Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
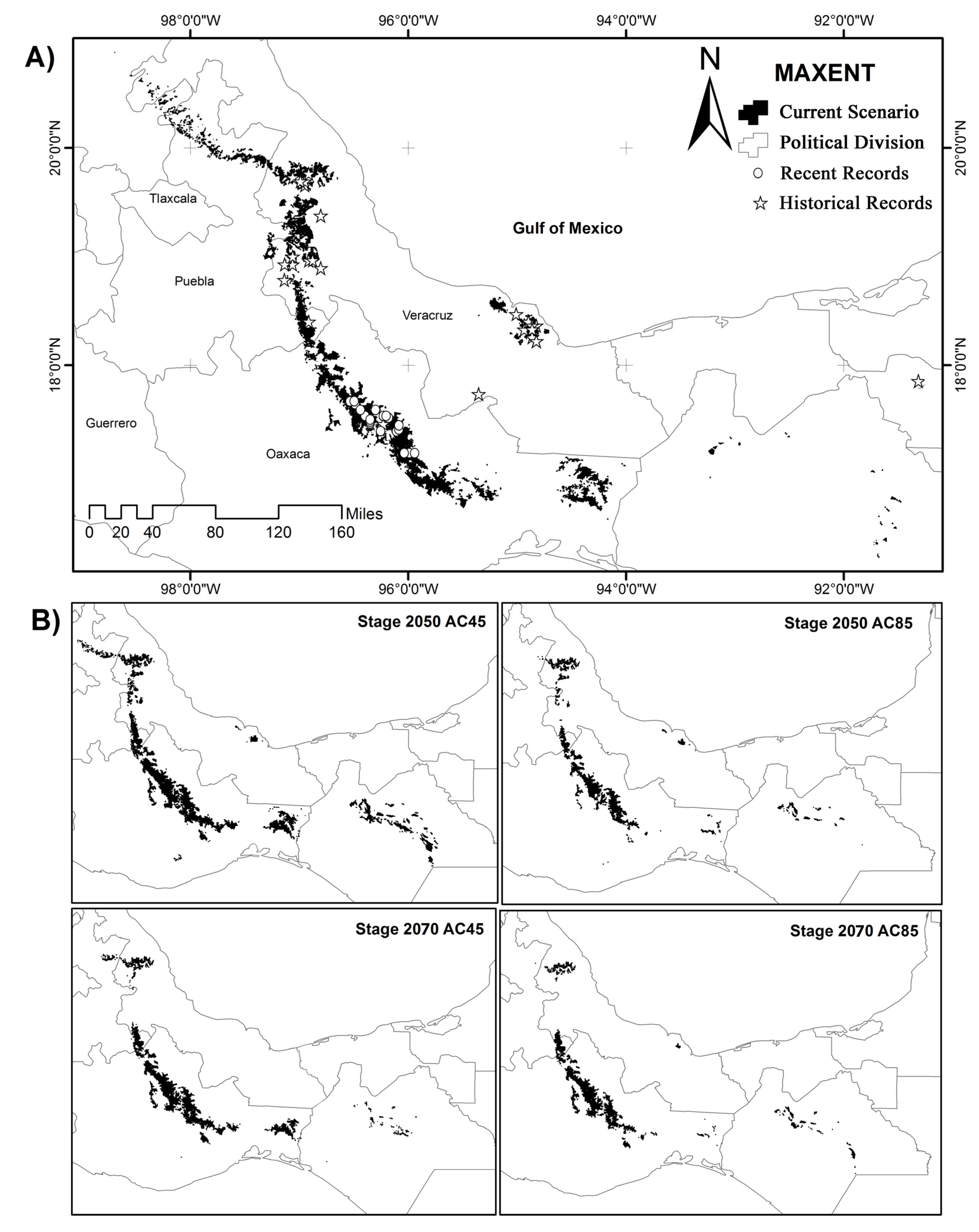
2.3. Potential Distribution and Future Climate Scenarios to Regional and National Level
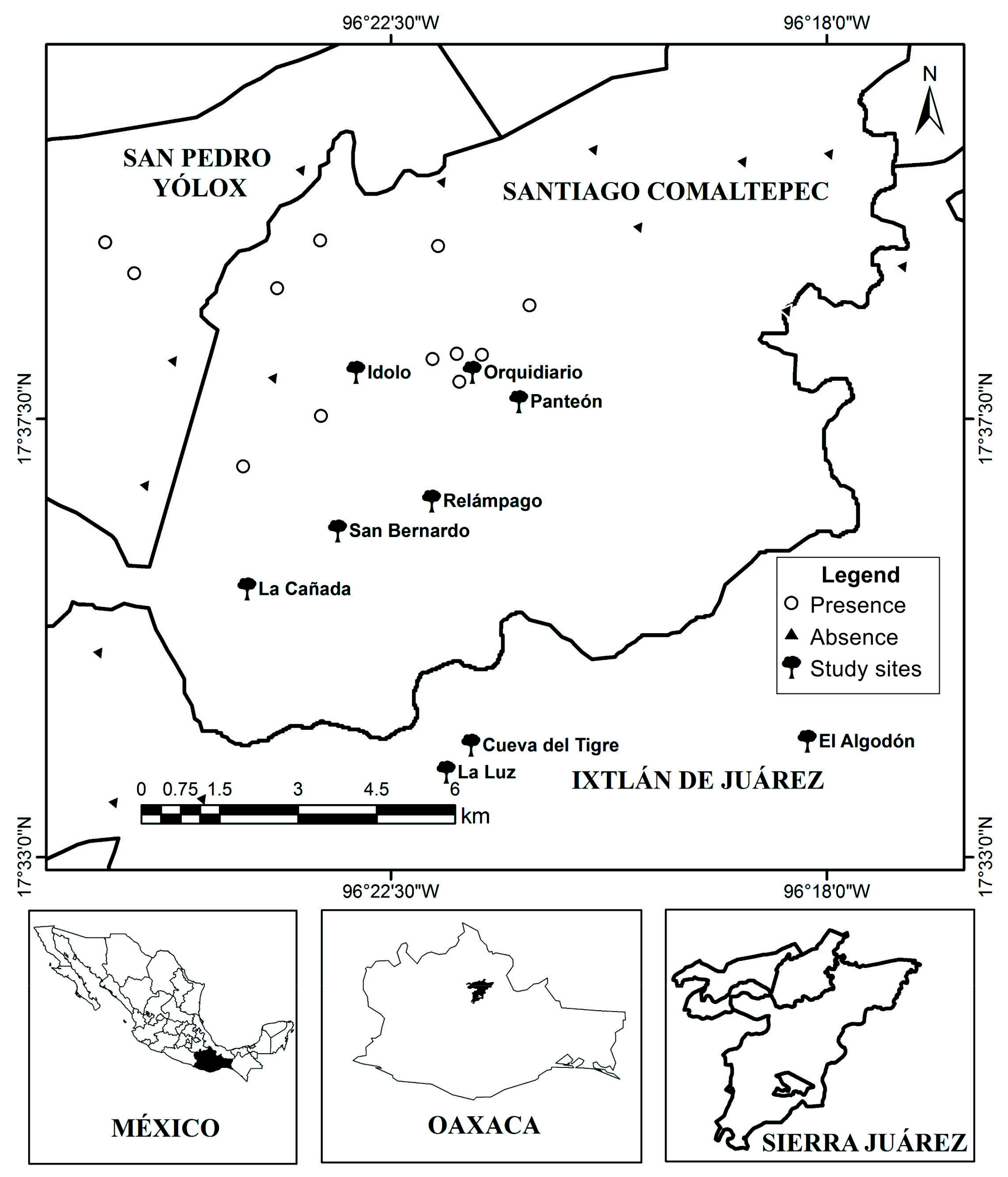
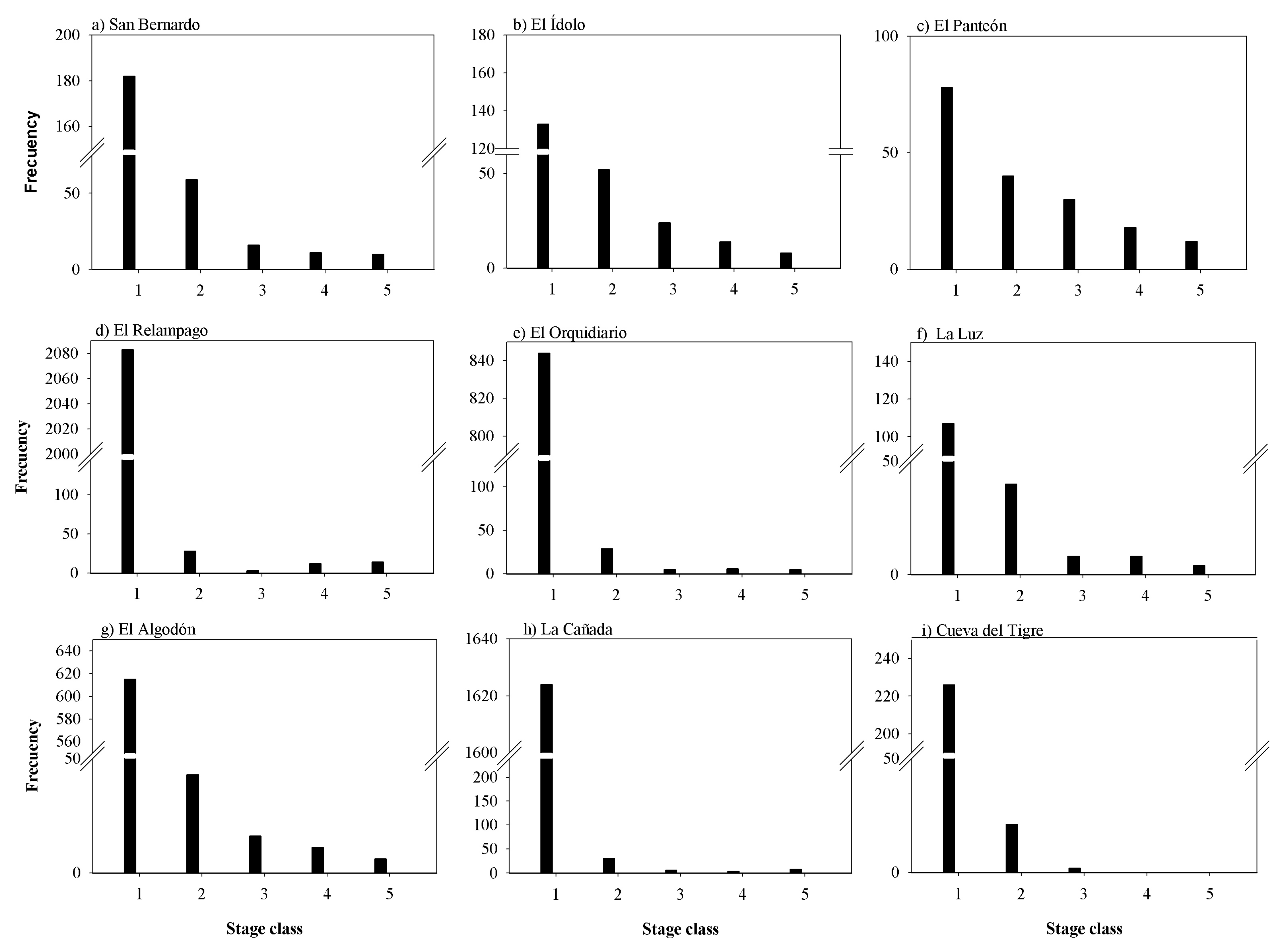
2.4. Abundance, Density and Spatial and Static Size Stage Distributions at Regional Level
2.5. Analysis of the O. mexicana Niche
3. Results
3.1. Potential Distribution and Future Climate Scenarios
3.2. Abundance, Density, Age Structure and Spatial Distribution at Regional Level
3.3. Analysis of the Oreomunnea mexicana Niche
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bubb, P.; May, I.; Miles, L.; Sayer, J. Cloud Forest Agenda; UNEP World Conservation Monitoring Centre: Cambridge, UK, 2004. [Google Scholar]
- Toledo-Aceves, A.; Meave, A.J.; González-Espinosa, M.; Ramírez-Marcial, N. Tropical montane cloud forests: Current threats and opportunities for their conservation and sustainable management in Mexico. J. Environ. Manag. 2011, 92, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, G.R.; Matzke, N.J.; Dawson, T.E. The incidence and implications of clouds for cloud forest plant water relations. Ecol. Lett. 2013, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Soto, O.R.; Sosa, V.; Ornelas, J.F. Forecasting cloud forest in Eastern and southern México: Conservation insights under future climate change scenarios. Biodivers. Conserv. 2012, 21, 2671–2690. [Google Scholar] [CrossRef]
- Ponce-Reyes, R.; Reynoso-Rosales, V.H.; Watson, J.E.; VanDerWal, J.; Fuller, R.A.; Pressey, R.L.; Possingham, H.P. Vulnerability of cloud forest reserves in Mexico to climate change. Nat. Clim. Chang. 2012, 2, 448–452. [Google Scholar] [CrossRef]
- Challenger, A.; Soberón, J. Los Ecosistemas Terrestres. In Capital Natural de México. Vol. I Conocimiento actual de la biodiversidad, 1st ed.; CONABIO: Mexico D.F., Mexico, 2008; pp. 87–108. [Google Scholar]
- González-Espinosa, M.; Meave, J.A.; Lorea-Hernández, F.G.; Ibarra-Manríquez, G.; Newton, A.C. The Red List of Mexican Cloud Forest Trees, 1st ed.; Fauna and Flora International: Cambridge, UK, 2011; pp. 1–148. ISBN 9781903703281. [Google Scholar]
- Téllez-Valdés, O.; Dávila-Aranda, P.; Lira-Saade, R. The effects of climate change on the long-term conservation of Fagus grandifolia var. mexicana, an important species of the cloud forest in Eastern Mexico. Biodivers. Conserv. 2006, 15, 1095–1107. [Google Scholar] [CrossRef]
- Graham, A. The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. Am. J. Bot. 2011, 98, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Williams-Linera, G.; Toledo-Garibaldi, M.; Gallardo-Hernández, C. How heterogeneous are the cloud forest communities in the central mountains of central Veracruz, México? Plant. Ecol. 2013, 214, 685–701. [Google Scholar] [CrossRef]
- Corrales, A.; Arnold, A.E.; Ferrer, A.; Turner, B.L.; Dalling, J.W. Variation in ectomycorrhizal fungal communities associated with Oreomunnea mexicana (Juglandaceae) in a Neotropical montane forest. Mycorrhiza 2016, 26, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zimmernann, M.; Vischer-Leopold, M.; Ellwanger, G.; Ssymank, A.; Schröder, E. The EC habitats directive and the German natura 2000 network of protected areas as tool for implementary. In Relic Species: Phylogeography and Conservation Biology, 1st ed.; Habel, J.C., Assaman, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 323–340. [Google Scholar]
- Habel, J.C.; Assmann, T.; Schmitt, T. Relic Species: From past to future. In Relic Species: Phylogeography and Conservation Biology, 1st ed.; Habel, J.C., Assaman, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–9. [Google Scholar]
- Rödder, D.; Dambach, J. Modeling futures trends of relic specie. In Relic Species: Phylogeography and Conservation Biology, 1st ed.; Habel, J.C., Assaman, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 385–400. [Google Scholar]
- Russell, J.; Cohn, R. Oreomunnea mexicana, 1st ed.; Bookvika publishing: Alplaus, NY, USA, 2012. [Google Scholar]
- Rzedowski, J.; Palacios-Chávez, R. El bosque de Engelhardtia (Oreomunnea mexicana) en la región de la Chinantla, Oaxaca, México, una reliquia del Cenozoico. Bol. Soc. Bot. Mex. 1977, 36, 93–123. [Google Scholar] [CrossRef]
- Anta-Fonseca, S.; Galindo-Leal, C.; González-Medrano, F.; Koleff-Osorio, P.; Meave del Castillo, J.; Moya-Moreno, H.; Victoria-Hernández, A. Sierra Norte de Oaxaca. In El bosque mesófilo de montaña en México: Amenazas y Oportunidades Para su Conservación y su Manejo Sostenible, 1st ed.; CONABIO: Mexico D.F., Mexico, 2010; pp. 108–115. [Google Scholar]
- Gaston, K.J. Rarity, 1st ed.; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Clark-Tapia, R.; Suárez-Mota, M.E.; Matías-Nolasco, M.; Velasco Hipólito, F. Clima: Pasado, presente y futuro. In Recursos Hídricos de la Sierra Norte de Oaxaca, Caracterización, Diagnóstico y Gestión, 1st ed.; Clark-Tapia, R., Ramos-Morales, M.F., Alfonso-Corrado, C., Mendoza-Díaz, M.G., Fuente-Carrasco, M., Eds.; Universidad de la Sierra Juárez: Oaxaca, Mexico, 2016; pp. 48–58. [Google Scholar]
- Naranjo-Luna, F.J. Ecología y Genética de Oreomunnea mexicana (Stand) J.F., (Juglandaceae), Especie Relicto del Bosque de Niebla de la Sierra Juárez, Oaxaca. Master’s Thesis, Universidad de la Sierra Juárez, Oaxaca, Mexico, 2014; p. 115. [Google Scholar]
- Unión de Comunidades Forestales Zapotecas-Chinantecas (UZACHI). Programa de Manejo Forestal para el Predio de Capulápam de Méndez, Municipio del Mismo Nombre (2003–2013); UZACHI: Oaxaca, Mexico, 2003; p. 200. [Google Scholar]
- Villaseñor, J.L.; Téllez-Valdés, O. Distribución potencial de las especies del género Jefea (Asteraceae) en México. An. Inst. Biol. Ser. Bot. Méx. 2004, 75, 205–220. [Google Scholar]
- Mota-Vargas, C.; Rojas-Soto, O.R. The importance of defining the geographic distribution of species for conservation: The case of the Bearded Wood-Partridge. J. Nat. Conserv. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility (GBIF). Available online: http://data.gbif.org (accessed on 14 July 2017).
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO). Available online: http://www.conabio.gob.mx/remib/doctos/remibnodosdb.html (accessed on 14 July 2017).
- Pearson, R.G.; Raxworthy, C.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 3, 102–117. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Estrada-Contreras, I.; Equihua, M.; Castillo-Campos, G.; Rojas-Soto, O. Climate change and effects on vegetation in Veracruz, Mexico: An approach using ecological niche modelling. Acta Bot. Mex. 2015, 112, 73–93. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Environmental Scientific Research Institute (ESRI). ArcView, version 3.x; ESRI: Redlands, CA, USA, 2000. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distribution. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent new extensions and comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Menon, S.; Choudhury, B.I.; Khan, M.L.; Peterson, A.T. Ecological niche modeling and local knowledge predict new populations of Gymnocladus assamicus a critically endangered tree species. Endanger. Species Res. 2010, 11, 175–181. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Estadística, Geografía e Informática (INEGI); Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; Instituto Nacional de Ecología. Ecorregiones terrestres de México; CONABIO: Mexico D.F., Mexico, 2008. [Google Scholar]
- Brook, R.K.; McLachlan, S.M. Trends and prospects for local knowledge in ecological and conservation research and monitoring. Biodivers. Conserv. 2008, 17, 3501–3512. [Google Scholar] [CrossRef]
- Alfonso-Corrado, C.; Clark-Tapia, R.; Mendoza, A. Demography and management of two clonal oaks: Quercus eduardii and Q. potosina (Fagaceae) in central México. For. Ecol. Manag. 2007, 251, 129–141. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT (2014) XLSTAT Software. Paris, France. Available online: https://www.xlstat.com/es/1995-2014 (accessed on 15 March 2015).
- Ter Braak, C.J. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Hammer, Q.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche separation in community analysis: A new method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Thioulouse, J.; Chessel, D.; Dole, S.; Olivier, J.M. ADE-4 a multivariate analysis and graphical display software. Stat. Comput. 1997, 7, 75–83. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidisa, I.; Karagiannakidoua, V.; Alifragisb, D. Niche analysis and conservation of the orchids of East Macedonia (NE Greece). Acta Oecol. 2008, 33, 27–35. [Google Scholar] [CrossRef]
- Deka, J.; Bharali, S.; Kr Das, K.; Tripath, O.P.; Latif Khan, M. Mapping the potential distribution of Pinus merkusii Jungh Et De Vries. A vulnerable gymnosperm in eastern Arunachal Pradesh using maximum entropy model. Asian J. Geoinform. 2013, 13, 16–22. [Google Scholar]
- Contreras-Medina, R.; Luna-Vega, I.; Ríos-Muñoz, C.A. Distribución de Taxus globosa (Taxaceae) en México: Modelos ecológicos de nicho, efecto del cambio del uso de suelo y conservación. Rev. Chil. Hist. Nat. 2010, 83, 421–433. [Google Scholar] [CrossRef]
- Vargas-Rodríguez, Y.L.; Platt, W.J. Remnant sugar maple (Acer saccharum subsp. skutchii) populations at their range edge: Characteristics, environmental constraints and conservation implications in tropical America. Biol. Conserv. 2012, 150, 111–120. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, E.; Ornelas, F.J. Phylogeography of Liquidambar styraciflua (Altingiaceae) in Mesoamerica: survivors of a Neogene widespread temperate forest (or cloud forest) in North America? Ecol. Evol. 2014, 4, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Quante, M. The Changing Climate: Past, Present, Future. In Relic Species: Phylogeography and Conservation Biology, 1st ed.; Habel, J.C., Assaman, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 10–57. [Google Scholar]
- Sandoval-García, R. Vulnerabilidad Hídríca de la Subcuenca Valle Nacional, Oaxaca. Master’s Thesis, Universidad de la Sierra Juárez, Oaxaca, Mexico, 2014. [Google Scholar]
- De Souza, I.; Souza, A.F.; Pizo, M.A.; Ganade, G. Using tree population tree size structures to assess the impacts of cattle grazing and eucalypts plantations in subtropical South America. Biodivers. Conserv. 2010, 19. [Google Scholar] [CrossRef]
- Pacheco-Cruz, N.J. Dinámica poblacional y regeneración de Oreomunnea mexicana, (Standl.) J.F. Leroy (Juglandaceae), Especie Relicto del Bosque de Niebla en la Sierra Juárez. Bachelor’s Thesis, Universidad de la Sierra Juárez, Oaxaca, Mexico, 2016. [Google Scholar]
- Brodie, H.J. The Bird´s Nest Fungi, 1st ed.; University of Toronto Press: Toronto, ON, Canada, 2016. [Google Scholar]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. Wiley Interdisciplinary. Rev. Clim. Chang. 2014, 5, 317–335. [Google Scholar] [CrossRef] [Green Version]
- Huber, D.C.; Gulledge, J. Extreme Weather & Climatic Change: Understanding the Link and Managing the Risk, 1st ed.; Center for Climate and Energy Solutions: Arlington, VA, USA, 2011; pp. 1–11. [Google Scholar]
- SEMARNAT. Plan Nacional de Desarrollo Sustentable. Programa Especial de Cambio Climático 2014–2018 (PECC), 1st ed.; SERMANAT: Mexico City, Mexico, 2013–2018; pp. 1–85. [Google Scholar]
- Bazzaz, F.A. Habitat selection in plants. Am. Nat. 1991, 137, 116–130. [Google Scholar] [CrossRef]
- Harms, K.E.; Condit, R.; Hubbell, S.P.; Foster, R.B. Habitat associations of trees and shrubs in a 50 ha Neotropical forest plot. J. Ecol. 2001, 89, 947–959. [Google Scholar] [CrossRef]
- Guerrero-Aguirre, G.F. Caracterización poblacional de cinco especies arbóreas ecológicamente importantes en el Corredor Biológico Turrialba Jiménez Costa Rica. Master’s Thesis, Centro Agronómico Tropical de Investigación y Enseñanza, Turrialba, Costa Rica, 2005. [Google Scholar]

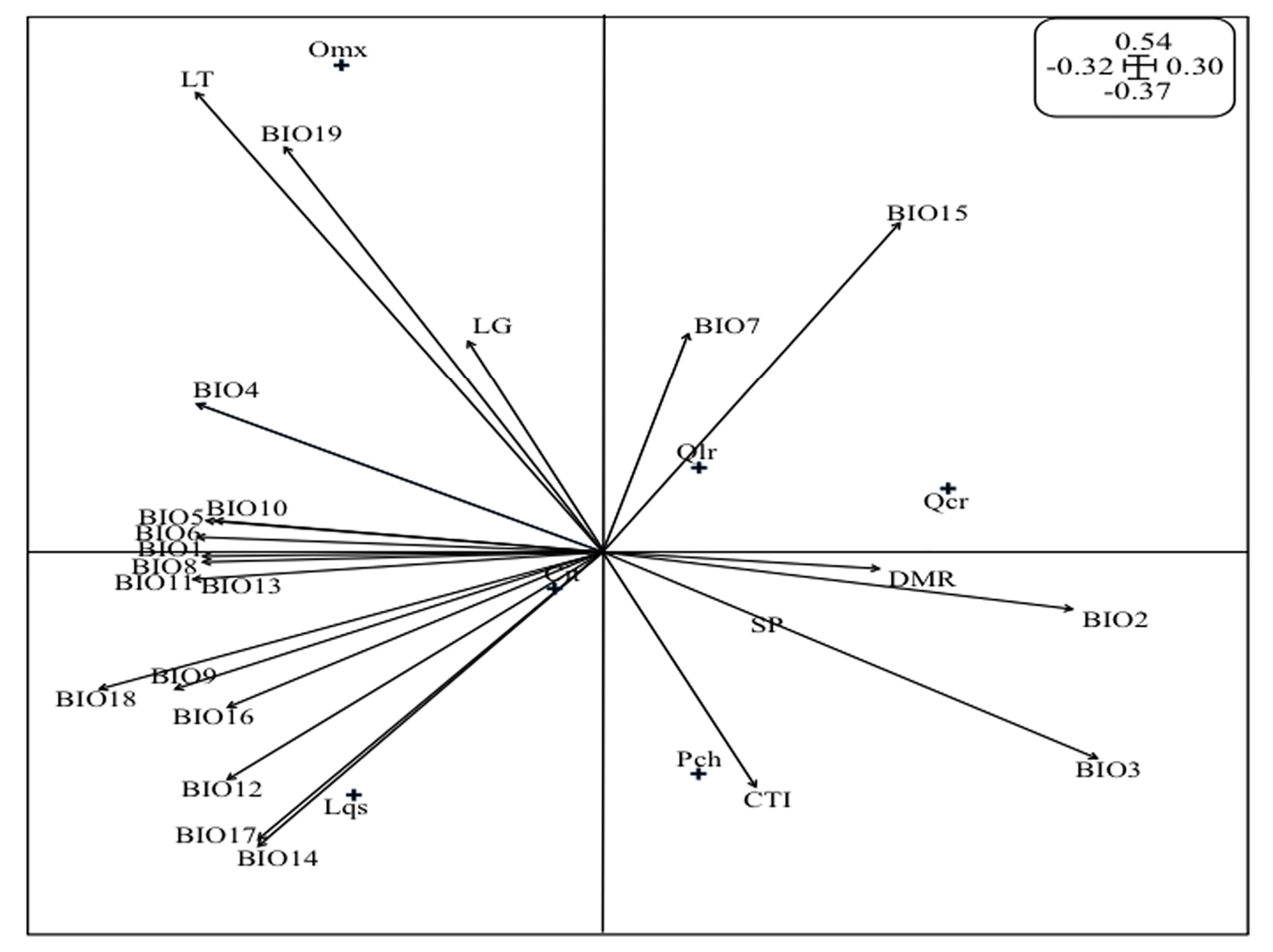
| Species | Inertia | OMI | T1 | T2 | P |
|---|---|---|---|---|---|
| O. mexicana (Omx) | 18.05 | 10.87 | 7.26 | 8.69 | 0.03 * |
| L. styraciflua (Lqs) | 29.7 | 0.115 | 14.5 | 28.8 | 0.45 NS |
| S. horrida (Sho) | 24.4 | 0.048 | 14.5 | 9.22 | 0.36 NS |
| P. chiapensis (Pch) | 31.2 | 1.06 | 11.0 | 19.1 | 0.35 NS |
| Q. corrugata (Qcr) | 23.9 | 7.38 | 6.42 | 10.1 | 0.02 * |
| Q. laurina (Qlr) | 29.7 | 2.35 | 9.31 | 17.1 | 0.29 NS |
| C. integerrima (Cit) | 26.3 | 0.122 | 13.6 | 18.26 | 0.48 NS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfonso-Corrado, C.; Naranjo-Luna, F.; Clark-Tapia, R.; Campos, J.E.; Rojas-Soto, O.R.; Luna-Krauletz, M.D.; Bodenhorn, B.; Gorgonio-Ramírez, M.; Pacheco-Cruz, N. Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest. Forests 2017, 8, 261. https://doi.org/10.3390/f8080261
Alfonso-Corrado C, Naranjo-Luna F, Clark-Tapia R, Campos JE, Rojas-Soto OR, Luna-Krauletz MD, Bodenhorn B, Gorgonio-Ramírez M, Pacheco-Cruz N. Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest. Forests. 2017; 8(8):261. https://doi.org/10.3390/f8080261
Chicago/Turabian StyleAlfonso-Corrado, Cecilia, Francisco Naranjo-Luna, Ricardo Clark-Tapia, Jorge E. Campos, Octavio R. Rojas-Soto, María Delfina Luna-Krauletz, Barbara Bodenhorn, Montserrat Gorgonio-Ramírez, and Nelly Pacheco-Cruz. 2017. "Effects of Environmental Changes on the Occurrence of Oreomunnea mexicana (Juglandaceae) in a Biodiversity Hotspot Cloud Forest" Forests 8, no. 8: 261. https://doi.org/10.3390/f8080261





