1. Introduction
Concerns over increasing atmospheric carbon emissions are driving the need to improve understanding of carbon sequestration within global ecosystems and investigate solutions to mitigate the effects of resulting climate change [
1,
2,
3,
4]. Coastal wetlands in particular are gaining increasing recognition as remarkably efficient carbon sinks [
5,
6]. Mangroves, sea grasses and tidal salt marshes are highly productive ecosystems, estimated to sequester carbon 10–50 times faster than terrestrial systems [
1,
7]. These ‘blue carbon’ ecosystems are capable of accumulating vast quantities of organic matter [
8] and have been shown to contain markedly greater stores of carbon than terrestrial forest ecosystems [
9]. A combination of high productivity, anaerobic conditions and high accumulation rates account for the high carbon storage capacity of mangrove ecosystems in particular [
5]. Consequently, these marine forest ecosystems have been reported to be the most carbon dense forest type in the tropics, contributing significantly to tropical blue carbon stores [
9,
10].
As one of the most productive biomes on Earth [
11], in addition to providing a climate change mitigation service through the storage of carbon, mangroves also supply a wide range of other ecosystem services [
12], both on a global and local scale [
1]. They can provide coastlines with protection against natural disasters such as tsunamis and hurricanes [
13,
14], local communities with products such as fuelwood and building materials [
15] and habitats with breeding and nursery grounds, supporting commercially important fish stocks [
16,
17]. However, despite, and in part due to, this provision of goods and services, mangrove ecosystems are under threat from increasing anthropogenic exploitation, reaching a loss of an alarming 1–3% year
−1 [
15,
18,
19] with half of the world’s mangroves estimated to have been lost in the past 50 years [
15,
20]. These losses are driven by growing pressures from coastal development, agriculture and aquaculture, as well as the extraction of timber for construction and charcoal. Pressures are further exacerbated by escalating natural losses caused by extreme weather events and sea level rise due to climate change. The degradation and loss of these blue carbon sinks not only jeopardizes their ability to store carbon by reducing carbon sequestration rates but also contributes towards emissions by releasing stored carbon [
19,
21].
Economic evaluation of ecosystems provides estimates of the value of the goods and services they provide [
14]. Associating ecosystems with economic values is considered to be an effective incentive for sustainable management [
22] and mangroves are reported to have the highest value per hectare of any blue carbon ecosystem [
10]. It is believed that many regulating and supporting services have often been undervalued, being harder to comprehend and evaluate [
15]. However, with increasing concern over climate change, efforts to evaluate the rate and value of carbon sequestration in forest systems has been increasing [
1,
10,
23].
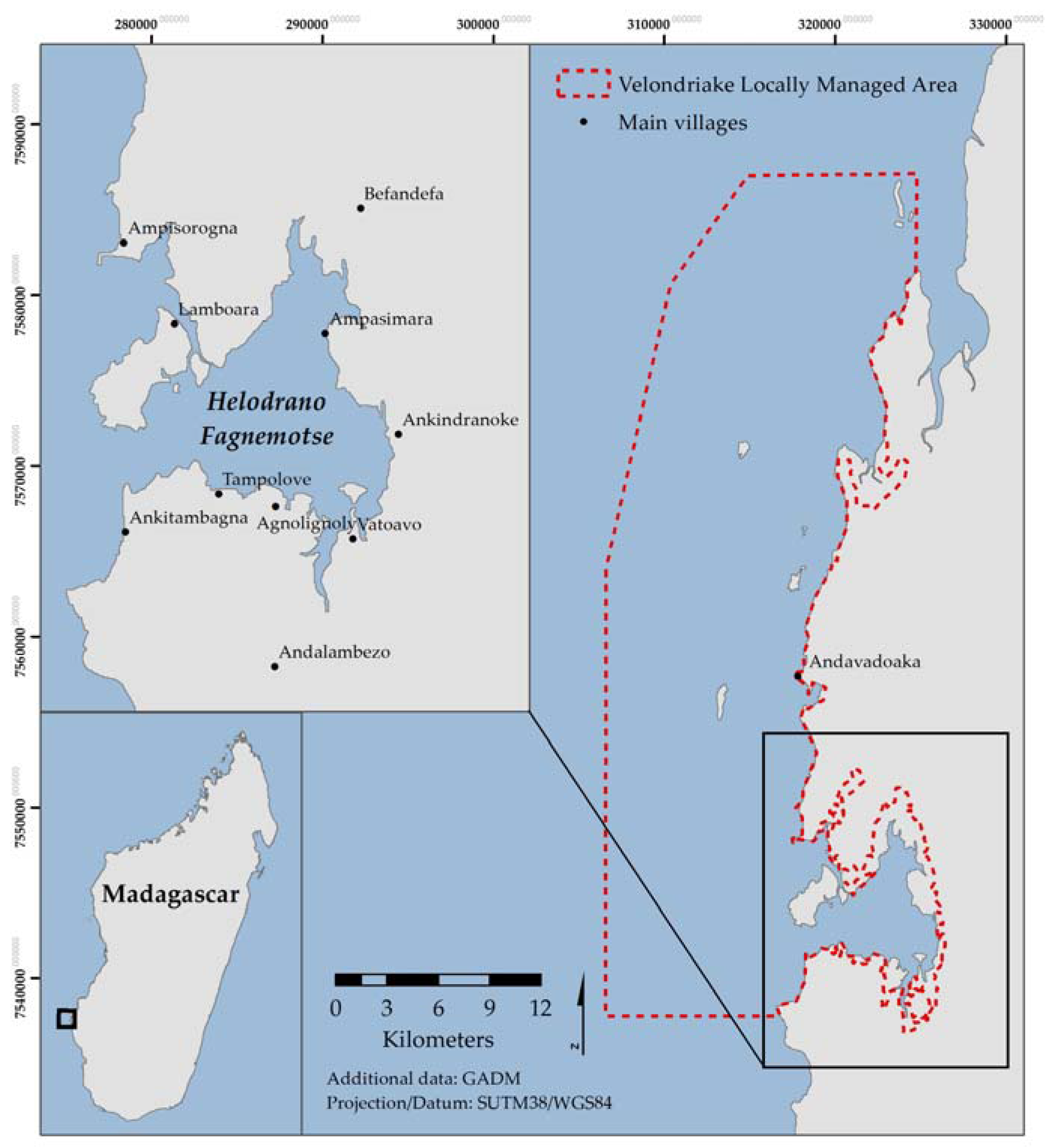
Madagascar contains Africa’s fourth largest extent of mangroves, which in 2010 comprised approximately 213,000 ha, representing 2% of the global mangrove cover [
20,
24]. Madagascar’s coastal communities are heavily dependent on the resources mangroves provide. In particular, mangroves meet the majority of the energy demands of many coastal communities and surrounding urban areas, in addition to providing an important source of timber for building construction [
25]. This heavy reliance on mangrove ecosystems is leading to increasing and wide-spread degradation and deforestation throughout Madagascar, with an estimated net loss of 21% between 1990 and 2010 [
24].
This study builds on a growing number of carbon stock inventories carried out throughout Africa [
25,
26,
27,
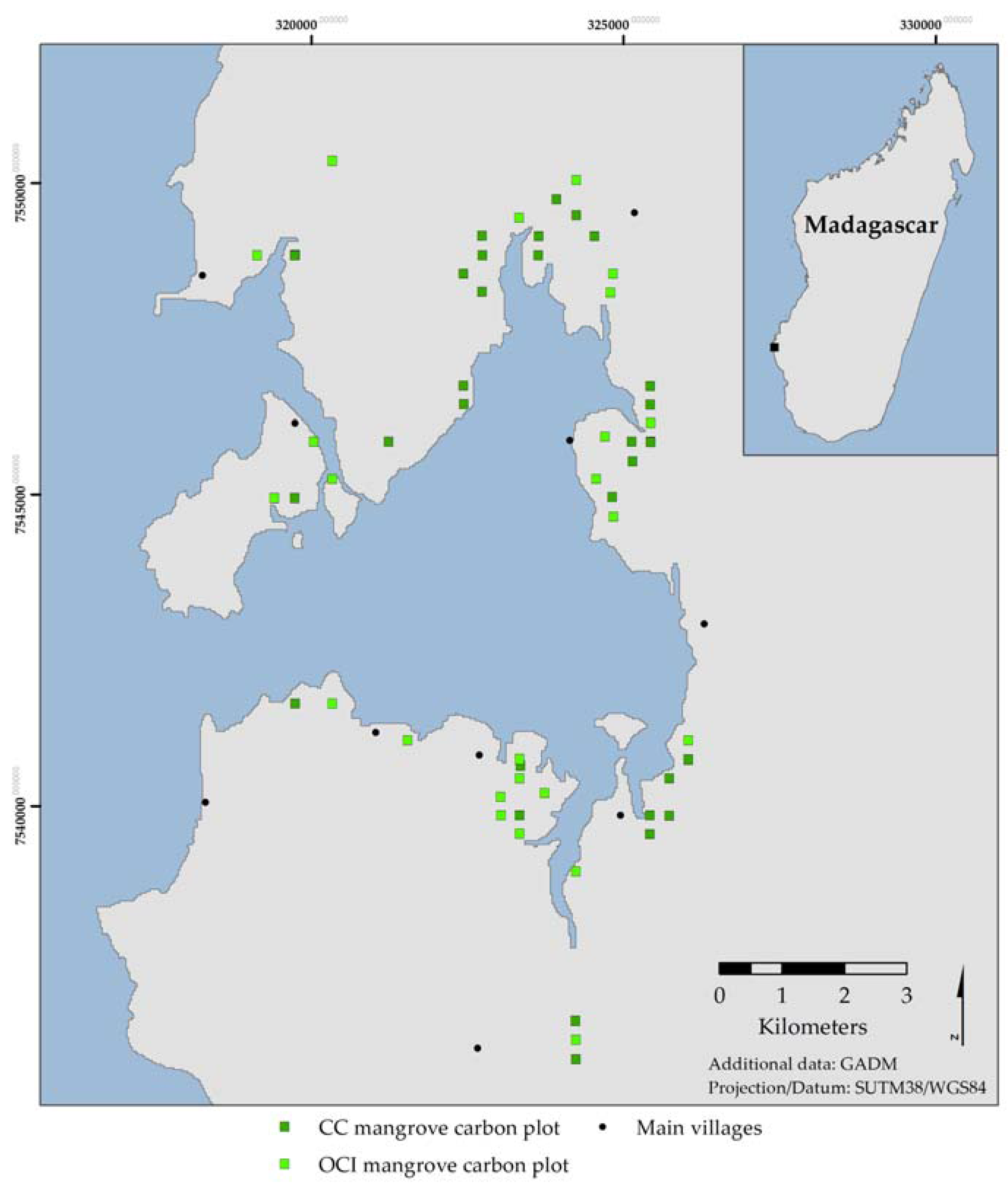
28], using well established inventory protocols to estimate the carbon stocks of the mangroves of Helodrano Fagnemotse (Baie des Assassins) in southwest Madagascar. In addition, it calculates forest land-cover change between 2002 and 2014 in order to assess the implications of forest exploitation on carbon storage and highlight the importance of a community-led mangrove management strategy aiming to conserve and restore the bay’s mangroves.
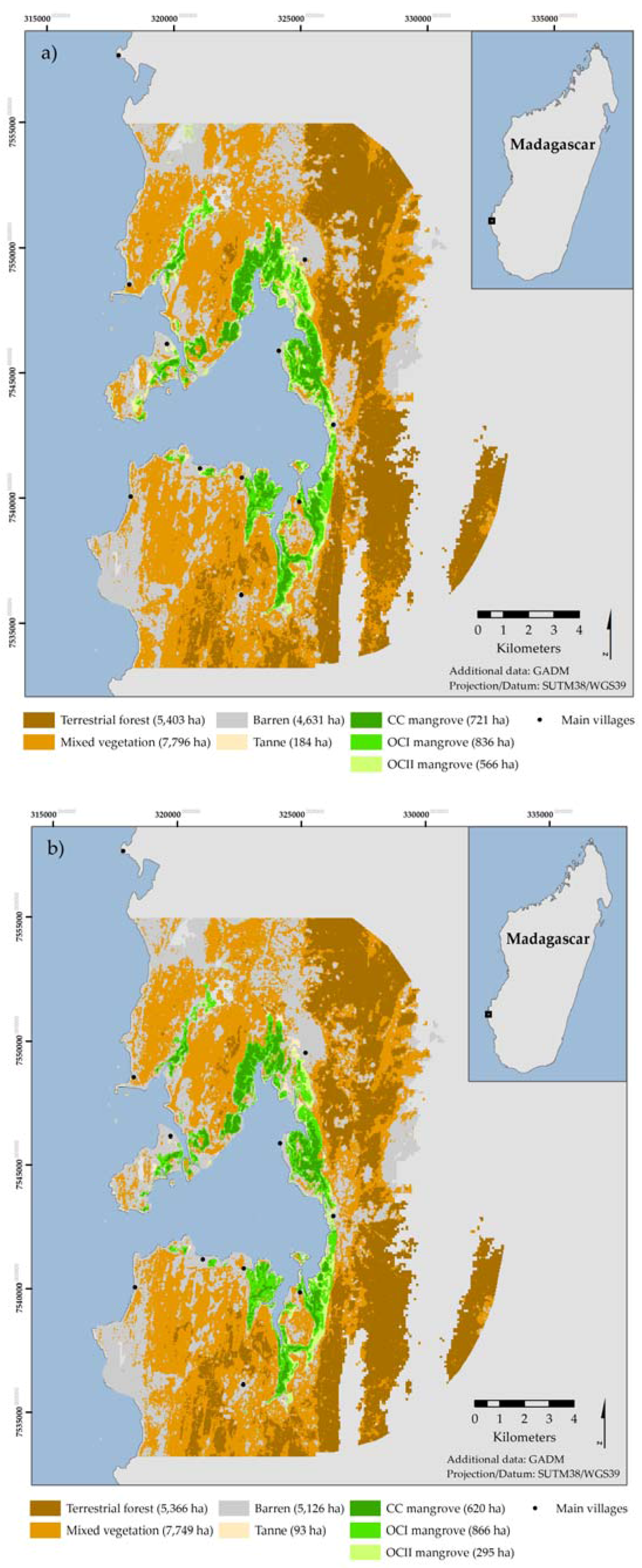
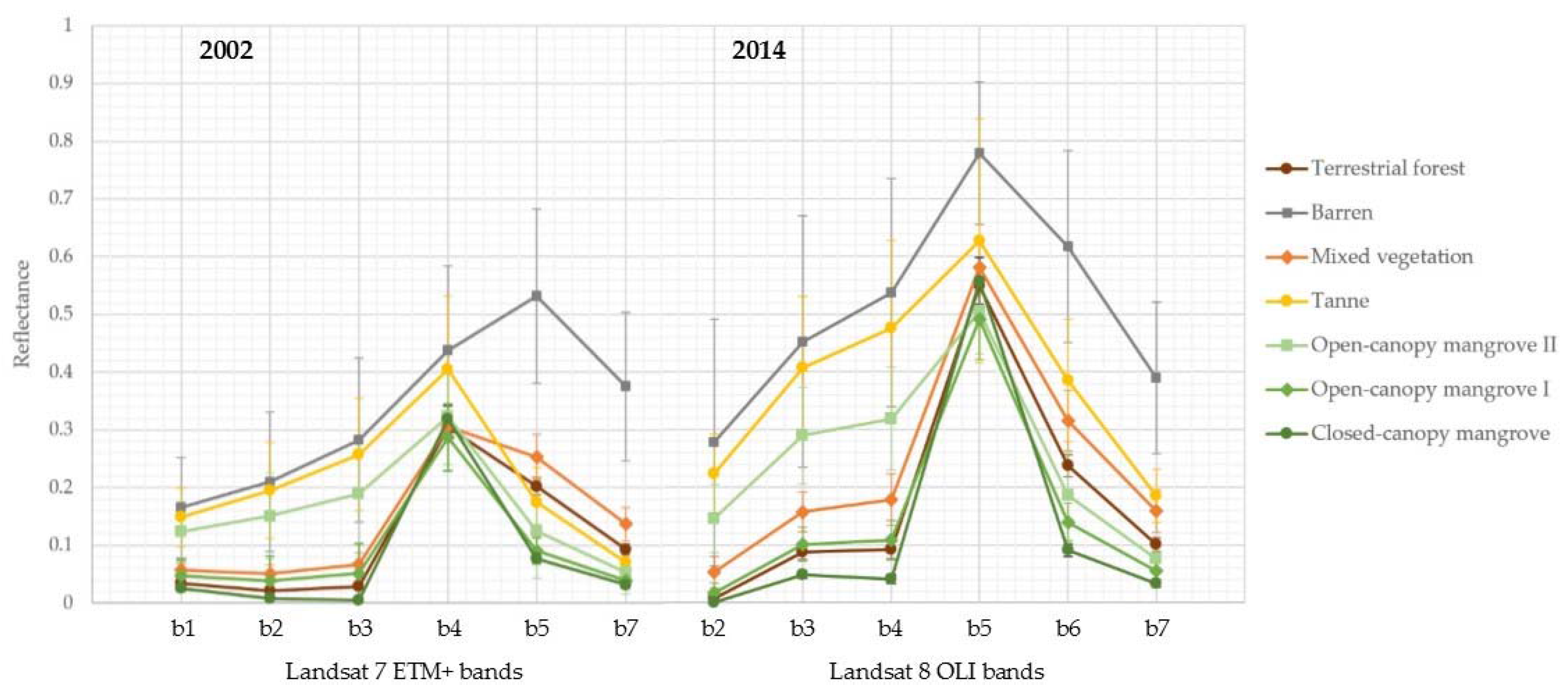
The remote sensing component of this study builds on the work of Jones et al. [
24], who present a land-cover classification for Helodrano Fagnemotse derived from a Landsat image acquired in April 2014 and analyze mangrove dynamics between 1990 and 2010 within the bay using national-level mangrove distribution maps derived from Landsat data by Giri [
29]. As discussed in Jones et al. [
24], while offering unprecedented national-level coverage, the data produced by Giri [
29] have only limited applicability at the local scale and, given that at the time of writing the primary data were captured over 6 years ago, also fail to capture recent patterns of mangrove gain or loss. This study further refines the 2014 classification presented in Jones et al. [
24] and replicates the methodology using a Landsat image acquired in April 2002, enabling a contemporary, localized analysis of mangrove dynamics within the bay between 2002 and 2014.
4. Discussion
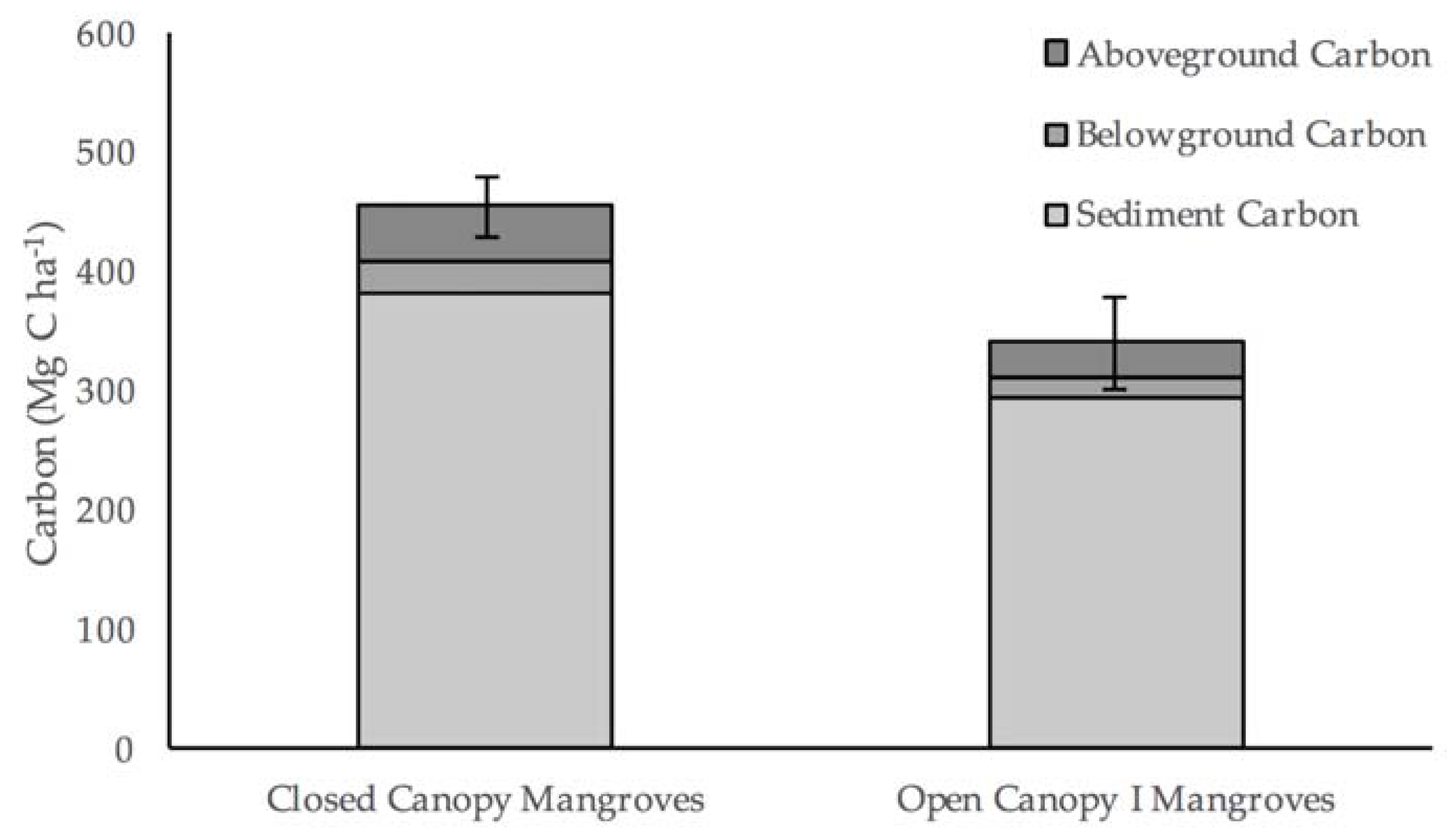
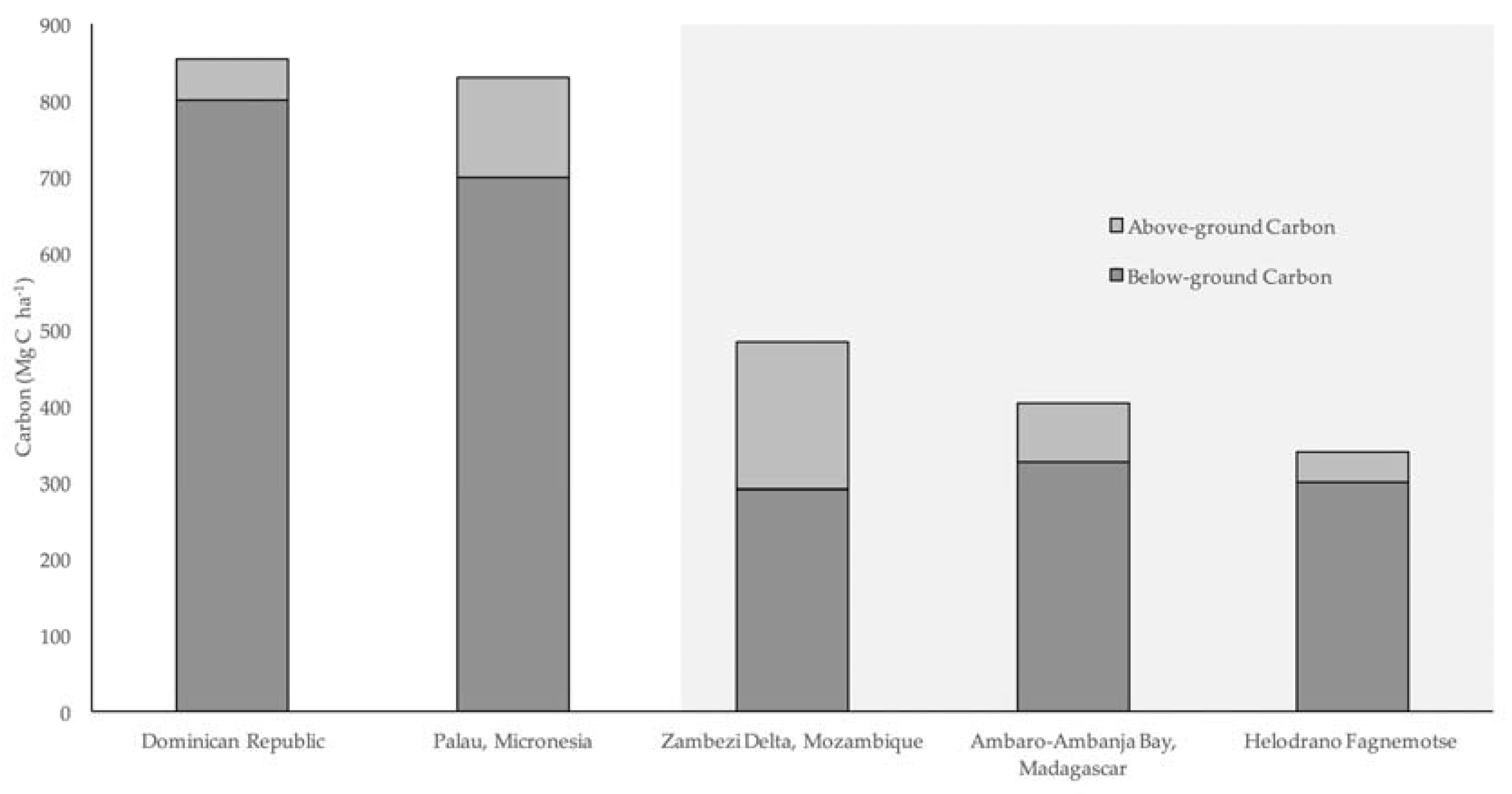
Total carbon stocks within both mangrove classes in Helodrano Fagnemotse were substantially lower than the global mean of approximately 965 Mg·C·ha
−1 [
57]. Although soil carbon stocks were comparable, total carbon stocks were also lower than values estimated by other mangrove carbon inventories carried out in East Africa in recent years [
25,
26] (
Figure 10). The difference in total stocks is mostly due to lower vegetation carbon values owing to the comparably smaller stature of mangroves in Helodrano Fagnemotse, which is most likely due to the climatic differences between the study areas. Due to the arid nature of the southwest region of Madagascar, this mangrove system receives less rainfall and is likely to be more saline in nature, both of which are environmental parameters that have previously been shown to impact mangrove productivity [
11,
57,
58,
59].
The percentage of total carbon stocks made up by soil stores falls into the reported range of 49–98% [
9]. Similarities within the Western Indian Ocean region as well as at the global scale were apparent in soil characteristics where mean carbon concentration fell within the global range of 2–5% [
60]. Bulk density values were estimated at the higher end of other reported values [
37,
42,
61] but were similar to other studies in the region [
26,
61], which is likely due to the high mineral content of the soil in the area.
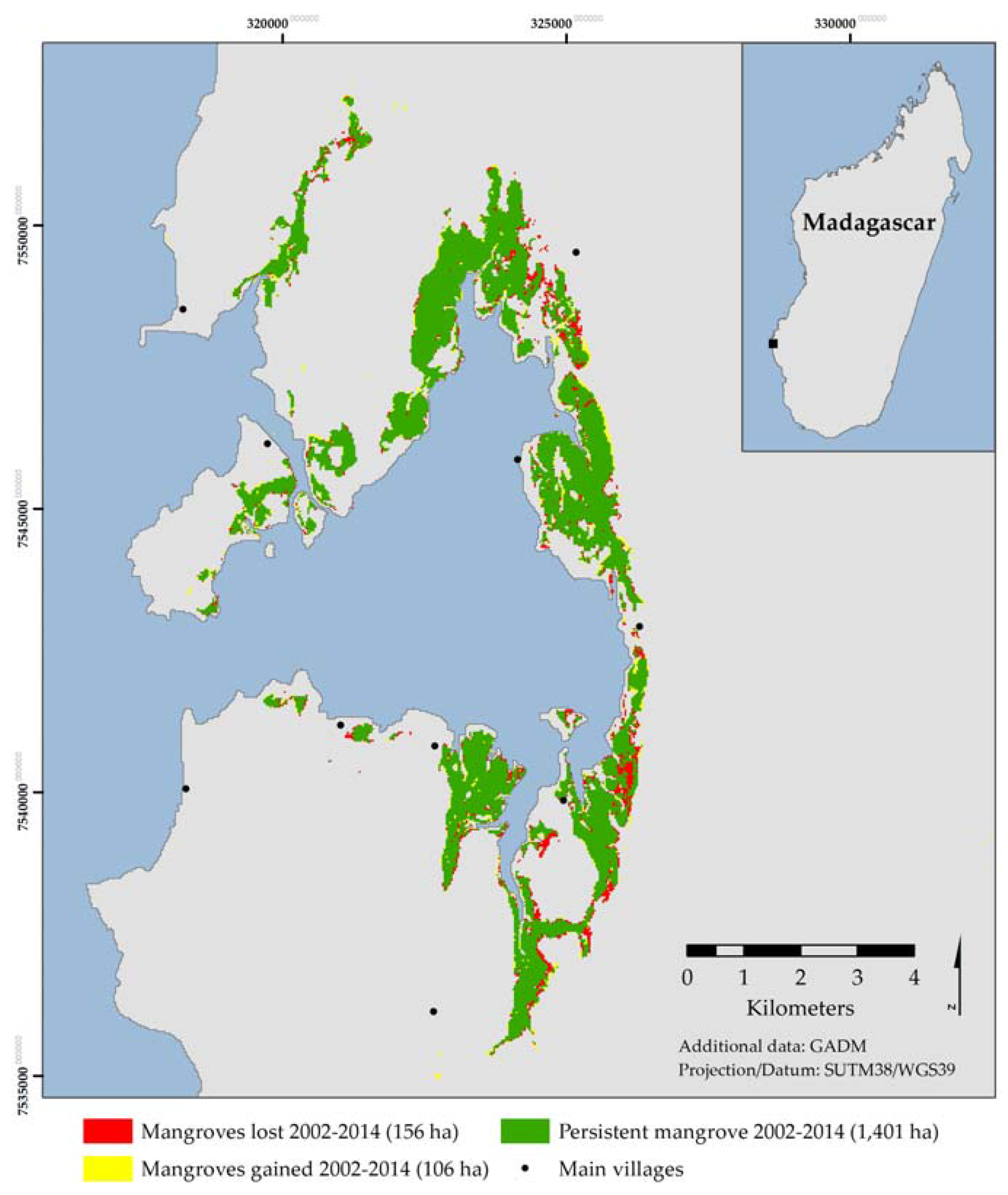
Although total carbon stocks were found to be lower than other studied mangrove systems, the mangroves of Helodrano Fagnemotse are clearly an extremely important resource for local coastal communities, which was highlighted by the evidence of mangrove loss observed in both the remote sensing and inventory data analyses. The remote sensing analysis indicated an overall net loss of 3.18% between 2002 and 2014. This is comparable to losses reported in Mahajamba Bay [
27], but substantially lower than those estimated in the Ambaro-Ambanja Bays in northwest Madagascar [
24,
25]. However, because the net loss figures only account for total deforestation—mangrove classes transitioning to non-vegetated classes—these figures alone do not accurately reflect the extent of mangrove exploitation within the bay. The dynamics analysis highlighted that 22.4% of closed-canopy mangroves transitioned to open-canopy mangrove I between 2002 and 2014 and a further 9.7% of open-canopy mangrove I transitioned to the more sparse open-canopy mangrove II. These trends suggest widespread, extensive degradation, an observation which is further reinforced by the high stump densities recorded during the carbon inventory surveys, particularly in the denser, taller, closed canopy
C. tagal dominated plots. Similar observations were also made by Scales et al. [
31] who found that 28.7% of trees in 60 randomly selected plots within Helodrano Fagnemotse showed signs of harvesting.
This study focusses on the regulating, carbon storage service that mangroves provide, however, the observed, decreasing mangrove ecosystem health is likely to have a negative impact on the ability of the system to provide additional services that are of importance to coastal communities in the immediate-term e.g., coastal protection and supporting commercially important fisheries [
57,
64,
65]. Approximately 80% of global fisheries are either directly or indirectly dependent on mangroves [
17]. This is of critical importance to the communities of Helodrano Fagnemotse where small-scale fisheries sustain local livelihoods, providing 82% of daily household income, preventing a fall into further poverty [
30].
Due to the strong local dependence on mangrove resources and the lack of viable alternatives in this remote and arid region—where the adjacent, endemic Mikea Forest ecosystem experienced the country’s highest deforestation rate between 1995 and 2005 [
66]—total, strict conservation is not a viable option if local livelihoods are to be unaffected. In addition, despite extensive national legislation governing mangrove forests and protected areas in Madagascar [
67], state authorities lack capacity for implementation and enforcement, especially in remote areas such as Helodrano Fagnemotse [
68,
69].
Mangroves are remarkably resilient species [
13,
70], making them suitable for locally-led sustainable harvesting and conservation regimes. However, this needs to be made a feasible option for people existing on the edge of, or below, the national poverty line. Despite the relatively low levels of carbon stored in the mangroves of Helodrano Fagnemotse, given the low levels of wealth in the area, harnessing the value that this conserved and restored carbon holds on the voluntary carbon market could generate revenue to support and incentivise locally-led sustainable mangrove management, improve livelihoods and alleviate anthropogenic pressures [
2,
71]. As the mangroves of Helodrano Fagnemotse sit within the Velondriake LMMA, there is also the potential for this revenue to support broader community-led marine conservation initiatives. The carbon stocks and the remote sensing-derived ‘business as usual’ deforestation rate presented herein could form the basis of a carbon project’s baseline scenario.
Despite the increasing interest in the use of carbon financing as an incentive for sustainable mangrove management, improvements to carbon project methodologies are needed in order to optimise them for use in wetland ecosystems. The fate of vegetation carbon following deforestation can be predicted with relative ease and the loss of soil organic carbon due to mangrove conversion for aquaculture is relatively well established [
62,
72]. However, there remains insufficient information on the fate of soil organic carbon following cutting for timber or charcoal, which is the predominant cause of mangrove deforestation both in Madagascar and the broader East Africa region [
17,
73]. In fact, it is estimated that 26% of mangrove forests worldwide are degraded due to over-exploitation for fuelwood and timber production [
74]. However, despite a growing number of studies highlighting the impact of ecosystem conversion and degradation on soil organic carbon stocks, the predictions of carbon losses through in-situ oxidation and soil export to estuarine and offshore areas are wide ranging [
2,
19,
71,
75].
Addressing these outstanding uncertainties should be a priority in order to include significant soil carbon stocks in blue carbon accounting and improve carbon sequestration accounting in the mangrove environment [
71]. This would ensure that the maximum potential offsets are achieved for specific wetlands conservation and/or restoration projects, increasing the carbon financing available for project activities and to support local communities.