus rapidly in the Eastern Tibetan Plateau. Biogeosciences 2016, 13, 5619–5631. [Google Scholar] [CrossRef]
- Hishinuma, T.; Azuma, J.I.; Osono, T.; Takeda, H. Litter quality control of decomposition of leaves, twigs, and sapwood by the white-rot fungus Trametes versicolor. Eur. J. Soil Biol. 2017, 80, 1–8. [Google Scholar] [CrossRef]
- Lidman, J.; Jonsson, M.; Burrows, R.M.; Bundschuh, M.; Sponseller, R.A. Composition of riparian litter input regulates organic matter decomposition: Implications for headwater stream functioning in a managed forest landscape. Ecol. Evol. 2017, 7, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.A.; Congdon, R.A.; Shoo, L.P.; Valdez Ramirez, V.; Williams, S.E. Spatial variability in litterfall, litter standing crop and litter quality in a tropical rain forest region. Biotropica 2014, 46, 378–386. [Google Scholar] [CrossRef]
- Zhang, H.C.; Yuan, W.P.; Dong, W.J.; Liu, S.G. Seasonal patterns of litterfall in forest ecosystem worldwide. Ecol. Complex. 2014, 20, 240–247. [Google Scholar] [CrossRef]
- Enright, N.J. Litterfall dynamics in a mixed conifer-angiosperm forest in northern New Zealand. J. Biogeogr. 2002, 26, 149–157. [Google Scholar] [CrossRef]
- Liu, C.; Ilvesniemi, H. Variation in Litterfall-Climate relationships between coniferous and broadleaf forests in Eurasia. Glob. Ecol. Biogeogr. 2004, 13, 105–114. [Google Scholar] [CrossRef]
- Yang, W.Q.; Wang, K.; Kelloma, S.K.; Gong, H.D. Litter dynamics of three subalpine forests in Western Sichuan. Pedosphere 2005, 16, 788–798. [Google Scholar] [CrossRef]
- Lin, B.; Liu, Q.; Wu, Y.; He, H. Nutrient and litter patterns in three subalpine coniferous forests of western Sichuan, China. Pedosphere 2006, 16, 380–389. [Google Scholar] [CrossRef]
- Hou, L.L.; Mao, Z.J.; Tao, S. Dynamic of litterfall in ten typical community types of Xiaoxing’an Mountain, China. Acta Ecol. Sin. 2013, 33, 1994–2002, (In Chinese with English abstract). [Google Scholar]
- Saarsalmi, A.; Starr, M.; Hokkanen, T.; Ukonmaanaho, L.; Kukkola, M.; Nojd, P.; Sievanen, R. Predicting annual canopy litterfall production for Norway spruce (Picea abies (L.) Karst.) stands. For. Ecol. Manag. 2007, 242, 578–586. [Google Scholar] [CrossRef]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Taylor, J.E.; Ellis, M.V.; Rayner, L.; Ross, K.A. Variability in allometric relationships for temperate woodland Eucalyptus trees. For. Ecol. Manag. 2016, 360, 122–132. [Google Scholar] [CrossRef]
- Taskinen, S.; Warton, D.I. Robust estimation and inference for bivariate line-fitting in allometry. Biom. J. 2011, 53, 652–672. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Z.; Cheng, D.L.; Zhong, Q.L.; Jin, B.J.; Xu, C.B.; Hu, B. Allometric relationships among different components of forest litterfall in China. Chin. J. Plant Ecol. 2014, 37, 1071–1079, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Cheng, D.L.; Ma, Y.Z.; Zhong, Q.L.; Xu, W.F. Allometric scaling relationship between above- and below-ground biomass within and across five woody seedlings. Ecol. Evol. 2014, 4, 3968–3977. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.C.; Cao, Y.; Wang, J.; Tang, Y. New patterns of establishment and growth of Picea abies and Betula tree species in subalpine forest gaps of Jiuzhaigou National Nature Reserve, Sichuan, southwestern China in a changing environment. For. Ecol. Manag. 2015, 356, 84–92. [Google Scholar] [CrossRef]
- Swamy, S.L.; Dutt, C.B.S.; Murthy, M.S.R.; Mishra, A.; Bargali, S.S. Floristics and dry matter dynamics of tropical wet evergreen forests of Western Ghats, India. Curr. Sci. 2010, 99, 353–364. [Google Scholar]
- Wu, F.Z.; Yang, W.Q.; Zhang, J.; Deng, R.J. Litter decomposition in two subalpine forests during the freeze-thaw season. Acta Oecol. 2010, 36, 135–140. [Google Scholar] [CrossRef]
- He, W.; Wu, F.Z.; Yang, W.Q.; Zhang, D.J.; Xu, Z.F.; Tan, B.; Zhao, Y.Y.; Justine, M.F. Gap locations influence the release of carbon, nitrogen and phosphorus in two shrub foliar litter in an alpine fir forest. Sci. Rep. 2016, 6, 22014. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.Y.; Yang, W.Q.; Tan, B.; He, J.; Xu, L.Y.; Li, H.; Wu, F.Z. Accelerated foliar litter humification in forest gaps: Dual feedbacks of carbon sequestration during winter and the growing season in an alpine forest. Geoderma 2015, 241–242, 136–144. [Google Scholar] [CrossRef]
- Xiao, S.; Wu, F.Z.; Yang, W.Q.; Chang, C.H.; Li, J.; Wang, B.; Cao, Y. Understory biomass and its characteristics as affected by forest gap in the alpine forest ecosystem in west Sichuan. J. Ecol. Environ. Sci. 2014, 23, 1515–1519, (In Chinese with English abstract). [Google Scholar]
- Zhang, X.P.; Wang, X.P.; Zhu, B.; Zong, Z.J.; Peng, C.H.; Fang, J.Y. Litter fall production in relation to environmental factors in northeast China’s forests. Chin. J. Plant Ecol. 2008, 32, 1031–1040, (In Chinese with English abstract). [Google Scholar]
- Giaccone, E.; Colombo, N.; Acquaotta, F.; Paro, L.; Fratianni, S. Climate variations in a high altitude Alpine basin and their effects on a glacial environment (Italian Western Alps). Atmosfera 2015, 28, 117–128. [Google Scholar] [CrossRef]
- Rustad, L.E.; Fernandez, I.J. Soil warming: consequences for foliar litter decay in a Spruce-fir forest in maine, USA. Soil Sci. Soc. Am. J. 1998, 62, 1072–1080. [Google Scholar] [CrossRef]
- Fukuda, M.; Koaze, T.; Nogami, M. Physical geography in cold regions: past and present. Geography 2008, 56, 103–111. [Google Scholar] [CrossRef]
- Gairola, S.; Rawal, R.S.; Dhar, U. Patterns of litterfall and return of nutrients across anthropogenic disturbance gradients in three subalpine forests of west Himalaya, India. J. For. Res. Jpn. 2009, 14, 73–80. [Google Scholar] [CrossRef]
- Miao, N.; Liu, S.R.; Yu, H.; Shi, Z.M.; Moermond, T.; Liu, Y. Spatial analysis of remnant tree effects in a secondary Abies-Betula forest on the eastern edge of the Qinghai-Tibetan Plateau, China. For. Ecol. Manag. 2014, 313, 104–111. [Google Scholar] [CrossRef]
- Clark, D.A.; Brown, S.; Kicklighter, D.W.; Chambers, J.Q.; Thomlinson, J.R.; Ni, J.; Holland, E.A. Net primary production in tropical forests: An evaluation and synthesis of existing field data. Ecol. Appl. 2008, 11, 371–384. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Kloeppel, B.D.; Ellsworth, D.S. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia 1995, 104, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyuki, M.; Kyoichi, O. Comparison of sapling-level daily light capture and carbon gain between a temperate deciduous and a co-occurring evergreen tree species in the growing season and in winter. Funct. Plant Biol. 2010, 37, 215–222. [Google Scholar]
- Öquist, G.; Huner, N.P.A. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 2003, 54, 329–355. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.G.; Zhou, G.S.; Xu, Z.Z. Forest litterfall and its composition: A new data set of observational data from China. Ecology 2016, 97, 1365. [Google Scholar] [CrossRef]
- Hennessey, T.C.; Dougherty, P.M.; Cregg, B.M.; Wittwer, R.F. Annual variation in needle fall of a loblolly pine stand in relation to climate and stand density. For. Ecol. Manag. 1992, 51, 329–338. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444. [Google Scholar] [CrossRef] [PubMed]
- Capell, B.; Dörffling, K. Low Temperature-Induced changes of abscisic acid contents in barley and cucumber leaves in relation to their water status. J. Plant Physiol. 1990, 135, 571–575. [Google Scholar] [CrossRef]
- Nihlgård, B. Plant biomass, primary production and distribution of chemical elements in a beech and a planted spruce forest in south Sweden. Oikos 1972, 23, 69–81. [Google Scholar] [CrossRef]
- Scoriza, R.N.; Piñarodrigues, F.C.M. Influence of precipitation and air temperature in production of litterfall in stretch of seasonal forest. Floresta 2014, 44, 687–696. [Google Scholar] [CrossRef]
- Wei, X.; Kimmins, J.P.; Peel, K.; Steen, O. Mass and nutrients in woody debris in harvested and wildfire-killed lodgepole pine forests in the central interior of British Columbia. Can. J. For. Res. 2011, 27, 148–155. [Google Scholar] [CrossRef]
- Samuelson, L.; Stokes, T.; Butnor, J.R.; Johnsen, K.H.; Gonzalez-Benecke, C.A.; Anderson, P.; Jackson, J.; Ferrari, L.; Martin, T.A.; Cropper, W.P. Ecosystem carbon stocks in Pinus palustris forests. Can. J. For. Res. 2014, 44, 476–486. [Google Scholar] [CrossRef]
- Woldendorp, G.; Keenan, R.J. Coarse woody debris in Australian forest ecosystems: A review. Austral Ecol. 2006, 30, 834–843. [Google Scholar] [CrossRef]
- Do, T.V.; Sato, T.; Saito, S.; Kozan, O. Fine-root production and litterfall: Main contributions to net primary production in an old-growth evergreen broad-leaved forest in southwestern Japan. Ecol. Res. 2015, 30, 921–930. [Google Scholar]
- McLaren, J.R.; Buckeridge, K.M.; van de Weg, M.J.; Shaver, G.R.; Schimel, J.P.; Gough, L. Shrub encroachment in Arctic tundra: Betula nana effects on above- and below- ground litter decomposition. Ecology 2017, 98, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Bebawi, F.F.; Campbell, S.D.; Mayer, R.J. Seed Fall, Seed fall, seed predation, twigging and litter fall of Cascabela thevetia (L.) Lippold. Rangeland J. 2017, 38, 569–577. [Google Scholar] [CrossRef]
- Boudreault, C.; Drapeau, P.; Bouchard, M.; Stlaurent, M.H.; Imbeau, L.; Bergeron, Y. Contrasting responses of epiphytic and terricolous lichens to variations in forest characteristics in northern boreal ecosystems. Can. J. For. Res. 2015, 45, 595–606. [Google Scholar] [CrossRef]
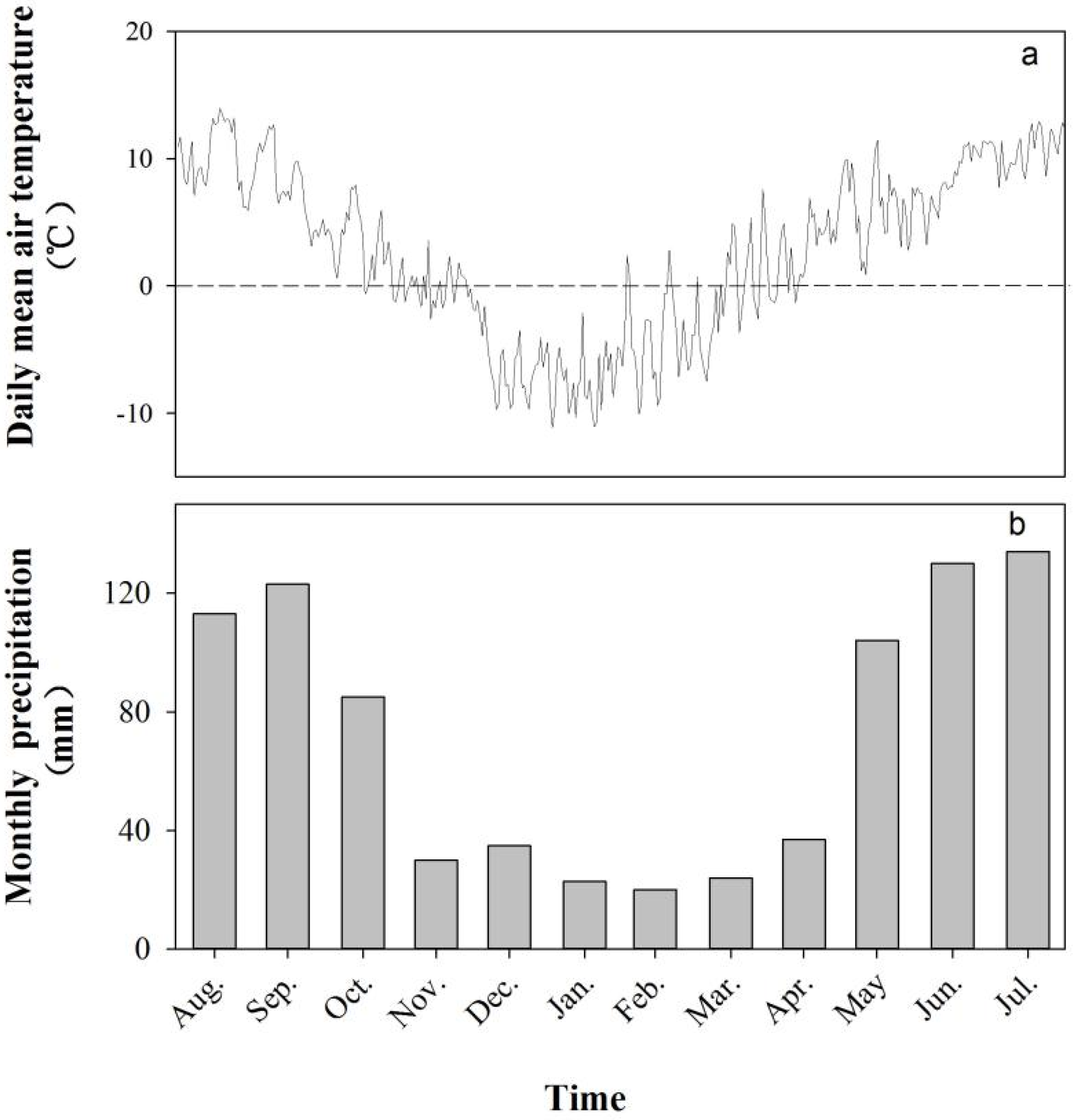

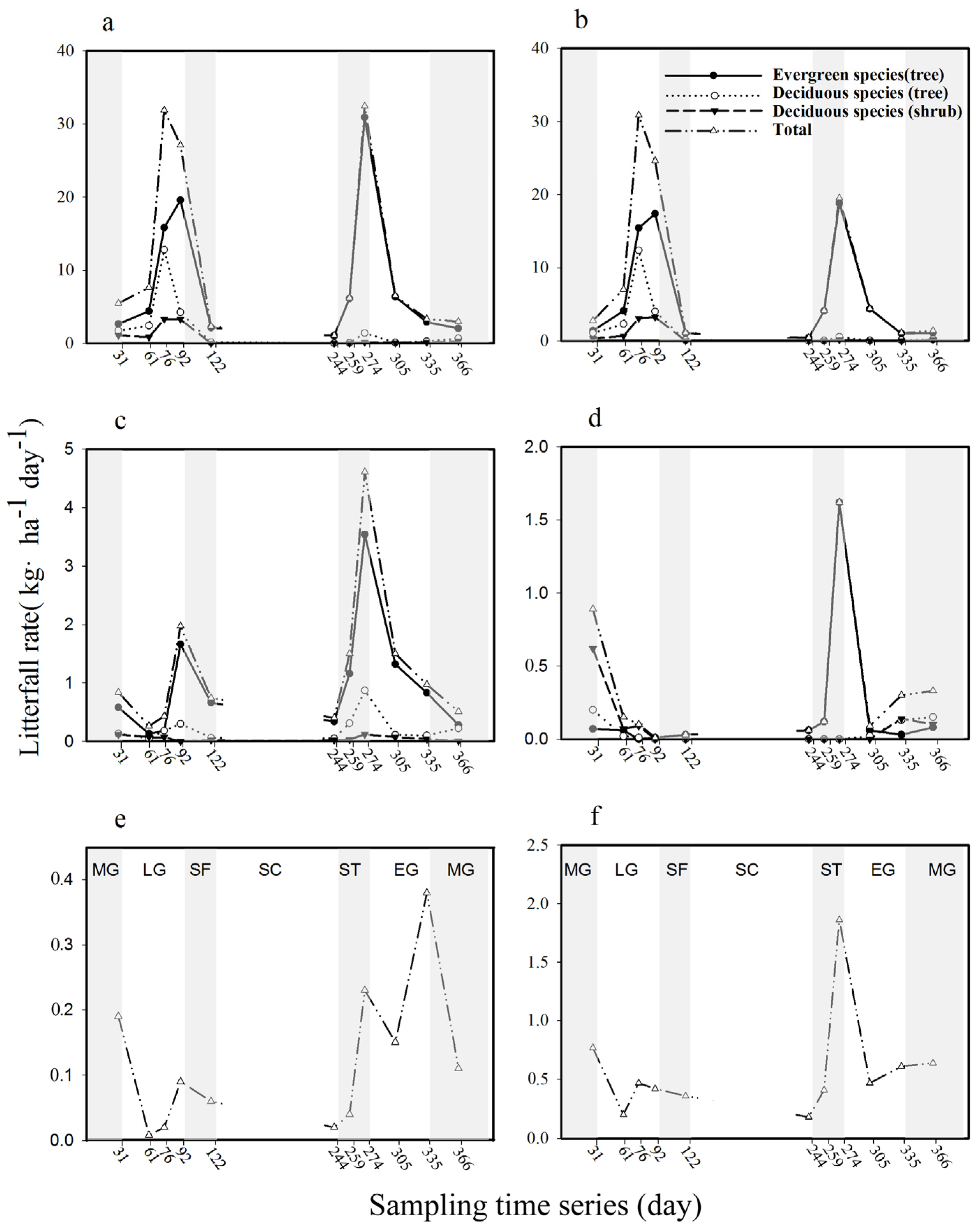

| Component Categories | Mean ± SD (kg·ha−1·year−1) | Percentage (%) | |
|---|---|---|---|
| Foliar litter a † | needle | 1263 ± 78 | 53.1 a |
| leaf | 488 ± 236 | 20.5 b | |
| Woody litter b | twig | 363 ± 145 | 15.2 bc |
| bark | 9 ± 3 | 0.4 d | |
| Reproductive litter b | flower | 14 ± 7 | 0.6 cd |
| fruit and seed | 57 ± 17 | 2.4 cd | |
| Epiphytic litter b | \ | 31 ± 18 | 1.3 cd |
| Miscellaneous b | \ | 155 ± 40 | 6.5 cd |
| Total | \ | 2380 ± 510 | 100.00 |
| Foliar Litter | Twig | Bark | Fruit and Seed | Flower | Epiphytic Litter | Miscellaneous | |
|---|---|---|---|---|---|---|---|
| Twig | 0.596 ** | 1 | |||||
| Bark | 0.386 ** | 0.477 ** | 1 | ||||
| Fruit and seed | 0.349 ** | 0.519 ** | 0.467 ** | 1 | |||
| Flower | 0.157 | 0.097 | 0.392 ** | 0.327 * | 1 | ||
| Epiphytic litter | 0.214 | 0.195 | 0.107 | 0.068 | −0.042 | 1 | |
| Miscellaneous | 0.752 ** | 0.707 ** | 0.465 ** | 0.407 ** | 0.188 | 0.238 | 1 |
| Litter Components | Sampling Time | Species | Sampling Time × Species | |||
|---|---|---|---|---|---|---|
| F Value | p Value | F Value | p Value | F Value | p Value | |
| Foliar litter | 5.64 | <0.001 | 1.92 | 0.009 | 2.67 | <0.001 |
| Twig litter | 0.45 | 0.92 | 2.05 | 0.009 | 1.68 | <0.001 |
| Bark litter | 0.62 | 0.80 | 1.31 | 0.17 | 0.61 | 1.00 |
| Fruit and seed litter | 0.60 | 0.81 | 1.01 | 0.44 | 1.48 | 0.009 |
| Flower litter | 5.85 | <0.001 | 8.05 | 0.009 | 4.13 | <0.001 |
| Total litter | 3.48 | <0.001 | 2.78 | <0.001 | 3.35 | <0.001 |
| Species | Peak (%) | Valley (%) | PVR |
|---|---|---|---|
| Vertical structure | |||
| Tree species | 32.7 ± 3.2 | 1.3 ± 0.8 | 25.2 ± 4.5 |
| Shrub species | 4.1 ± 1.2 | 0.01 ± 0.01 | 410.0 ± 178.1 |
| Functional type | |||
| Evergreen species | 22.7 ± 4.8 | 1.3 ± 0.9 | 17.5 ± 6.1 |
| Deciduous species | 14.6 ± 3.4 | 0.03 ± 0.1 | 486.7 ± 98.5 |
| Comparison | n | R2 | β (95%CI) | logγ (95%CI) | Test if β Equals a Specific Value (β0 = 1), F |
|---|---|---|---|---|---|
| Litter components | |||||
| LF∝LT | 60 | 0.98 ** | 0.99 (0.95, 1.03) | −0.1 (−0.18, −0.01) | 0.54 |
| LW∝LT | 60 | 0.63 ** | 1.40 (1.19, 1.64) | −1.96 (−2.46, −1.47) | 18.14 ** |
| LR∝LT | 60 | 0.31 ** | 0.98 (0.79, 1.22) | −1.66 (−2.14, −1.19) | 0.03 |
| LE∝LT | 60 | 0.04 | 0.93 (0.72, 1.19) | −1.88 (−2.42, −1.34) | 0.36 |
| LM∝LT | 60 | 0.74 ** | 0.82 (0.71, 0.93) | −0.71 (−0.95, −0.46) | 9.15 ** |
| LF∝LW | 60 | 0.53 ** | 0.71 (0.59, 0.84) | 1.29 (1.11, 1.46) | 15.64 ** |
| LF∝LR | 60 | 0.28 ** | 1.00 (0.80, 1.25) | 1.57 (1.39, 1.76) | 0.001 |
| LF∝LE | 60 | 0.04 | 1.06 (0.83, 1.37) | 1.9 (1.69, 2.11) | 0.24 |
| LF∝LM | 60 | 0.65 ** | 1.21 (1.03, 1.41) | 0.76 (0.54, 0.97) | 5.96 * |
| LW∝LR | 60 | 0.31 ** | 1.42 (1.15, 1.77) | 0.40 (0.15, 0.65) | 10.89 ** |
| LW∝LE | 60 | 0.05 | 1.51 (1.17, 1.94) | 0.87 (0.58, 1.156) | 10.91 ** |
| LW∝LM | 60 | 0.65 ** | 1.71 (1.46, 2.00) | −0.75 (−1.06, −0.45) | 51.97 ** |
| LR∝LE | 60 | 0.08 * | 1.06 (0.83, 1.36) | 0.33 (0.13, 0.52) | 0.22 |
| LR∝LM | 60 | 0.34 ** | 1.20 (0.97, 1.49) | −0.81 (−1.11, −0.51) | 3.03 |
| LE∝LM | 60 | 0.05 | 1.13 (0.88, 1.46) | −1.08 (−1.43, −0.51) | 0.97 |
| Tree versus shrub | |||||
| Lty∝LT | 60 | 0.96 ** | 1.05 (1.00, 1.11) | −0.22 (−0.34, −0.09) | 3.57 |
| Lsy∝LT | 60 | 0.05 | 1.32 (1.03, 1.70) | −2.37 (−3.14, −1.60) | 4.83 * |
| Lty∝Lsy | 60 | 0.01 | 0.80 (0.62, 1.03) | 1.68 (1.43, 1.93) | 3.04 |
| Evergreen versus deciduous | |||||
| Lev∝LT | 60 | 0.83 ** | 1.14 (1.02, 1.27) | −0.52 (−0.80, −0.25) | 5.89 * |
| Lde∝LT | 60 | 0.35 ** | 1.58 (1.28, 1.94) | −2.24 (−2.98, −1.50) | 19.73 ** |
| Lev∝Lde | 60 | 0.13 * | 0.72 (0.57, 0.92) | 1.10 (0.81, 1.38) | 7.19 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.; Yang, W.; Tan, B.; Xu, Z.; Zhang, Y.; Yang, J.; Ni, X.; Wu, F. Seasonal Dynamics of Litterfall in a Sub-Alpine Spruce-Fir Forest on the Eastern Tibetan Plateau: Allometric Scaling Relationships Based on One Year of Observations. Forests 2017, 8, 314. https://doi.org/10.3390/f8090314
Fu C, Yang W, Tan B, Xu Z, Zhang Y, Yang J, Ni X, Wu F. Seasonal Dynamics of Litterfall in a Sub-Alpine Spruce-Fir Forest on the Eastern Tibetan Plateau: Allometric Scaling Relationships Based on One Year of Observations. Forests. 2017; 8(9):314. https://doi.org/10.3390/f8090314
Chicago/Turabian StyleFu, Changkun, Wanqin Yang, Bo Tan, Zhenfeng Xu, Yu Zhang, Jiaping Yang, Xiangyin Ni, and Fuzhong Wu. 2017. "Seasonal Dynamics of Litterfall in a Sub-Alpine Spruce-Fir Forest on the Eastern Tibetan Plateau: Allometric Scaling Relationships Based on One Year of Observations" Forests 8, no. 9: 314. https://doi.org/10.3390/f8090314





