Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Litter Input Collection and Litterbag Experiment
2.3. Forest Floor and Topsoil Sampling
2.4. Chemical Analyses
2.5. Statistical Analysis
3. Results
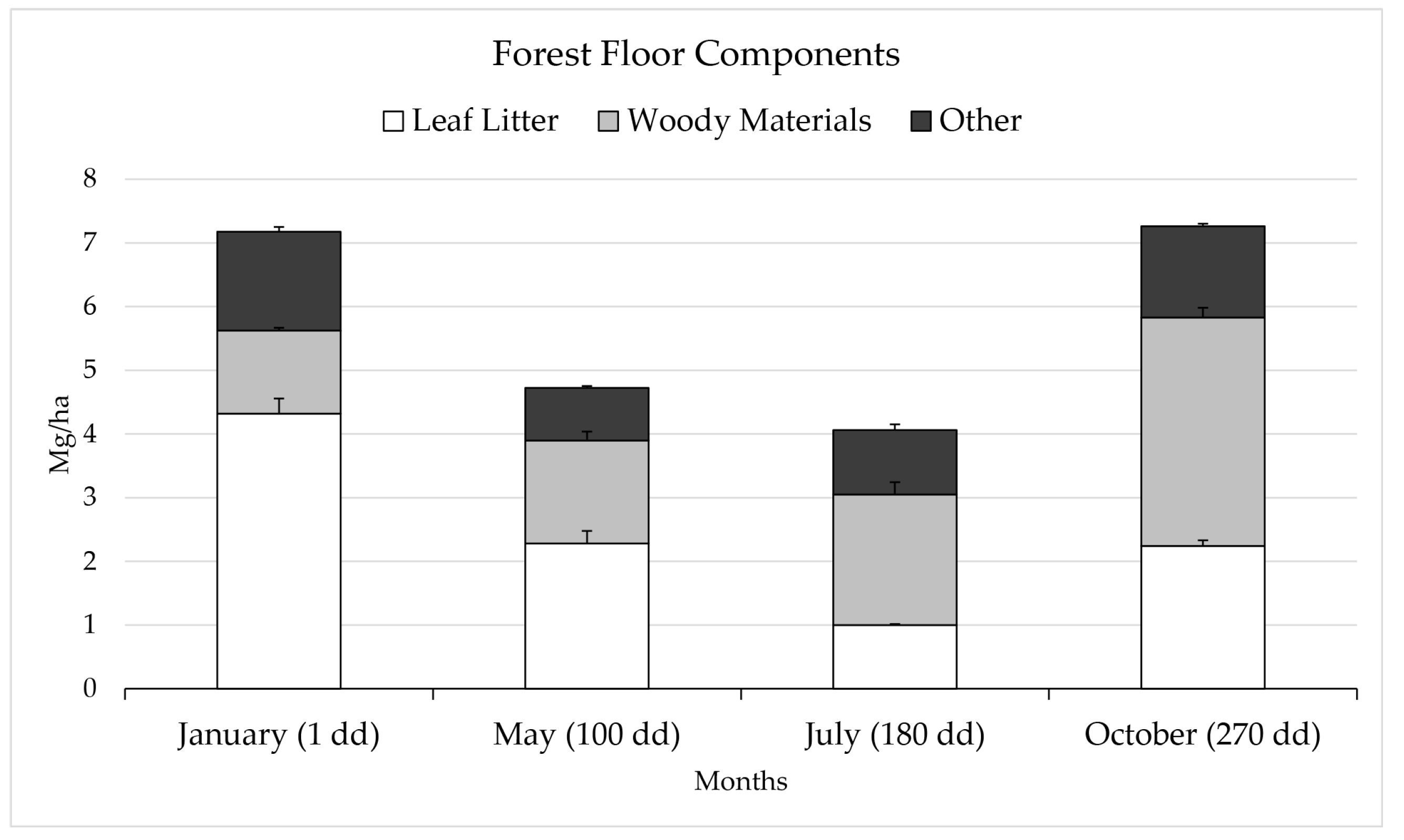
3.1. Litter Fall, Decomposition Dynamics and Forest Floor
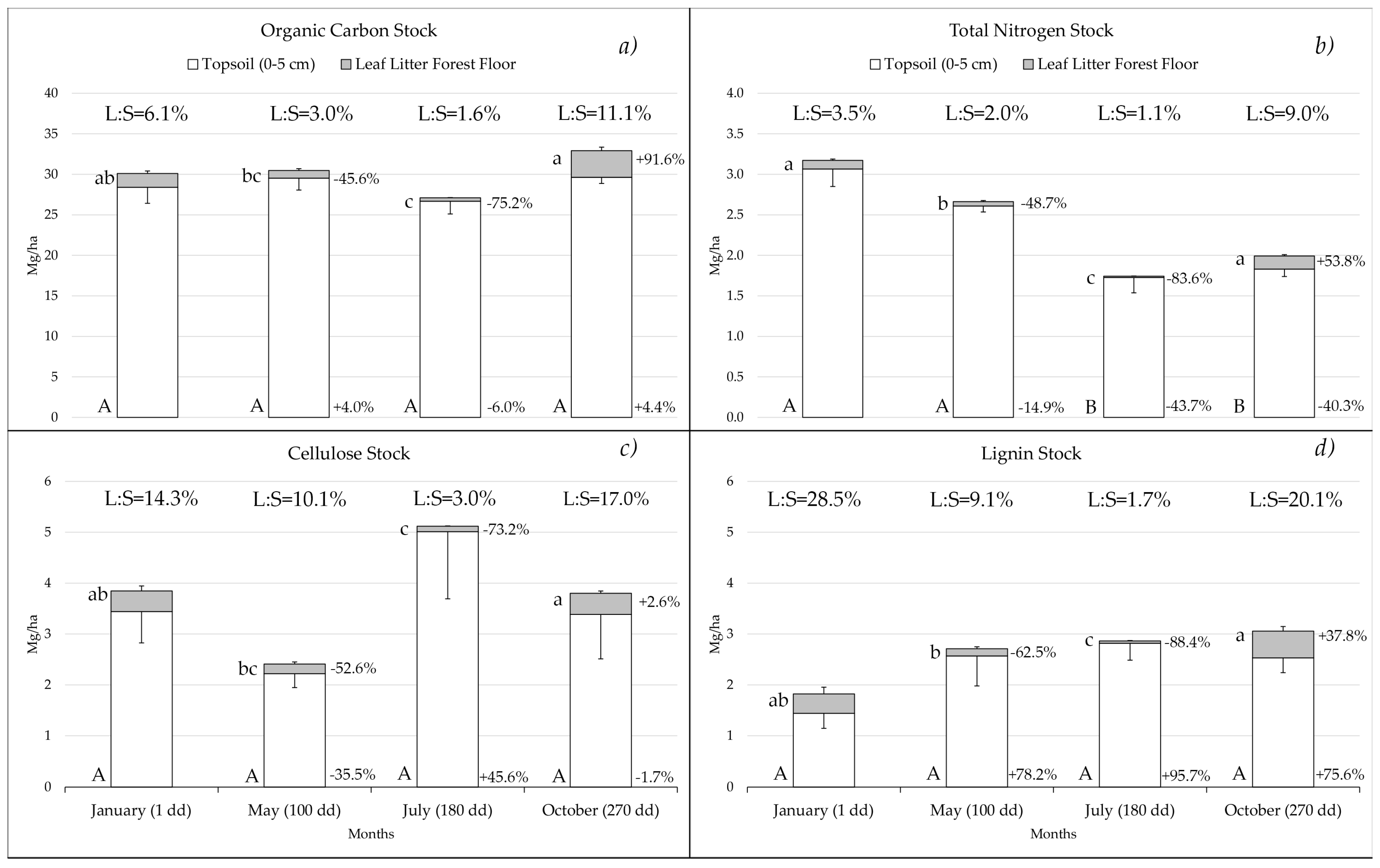
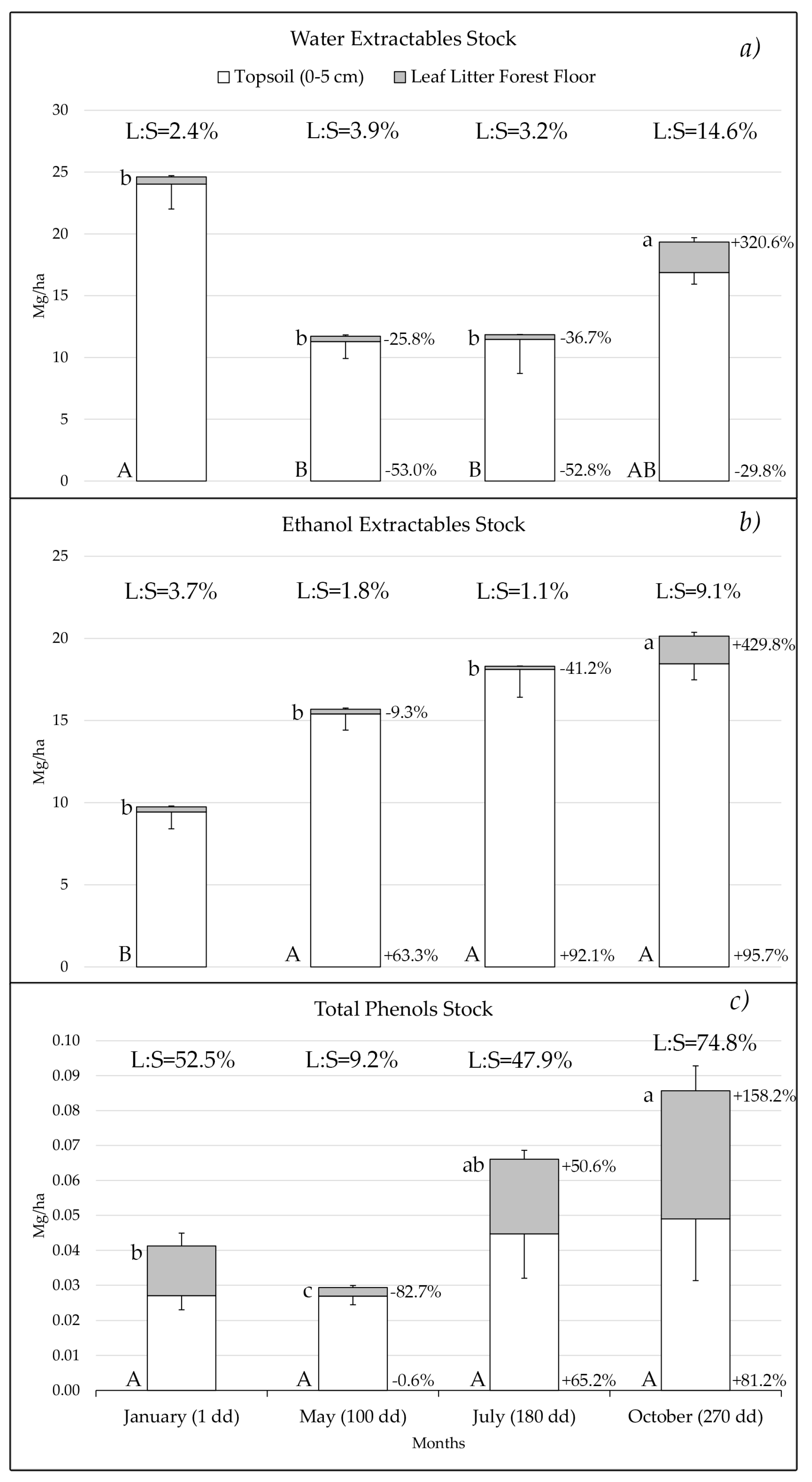
3.2. Dynamics of the Stocks in the Forest Floor Leaf Litter and Topsoil
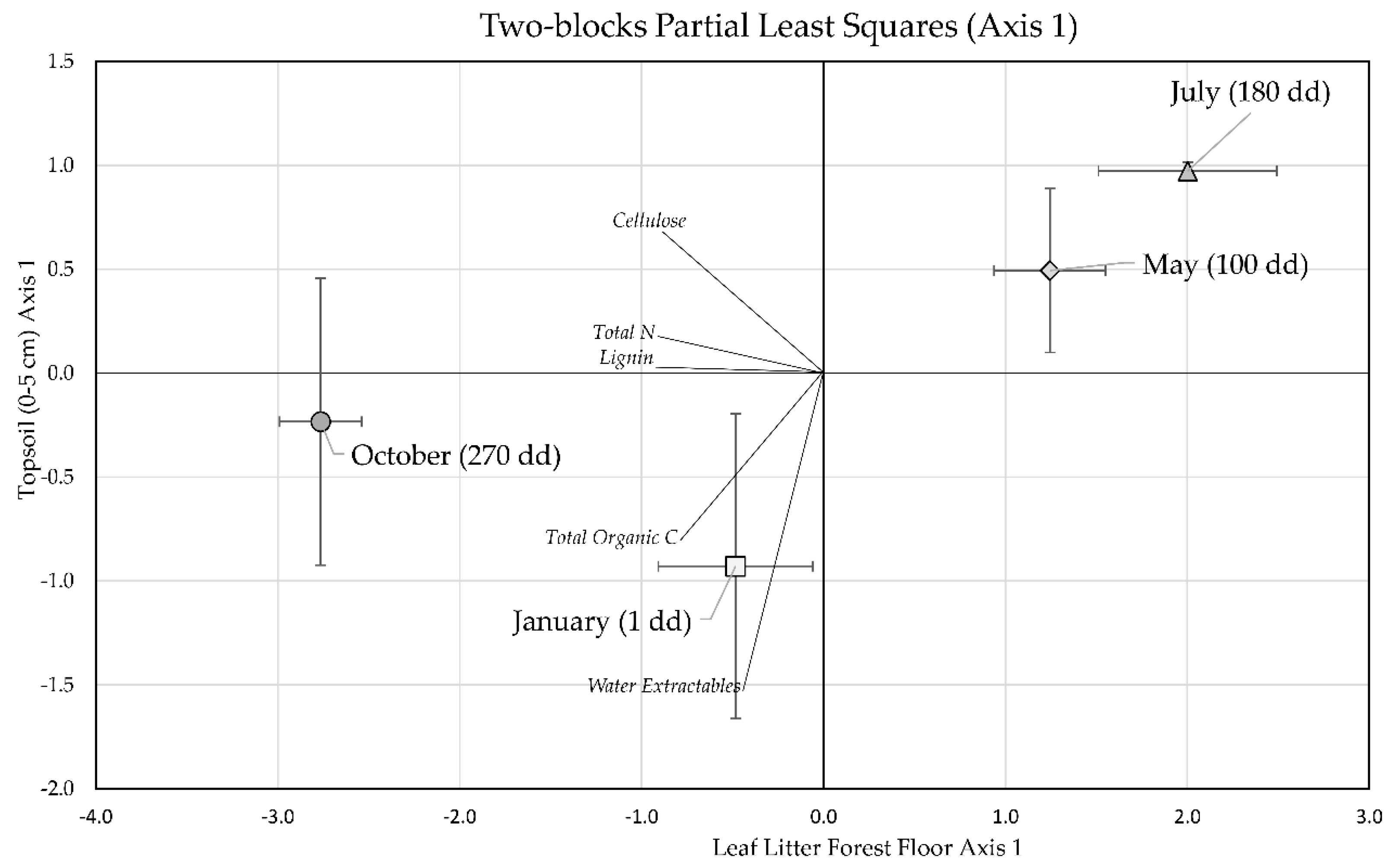
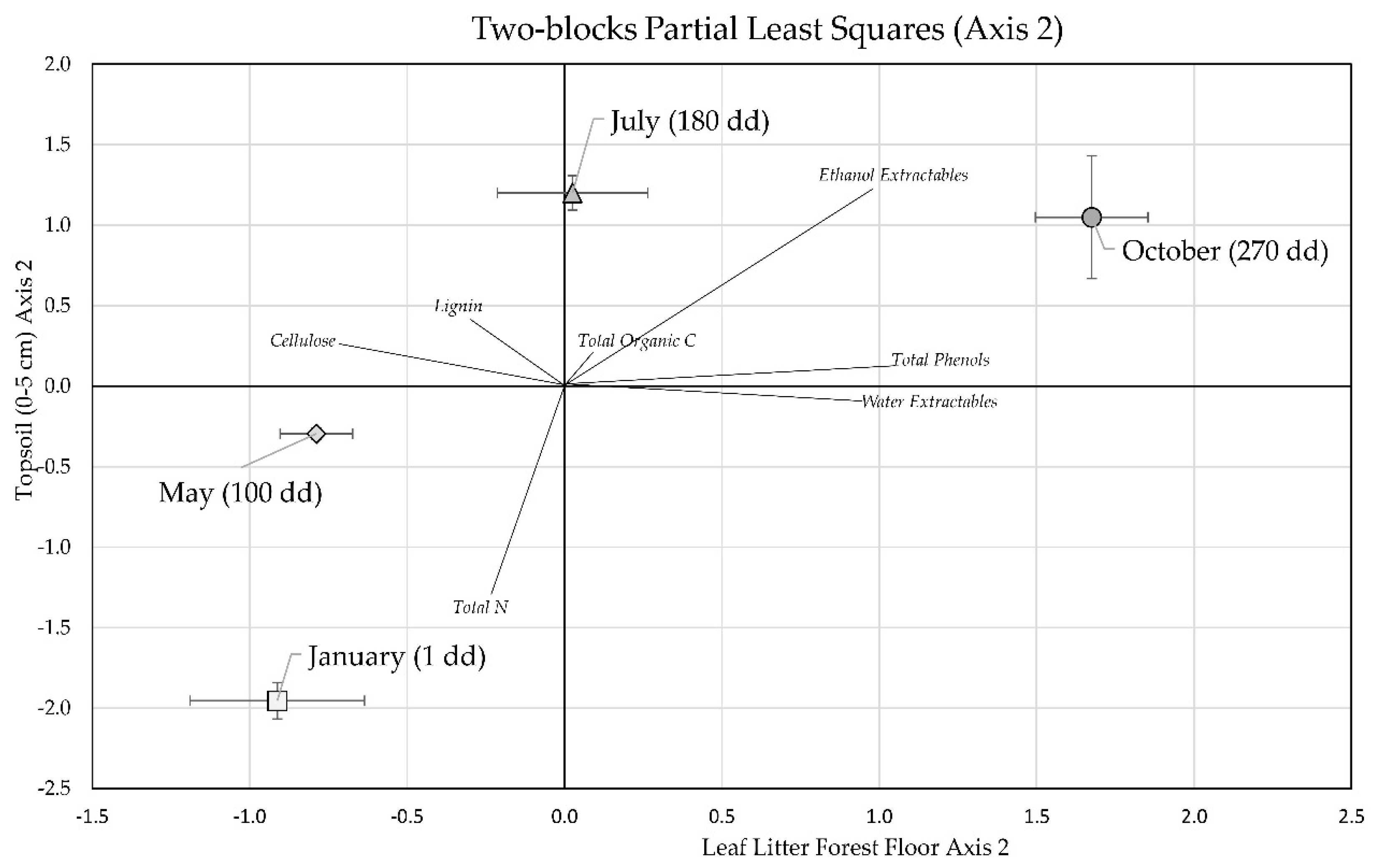
3.3. Two-Block Partial Least Squares Analysis of Forest Floor Leaf Litter and Topsoil Stocks
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.K.; Brown, S.; Houghton, R.A.; Solomon, A.M.; Trexler, M.C.; Wisniewski, J. Carbon pools and flux of global forest ecosystems. Science 1994, 263, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.A.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; Euskirchen, E.S. Carbon cycling and storage in world forests: Biome patterns related to forest age. Glob. Chang. Biol. 2004, 10, 2052–2077. [Google Scholar] [CrossRef]
- Triadiati, S.; Tjitrosemito, B.; Guhardja, E.; Sudarsono, I.; Qayim, I.; Leuschner, C. Litterfall production and leaf-litter decomposition at natural forest and cacao agroforestry in Central Sulawesi, Indonesia. Asian J. Biol. Sci. 2011, 4, 221–234. [Google Scholar]
- Innangi, M.; Schenk, M.K.; D’Alessandro, F.; Pinto, S.; Menta, C.; Papa, S.; Fioretto, A. Field and microcosms decomposition dynamics of European beech leaf litter: Influence of climate, plant material and soil with focus on N and Mn. Appl. Soil Ecol. 2015, 93, 88–97. [Google Scholar] [CrossRef]
- Bradford, M.A.; Berg, B.; Maynard, D.S.; Wieder, W.R.; Wood, S.A. Understanding the dominant controls on litter decomposition. J. Ecol. 2016, 104, 229–238. [Google Scholar] [CrossRef]
- Muñoz-rojas, M.; Doro, L.; Ledda, L.; Francaviglia, R. Application of CarboSOIL model to predict the effects of climate change on soil organic carbon stocks in agro-silvo-pastoral Mediterranean management systems. Agric. Ecosyst. Environ. 2014, 16, 9–10. [Google Scholar] [CrossRef]
- Uri, V.; Aosaar, J.; Varik, M.; Becker, H.; Ligi, K.; Padari, A.; Kanal, A.; Lõhmus, K. The dynamics of biomass production, carbon and nitrogen accumulation in grey alder (Alnus incana (L.) Moench) chronosequence stands in Estonia. For. Ecol. Manag. 2014, 327, 106–117. [Google Scholar] [CrossRef]
- Innangi, M.; D’Alessandro, F.; Fioretto, A.; Di Febbraro, M. Modeling distribution of Mediterranean beech forests and soil carbon stock under climate change scenarios. Clim. Res. 2015, 66, 25–36. [Google Scholar] [CrossRef]
- Lucas-Borja, M.E.; Candel, D.; Jindo, K.; Moreno, J.L.; Andrés, M.; Bastida, F. Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 2012, 354, 359–370. [Google Scholar] [CrossRef]
- Baldrian, P.; Kolařík, M.; Stursová, M.; Kopecký, J.; Valášková, V.; Větrovský, T.; Zifčáková, L.; Snajdr, J.; Rídl, J.; Vlček, C.; et al. Active and total microbial communities in forest soil are largely different and highly stratified during decomposition. ISME J. 2012, 6, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, A.; Ascher, J.; Corti, G.; Ceccherini, M.T.; Nannipieri, P.; Pietramellara, G. Distribution of microbial communities in a forest soil profile investigated by microbial biomass, soil respiration and DGGE of total and extracellular DNA. Soil Biol. Biochem. 2004, 36, 859–868. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W.; Valverde-Barrantes, O.J.; Fisher, R.F. Tree Species Effects on Soil Properties in Experimental Plantations in Tropical Moist Forest. Soil Sci. Soc. Am. J. 2007, 71, 1389–1397. [Google Scholar] [CrossRef]
- Schulp, C.; Nabuurs, G. Effect of tree species on carbon stocks in forest floor and mineral soil and implications for soil carbon inventories. For. Ecol. Manag. 2008, 256, 482–490. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Noble, I.; Bolin, B.; Ravindranath, N.H.; Verardo, D.J.; Dokken, D.J. Land Use, Land Use Change, and Forestry; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Aosaar, J.; Varik, M.; Lõhmus, K.; Ostonen, I.; Becker, H.; Uri, V. Long-term study of above- and below-ground biomass production in relation to nitrogen and carbon accumulation dynamics in a grey alder (Alnus incana (L.) Moench) plantation on former agricultural land. Eur. J. For. Res. 2013, 132, 737–749. [Google Scholar] [CrossRef]
- Bárcena, T.G.; Kiær, L.P.; Vesterdal, L.; Stefánsdóttir, H.M.; Gundersen, P.; Sigurdsson, B.D. Soil carbon stock change following afforestation in Northern Europe: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.I.; Polglase, P.J.; Nyakuengama, J.G.; Khanna, P.K. Change in Soil Carbon Following Afforestation or Reforestation. For. Ecol. Manag. 2000, 168, 241–257. [Google Scholar] [CrossRef]
- Pérez-Corona, M.E.; Hernández, M.C.P.; de Castro, F.B. Decomposition of Alder, Ash, and Poplar Litter in a Mediterranean Riverine Area. Commun. Soil Sci. Plant Anal. 2006, 37, 1111–1125. [Google Scholar] [CrossRef]
- Aosaar, J.; Varik, M.; Uri, V. Biomass production potential of grey alder (Alnus incana (L.) Moench.) in Scandinavia and Eastern Europe: A review. Biomass Bioenergy 2012, 45, 11–26. [Google Scholar] [CrossRef]
- San-Miguel-Ayanz, J.; de Rigo, D.; Caudullo, G.; Houston Durrant, T.; Mauri, A. (Eds.) European Atlas of Forest Tree Species; European Commision: Luxembourg, 2016. [Google Scholar]
- Cutini, A.; Giannini, T. Effects of thinning and mixed plantations with Alnus cordata on growth and efficiency of common walnut (Juglans regia L.). Forest@ 2009, 6, 29–38. [Google Scholar] [CrossRef]
- Incerti, G.; Bonanomi, G.; Giannino, F.; Cartenì, F.; Spaccini, R.; Mazzei, P.; Piccolo, A.; Mazzoleni, S. OMDY: A new model of organic matter decomposition based on biomolecular content as assessed by 13C-CPMAS-NMR. Plant Soil 2016, 411, 377–394. [Google Scholar] [CrossRef]
- Tani, A.; Maltoni, A.; Mariotti, B.; Buresti Lattes, E. Juglans regia L. tree plantations for wood production in mining area of S. Barbara (AR). Evaluation of N-fixing accessory trees effect. Forest@ 2006, 3, 588–597. [Google Scholar] [CrossRef]
- Blasi, C.; Burrascano, S.; Maturani, A.; Sabatini, F.M. Foreste Vetuste in Italia; Ministerio dell’Ambiente e della Tutela del Territorio e del Mare: Roma, Italy, 2010. [Google Scholar]
- Di Gennaro, A.; Aronne, G.; De Mascellis, R.; Vingiani, S.; Sarnataro, M.; Abalsamo, P.; Cona, F.; Vitelli, L.; Arpia, G. I Sistemi di Terre Della Campania; S.EL.CA.: Firenze, Italy, 2002. [Google Scholar]
- De Marco, A.; Fioretto, A.; Giordano, M.; Innangi, M.; Menta, C.; Papa, S.; Virzo De Santo, A. C Stocks in Forest Floor and Mineral Soil of Two Mediterranean Beech Forests. Forests 2016, 7, 181. [Google Scholar] [CrossRef]
- Olson, J. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Throop, H.L.; Archer, S.R.; Monger, H.C.; Waltman, S. When bulk density methods matter: Implications for estimating soil organic carbon pools in rocky soils. J. Arid Environ. 2012, 77, 66–71. [Google Scholar] [CrossRef]
- Wright, F.; Bailey, J.S. Organic Carbon, Total Carbon, and Total Nitrogen Determinations in Soils of Variable Calcium Carbonate Contents Using a Leco CN-2000 Dry Combustion Analyzer. Commun. Soil Sci. Plant Anal. 2001, 32, 3243–3258. [Google Scholar] [CrossRef]
- Berg, B.; Kjønaas, O.J.; Johansson, M.-B.; Erhagen, B.; Åkerblom, S. Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. For. Ecol. Manag. 2015, 358, 41–47. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer: Berlin, Germany, 2003. [Google Scholar]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed Sci. Technol. 2015, 201, 25–37. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Corti, M. Use of two-block partial least-squares to study covariation in shape. Syst. Biol. 2000, 49, 740–753. [Google Scholar] [PubMed]
- Viscosi, V.; Fortini, P.; Slice, D.E.; Loy, A.; Blasi, C. Geometric morphometric analyses of leaf variation in four oak species of the subgenus Quercus (Fagaceae). Plant Biosyst. 2009, 143, 575–587. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Miletić, Z.; Knežević, M.; Stajić, S.; Košanin, O.; Dordević, I. Effect of European black alder monocultures on the characteristics of reclaimed mine soil. Int. J. Environ. Res. 2012, 6, 703–710. [Google Scholar]
- Sharma, E.; Ambasht, R. Litterfall, decomposition and nutrient release in an age sequence of Alnus nepalensis plantation stands in the eastern Himalaya. J. Ecol. 1987, 75, 997–1010. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Edmonds, R.L.; Tuttle, K.M. Red alder leaf decomposition and nutrient release in alder and conifer riparian patches in western Washington, USA. For. Ecol. Manag. 2010, 259, 2375–2381. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, R.; Sharma, E. Influence of stand age on nutrient and energy release through decomposition in alder-cardamom agroforestry systems of the eastern Himalayas. Ecol. Res. 2008, 23, 99–106. [Google Scholar] [CrossRef]
- Berg, B.; Ekbohm, G. Litter mass-loss rates and decomposition patterns in some needles and leaf litter types. Long-term decomposition in a Scots pine forest 7. Can. J. Bot. 1991, 69, 1449–1456. [Google Scholar] [CrossRef]
- Berg, B. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol. Biochem. 2014, 78, 222–232. [Google Scholar] [CrossRef]
- Dilly, O.; Munch, J.C.J. Microbial biomass content, basal respiration and enzyme activities during the course of decomposition of leaf litter in a black alder (Alnus glutinosa (L.) Gaertn.). Soil Biol. Biochem. 1996, 28, 1073–1081. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Berg, B.; Ekbohm, G.; Johansson, M.B.; McClaugherty, C.; Rutigliano, F.A.; Virzo De Santo, A. Maximum decomposition limits of forest litter types: A synthesis. Can. J. Bot. 1996, 74, 659–672. [Google Scholar] [CrossRef]
- Berg, B.; Cortina, J. Nutrient dynamics in some decomposing leaf and needle litter types in a Pinus sylvestris forest. Scand. J. For. Res. 1995, 10, 1–11. [Google Scholar] [CrossRef]
- Canhoto, C.; Graça, M.A.S. Decomposition of Eucalyptus globulus leaves and three native leaf species (Alnus glutinosa, Castanea sativa and Quercus faginea) in a Portuguese low order stream. Hydrobiologia 1996, 333, 79–85. [Google Scholar] [CrossRef]
- Wuest, S. Seasonal variation in soil organic carbon. Soil Sci. Soc. Am. J. 2014, 78, 1442–1447. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Anderson, J.M.; Hetherington, S.L. Temperature, nitrogen availability and mixture effects on the decomposition of heather [Calluna vulgaris (L.) Hull] and bracken [Pteridium aquilinum (L.) Kuhn] litters. Funct. Ecol. 1999, 13, 116–124. [Google Scholar] [CrossRef]
- Frankland, J. Succession of Fungi on Decaying Petioles of Pteridium aquilinum. J. Ecol. 1966, 54, 41–63. [Google Scholar] [CrossRef]
- Magill, A.H.; Aber, J.D. Dissolved organic carbon and nitrogen relationships in forest litter as affected by nitrogen deposition. Soil Biol. Biochem. 2000, 32, 603–613. [Google Scholar] [CrossRef]
- Killham, K. Soil Ecology; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Michalzik, B.; Kalbitz, K.; Park, J.; Solinger, S.; Matzner, E. Fluxes and concentrations of dissolved organic carbon and nitrogen—A synthesis for temperate forests. Biogeochemistry 2001, 52, 173–205. [Google Scholar] [CrossRef]
- McDowell, W.H. Dissolved organic matter in soils—Future directions and unanswered questions. Geoderma 2003, 113, 179–186. [Google Scholar] [CrossRef]
- Kaiser, K.; Kalbitz, K. Cycling downwards—Dissolved organic matter in soils. Soil Biol. Biochem. 2012, 52, 29–32. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Guggenberger, G.; Zech, W. Composition and dynamics of dissolved carbohydrates and lignin-degradation products in two coniferous forests, N.E. Bavaria, Germany. Soil Biol. Biochem. 1994, 26, 19–27. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Haumaier, L.; Zech, W. Seasonal variations in the chemical composition of dissolved organic matter in organic forest floor layer leachates of old-growth Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) stands in northeastern Bavaria, Germany. Biogeochemistry 2001, 55, 103–143. [Google Scholar] [CrossRef]
- Hernes, P.J.; Kaiser, K.; Dyda, R.Y.; Cerli, C. Molecular trickery in soil organic matter: Hidden lignin. Environ. Sci. Technol. 2013, 47, 9077–9085. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, M.; Dignac, M.F.M.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Kalbitz, K.; Schwesig, D.; Schmerwitz, J.; Kaiser, K.; Haumaier, L.; Glaser, B.; Ellerbrock, R.; Leinweber, P. Changes in properties of soil-derived dissolved organic matter induced by biodegradation. Soil Biol. Biochem. 2003, 35, 1129–1142. [Google Scholar] [CrossRef]
- Scheel, T.; Haumaier, L.; Ellerbrock, R.H.; Rühlmann, J.; Kalbitz, K. Properties of organic matter precipitated from acidic forest soil solutions. Org. Geochem. 2008, 39, 1439–1453. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J.F. Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta 1995, 59, 219–229. [Google Scholar] [CrossRef]
- Kaiser, K.; Guggenberger, G.; Haumaier, L. Changes in dissolved lignin-derived phenols, neutral sugars, uronic acids, and amino sugars with depth in forested Haplic Arenosols and Rendzic Leptosols. Biogeochemistry 2004, 70, 135–151. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef] [PubMed]
| Chemical Variable | Mean ± SEM |
|---|---|
| Nitrogen (mg/g d.w.) | 30.3 ± 0.6 |
| Water extractables (mg/g d.w.) | 246.8 ± 1.3 |
| Ethanol extractables (mg/g d.w.) | 149.5 ± 2.6 |
| Total Phenols (mg/g d.w.) | 20.2 ± 2.3 |
| Cellulose (mg/g d.w.) | 108.1 ± 2.9 |
| Lignin (mg/g d.w.) | 100.6 ± 6.0 |
| C:N | 16.5 ± 0.3 |
| Lignin:N | 3.3 ± 0.2 |
| Variable | Decomposition Days | ||
|---|---|---|---|
| 100 | 180 | 270 | |
| Total residual mass (%) | 76.6 ± 2.2 | 67.0 ± 4.1 | 60.0 ± 1.4 |
| Residual water extractables (%) | 67.9 ± 2.5 | 57.4 ± 5.2 | 50.8 ± 4.1 |
| Residual ethanol extractables (%) | 67.9 ± 3.3 | 64.2 ± 5.0 | 56.9 ± 4.1 |
| Residual total phenols (%) | 10.1 ± 0.8 | 6.2 ± 1.4 | 0.8 ± 0.0 |
| Residual nitrogen (%) | 66.2 ± 4.3 | 50.5 ± 5.4 | 44.0 ± 3.3 |
| Residual cellulose (%) | 73.8 ± 8.5 | 38.4 ± 5.9 | 18.1 ± 2.9 |
| Residual lignin (%) | 62.1 ± 4.8 | 29.8 ± 6.8 | 22.3 ± 2.5 |
| C:N | 16.8 ± 0.6 | 17.2 ± 0.3 | 16.9 ± 1.0 |
| Lignin:N | 3.2 ± 0.3 | 2.1 ± 0.5 | 1.7 ± 0.1 |
| Forest Floor Leaf Litter | ||||||
| Organic Carbon | Total Nitrogen | Water Extractables | Ethanol Extractables | Total Phenols | Cellulose | |
| Total Nitrogen | 0.969 *** | |||||
| Water Extractables | 0.925 *** | 0.856*** | ||||
| Ethanol Extractables | 0.930 *** | 0.875 *** | 0.983 *** | |||
| Total Phenols | 0.455 n.s. | 0.365 n.s. | 0.549 ** | 0.522 ** | ||
| Cellulose | 0.893 *** | 0.866 *** | 0.805 *** | 0.811 *** | 0.368 n.s. | |
| Lignin | 0.915 *** | 0.943 *** | 0.815 *** | 0.832 *** | 0.312 n.s. | 0.900 *** |
| Topsoil (0–5 cm) | ||||||
| Organic Carbon | Total Nitrogen | Water Extr. | Ethanol Extr. | Total Phenols | Cellulose | |
| Total Nitrogen | 0.403 n.s. | |||||
| Water Extractables | 0.083 n.s. | 0.335 n.s. | ||||
| Ethanol Extractables | −0.017 n.s. | −0.675 *** | −0.303 n.s. | |||
| Total Stock | 0.234 n.s. | −0.104 n.s. | −0.154 n.s. | 0.415 * | ||
| Cellulose | −0.019 n.s. | −0.165 n.s. | −0.087 n.s. | −0.011 n.s. | 0.084 n.s. | |
| Lignin | −0.083 n.s. | −0.482 * | −0.338 n.s. | 0.349 n.s. | −0.057 n.s. | −0.088 n.s. |
| Stock | Loadings Axis 1 | Loadings Axis 2 | ||
|---|---|---|---|---|
| Forest Floor Leaf Litter | Topsoil (0–5 cm) | Forest Floor Leaf Litter | Topsoil (0–5 cm) | |
| Total Organic C | −0.42 | −0.43 | 0.06 | 0.13 |
| Total N | −0.49 | 0.09 | −0.12 | −0.69 |
| Total Phenols | −0.10 | 0.18 | 0.55 | 0.07 |
| Ethanol Extractables | −0.22 | 0.02 | 0.51 | 0.65 |
| Water Extractables | −0.25 | −0.80 | 0.50 | −0.05 |
| Cellulose | −0.47 | 0.37 | −0.38 | 0.14 |
| Lignin | −0.49 | 0.01 | −0.16 | 0.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innangi, M.; Danise, T.; D’Alessandro, F.; Curcio, E.; Fioretto, A. Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem. Forests 2017, 8, 240. https://doi.org/10.3390/f8070240
Innangi M, Danise T, D’Alessandro F, Curcio E, Fioretto A. Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem. Forests. 2017; 8(7):240. https://doi.org/10.3390/f8070240
Chicago/Turabian StyleInnangi, Michele, Tiziana Danise, Francesco D’Alessandro, Elena Curcio, and Antonietta Fioretto. 2017. "Dynamics of Organic Matter in Leaf Litter and Topsoil within an Italian Alder (Alnus cordata (Loisel.) Desf.) Ecosystem" Forests 8, no. 7: 240. https://doi.org/10.3390/f8070240






