Figure 1.
Distribution of sampling sites. The map was generated by ArcMap software (Version 10, ESRI, RedLands, CA, USA).
Figure 1.
Distribution of sampling sites. The map was generated by ArcMap software (Version 10, ESRI, RedLands, CA, USA).
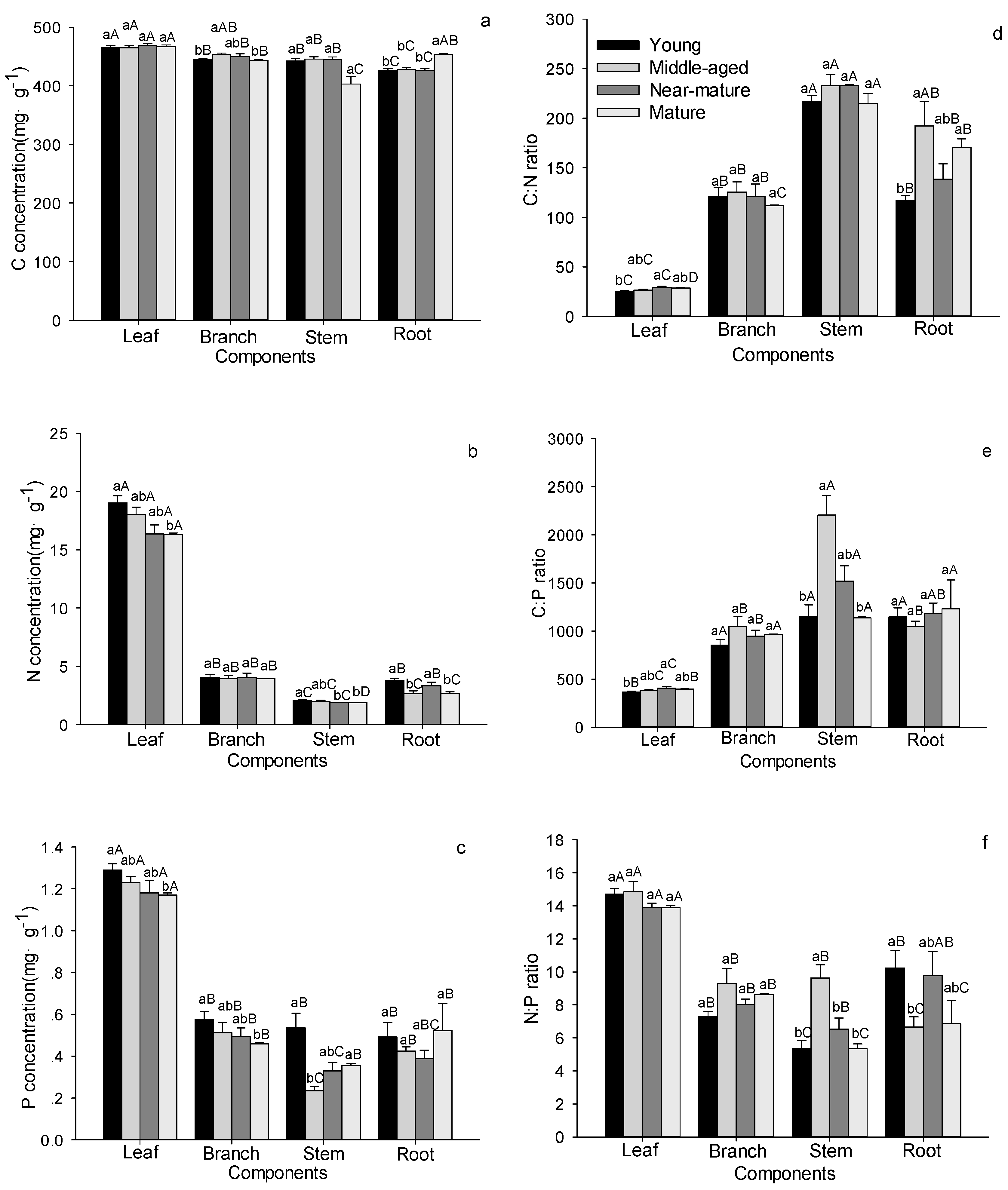
Figure 2.
C, N, and P stoichiometric characteristics in tree layer in Quercus variabilis natural secondary forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in tree layer of different age classes; (b) N concentration in tree layer of different age classes; (c) P concentration in tree layer of different age classes; (d) C:N ratio in tree layer of different age classes; (e) C:P ratio in tree layer of different age classes; (f) N:P ratio in tree layer of different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes, and different capital letters indicated significant difference among different components in the same forest age classes (p < 0.05).
Figure 2.
C, N, and P stoichiometric characteristics in tree layer in Quercus variabilis natural secondary forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in tree layer of different age classes; (b) N concentration in tree layer of different age classes; (c) P concentration in tree layer of different age classes; (d) C:N ratio in tree layer of different age classes; (e) C:P ratio in tree layer of different age classes; (f) N:P ratio in tree layer of different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes, and different capital letters indicated significant difference among different components in the same forest age classes (p < 0.05).
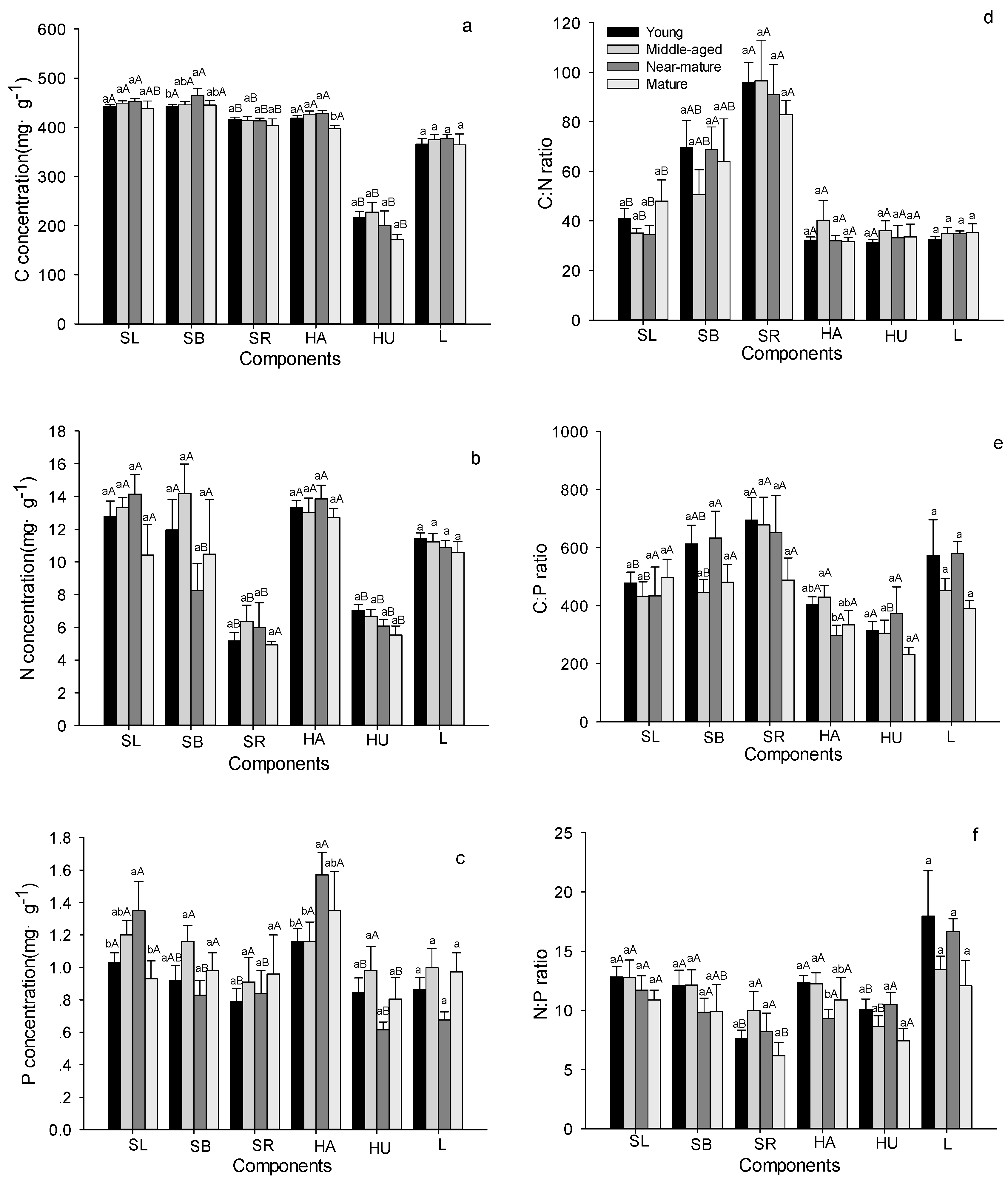
Figure 3.
C, N, and P stoichiometric characteristics in shrub, herb and litter layers in Quercus variabilis natural secondary forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in shrub, herb and litter layers of different age classes; (b) N concentration in shrub, herb and litter layers of different age classes; (c) P concentration in shrub, herb and litter layers of different age classes; (d) C:N ratio in shrub, herb and litter layers of different age classes; (e) C:P ratio in shrub, herb and litter layers of different age classes; (f) N:P ratio in shrub, herb and litter layers of different age classes. Different small letters indicated significant difference in the same indicators among age classes, and different capital letters indicated significant difference among different components in SL, SB, SR or in HA, HU in the same forest age class (p < 0.05). SL: shrub leaf, SB: shrub branch, SR: shrub roots, HA: herbage aboveground, HU: herbage underground, L: litter.
Figure 3.
C, N, and P stoichiometric characteristics in shrub, herb and litter layers in Quercus variabilis natural secondary forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in shrub, herb and litter layers of different age classes; (b) N concentration in shrub, herb and litter layers of different age classes; (c) P concentration in shrub, herb and litter layers of different age classes; (d) C:N ratio in shrub, herb and litter layers of different age classes; (e) C:P ratio in shrub, herb and litter layers of different age classes; (f) N:P ratio in shrub, herb and litter layers of different age classes. Different small letters indicated significant difference in the same indicators among age classes, and different capital letters indicated significant difference among different components in SL, SB, SR or in HA, HU in the same forest age class (p < 0.05). SL: shrub leaf, SB: shrub branch, SR: shrub roots, HA: herbage aboveground, HU: herbage underground, L: litter.
![Forests 08 00281 g003]()
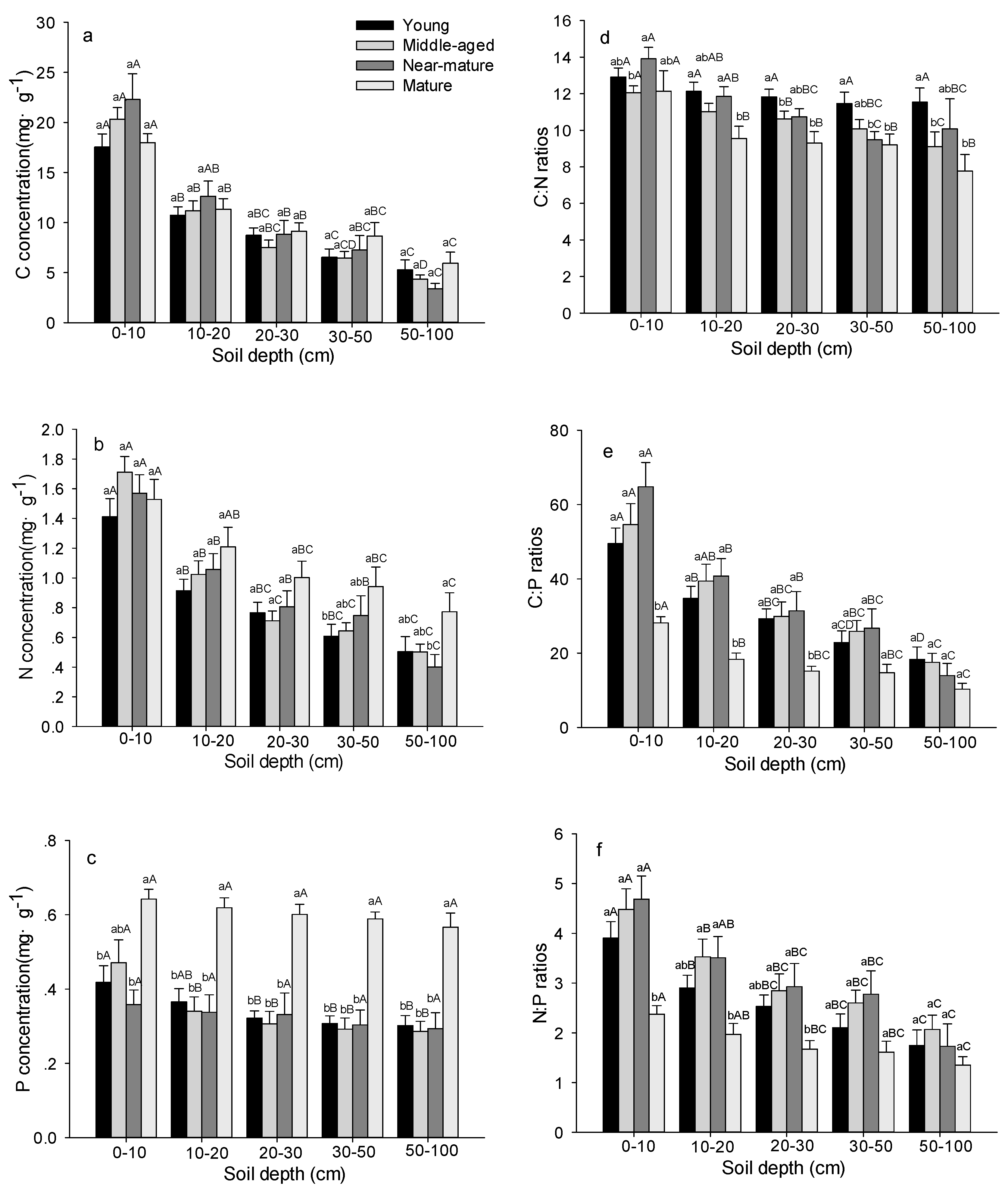
Figure 4.
C, N, and P stoichiometric characteristics in soil layer in Quercus variabilis natural forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in soil layer of different age classes; (b) N concentration in soil layer of different age classes; (c) P concentration in soil layer of different age classes; (d) C:N ratio in soil layer of different age classes; (e) C:P ratio in soil layer of different age classes; (f) N:P ratio in soil layer of different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes, and different capital letters indicated significant difference among different components in the same forest age class (p < 0.05).
Figure 4.
C, N, and P stoichiometric characteristics in soil layer in Quercus variabilis natural forest of different age classes (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) C concentration in soil layer of different age classes; (b) N concentration in soil layer of different age classes; (c) P concentration in soil layer of different age classes; (d) C:N ratio in soil layer of different age classes; (e) C:P ratio in soil layer of different age classes; (f) N:P ratio in soil layer of different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes, and different capital letters indicated significant difference among different components in the same forest age class (p < 0.05).
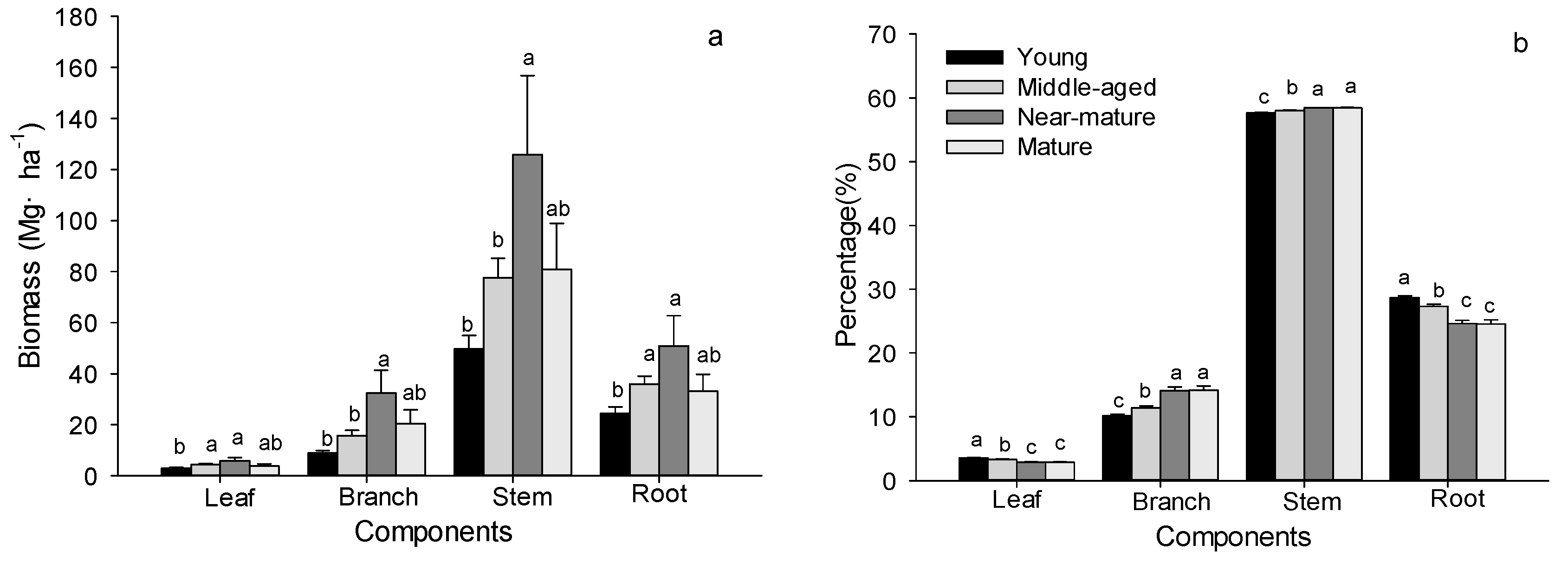
Figure 5.
Biomass and the distribution of tree components in Quercus variabilis stands (Means ± SE, Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) Biomass of each component; (b) percentage of biomass. Different small letters indicated significant difference in the same indicators among different forest age classes.
Figure 5.
Biomass and the distribution of tree components in Quercus variabilis stands (Means ± SE, Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) Biomass of each component; (b) percentage of biomass. Different small letters indicated significant difference in the same indicators among different forest age classes.
Figure 6.
Carbon storage in soil and plant layer of Quercus variabilis stands (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) Soil carbon storage at different depth; (b) forest ecosystem carbon storage among different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes.
Figure 6.
Carbon storage in soil and plant layer of Quercus variabilis stands (Means ± SE, n = 8, 6, 3 and 3 for young, middle-aged, near-mature and mature stands, respectively). (a) Soil carbon storage at different depth; (b) forest ecosystem carbon storage among different age classes. Different small letters indicated significant difference in the same indicators among different forest age classes.
Table 1.
Estimation equations for tree layer in Quercus variabilis natural forest.
Table 1.
Estimation equations for tree layer in Quercus variabilis natural forest.
| Components | Equation | R2 |
|---|
| Leaf | W = 0.012870 × D2.101 | 0.992 |
| Branch | W = 0.004734 × D2.984 | 0.983 |
| Stem | W = 0.055974 × D2.562 | 0.971 |
| Bark | W = 0.045820 × D2.104 | 0.984 |
| Roots | W = 0.083084 × D2.198 | 0.963 |
Table 2.
F and p values for the effects of stand age, tree, shrub, herb tissue and soil depth on plant and soil C:N:P stoichiometric characteristics.
Table 2.
F and p values for the effects of stand age, tree, shrub, herb tissue and soil depth on plant and soil C:N:P stoichiometric characteristics.
| Components | Factors | F (p) Value |
|---|
| C | N | P | C:N | C:P | N:P |
|---|
| Tree layer | Stand age | 1.410 (0.241) | 4.993 (0.002) | 6.032 (0.001) | 4.548 (0.004) | 6.912 (0.000) | 1.651 (0.179) |
| Tree tissue | 46.139 (0.000) | 1003.615 (0.000) | 146.409 (0.000) | 159.632 (0.000) | 52.790 (0.000) | 55.657 (0.000) |
| Stand age × Tree tissue | 6.518 (0.0000) | 2.420 (0.012) | 1.269 (0.256) | 2.635 (0.007) | 6.017 (0.000) | 4.872 (0.000) |
| Shrub layer | Stand age | 0.497 (0.685) | 1.549 (0.204) | 1.911 (0.130) | 0.437 (0.727) | 0.774 (0.510) | 1.378 (0.251) |
| Shrub tissue | 30.194 (0.000) | 25.233 (0.000) | 5.135 (0.007) | 20.265 (0.000) | 5.148 (0.007) | 8.245 (0.000) |
| Stand age × Shrub tissue | 1.096 (0.353) | 1.002 (0.394) | 0.672 (0.571) | 0.652 (0.583) | 0.868 (0.459) | 0.436 (0.728) |
| Herb layer | Stand age | 1.946 (0.127) | 0.747 (0.526) | 0.261 (0.853) | 1.126 (0.342) | 0.891 (0.448) | 1.305 (0.277) |
| Herb tissue | 347.801 (0.000) | 200.72 (0.000) | 24.823 (0.000) | 0.021 (0.886) | 2.785 (0.098) | 6.583 (0.012) |
| Stand age × Herb tissue | 0.457 (0.713) | 0.576 (0.632) | 3.092 (0.030) | 0.163 (0.921) | 1.592 (0.196) | 1.827 (0.147) |
| Soil layer | Stand age | 1.028 (0.381) | 3.561 (0.015) | 27.906 (0.000) | 10.801 (0.000) | 9.846 (0.000) | 9.092 (0.000) |
| Soil depth | 76.843 (0.000) | 44.147 (0.000) | 3.559 (0.008) | 11.902 (0.000) | 28.427 (0.000) | 15.572 (0.000) |
| Stand age × Soil depth | 1.038 (0.414) | 0.755 (0.696) | 0.382 (0.969) | 0.869 (0.579) | 0.836 (0.614) | 0.389 (0.967) |
Table 3.
Biomass of tree layer in Quercus variabilis stands.
Table 3.
Biomass of tree layer in Quercus variabilis stands.
| Age class | Stand Density (tree·ha−1) | Single Tree Biomass (kg·tree−1) | Biomass Per Hectare (Mg·ha−1) |
|---|
| Young | 1807.89 ± 196.43 a | 53.22 ± 5.37 b | 86.03 ± 9.21 b |
| Middle-aged | 1717.35 ± 178.71 a | 93.36 ± 19.13 b | 133.7 ± 13.06 b |
| Near-mature | 808.16 ± 79.88 b | 243.89 ± 44.81 a | 215.01 ± 53.07 a |
| Mature | 574.98 ± 62.09 b | 248.66 ± 57.48 a | 138.35 ± 30.80 ab |
Table 4.
Biomass and the distribution of Quercus variabilis natural forest.
Table 4.
Biomass and the distribution of Quercus variabilis natural forest.
| Age Class | Tree | Shrub | Herb | Litter | Total |
|---|
| Biomass (Mg·ha−1) | Percentage (%) | Biomass (Mg·ha−1) | Percentage (%) | Biomass (Mg·ha−1) | Percentage (%) | Biomass (Mg·ha−1) | Percentage (%) | Biomass (Mg·ha−1) | Percentage (%) |
|---|
| Young | 86.03 ± 9.21 b | 88.53 b | 2.49 ± 0.33 bc | 3.59 a | 1.03 ± 0.13 ab | 1.33 a | 6.21 ± 0.79 a | 6.54 a | 95.77 ± 9.73 b | 100 |
| Middle-aged | 133.7 ± 13.06 b | 94.23 a | 1.39 ± 0.19 c | 1.22 b | 0.60 ± 0.09 c | 0.52 b | 5.42 ± 0.78 a | 4.02 b | 141.11 ± 13.19 b | 100 |
| Near-mature | 215.01 ± 53.07 a | 93.43 a | 6.87 ± 2.26 a | 3.41 ab | 0.67 ± 0.08 bc | 0.45 b | 3.64 ± 0.59 a | 2.71 b | 226.20 ± 52.36 a | 100 |
| Mature | 138.35 ± 30.80 ab | 91.94 ab | 3.80 ± 1.84 ab | 3.11 ab | 1.48 ± 0.30 a | 1.21 a | 4.98 ± 1.25 a | 3.73 b | 148.61 ± 31.25 ab | 100 |
Table 5.
Carbon storage allocation of Quercus variabilis natural forests (Mg·ha−1).
Table 5.
Carbon storage allocation of Quercus variabilis natural forests (Mg·ha−1).
| Age Class | Tree | Shrub | Herb | Litter | Plant |
|---|
| Leaf | Branch | Stem | Root |
|---|
| Young | 1.39 ± 0.14 b | 3.95 ± 0.44 b | 21.82 ± 2.32 c | 10.55 ± 1.16 b | 1.07 ± 0.14 b | 0.28 ± 0.03 ab | 2.15 ± 0.24 a | 41.20 ± 4.19 b |
| Middle-aged | 2.04 ± 0.18 a | 7.17 ± 0.97 b | 34.80 ± 3.54 b | 15.40 ± 1.32 ab | 0.60 ± 0.08 b | 0.17 ± 0.02 c | 1.96 ± 0.26 a | 62.13 ± 6.00 b |
| Near-mature | 2.77 ± 0.64 a | 14.80 ± 4.12 a | 56.45 ± 14.14 a | 21.89 ± 5.15 a | 2.94 ± 1.00 a | 0.19 ± 0.02 bc | 1.36 ± 0.22 a | 100.40 ± 23.71 a |
| Mature | 1.78 ± 0.34 ab | 9.08 ± 2.34 ab | 31.99 ± 6.53 bc | 14.89 ± 3.01 ab | 1.56 ± 0.73 ab | 0.33 ± 0.05 a | 1.73 ± 0.40 a | 61.45 ± 12.28 b |
Table 6.
Relationships between biomass of different layers and C, N, and P stoichiometry in tree, shrub, herb leaves and litter among different age classes.
Table 6.
Relationships between biomass of different layers and C, N, and P stoichiometry in tree, shrub, herb leaves and litter among different age classes.
| Age Class | Biomass | C | N | P | C:N | C:P | N:P |
|---|
| Young | Tree | −0.507 * | 0.288 | 0.486 * | −0.349 | −0.496 * | −0.057 |
| Shrub | 0.594 ** | 0.542 ** | 0.466 ** | −0.486 * | −0.493 * | 0.059 |
| Herb | −0.087 | −0.300 | −0.243 | 0.133 | 0.179 | 0.172 |
| Litter | −0.652 ** | −0.215 | −0.289 | −0.410 * | −0.131 | −0.092 |
| Middle-aged | Tree | 0.504 * | −0.355 | −0.251 | 0.39 | 0.351 | −0.173 |
| Shrub | −0.122 | −0.282 | 0.036 | 0.268 | −0.042 | −0.107 |
| Herb | −0.074 | −0.321 | −0.582 * | 0.132 | 0.316 | 0.297 |
| Litter | −0.487 * | −0.177 | 0.248 | −0.156 | −0.235 | −0.249 |
| Near-mature | Tree | 0.523 | 0.608 | 0.446 | −0.608 | −0.464 | 0.386 |
| Shrub | 0.003 | −0.608 | −0.556 | 0.722 * | 0.770 * | 0.659 |
| Herb | −0.047 | 0.115 | 0.113 | −0.063 | −0.213 | −0.093 |
| Litter | −0.205 | −0.222 | −0.719 * | 0.074 | 0.743 * | 0.790 * |
| Mature | Tree | −0.526 | 0.725 | −0.526 | −0.762 | −0.526 | 0.766 |
| Shrub | −0.049 | 0.601 | 0.559 | −0.52 | −0.465 | 0.506 |
| Herb | −0.726 | 0.415 | −0.76 | −0.542 | 0.73 | 0.813 * |
| Litter | −0.634 | 0.501 | −0.79 | −0.676 | 0.837 * | 0.663 |