Characteristics of Fine Roots of Pinus massoniana in the Three Gorges Reservoir Area, China
Abstract
:1. Introduction
2. Materials and Methods
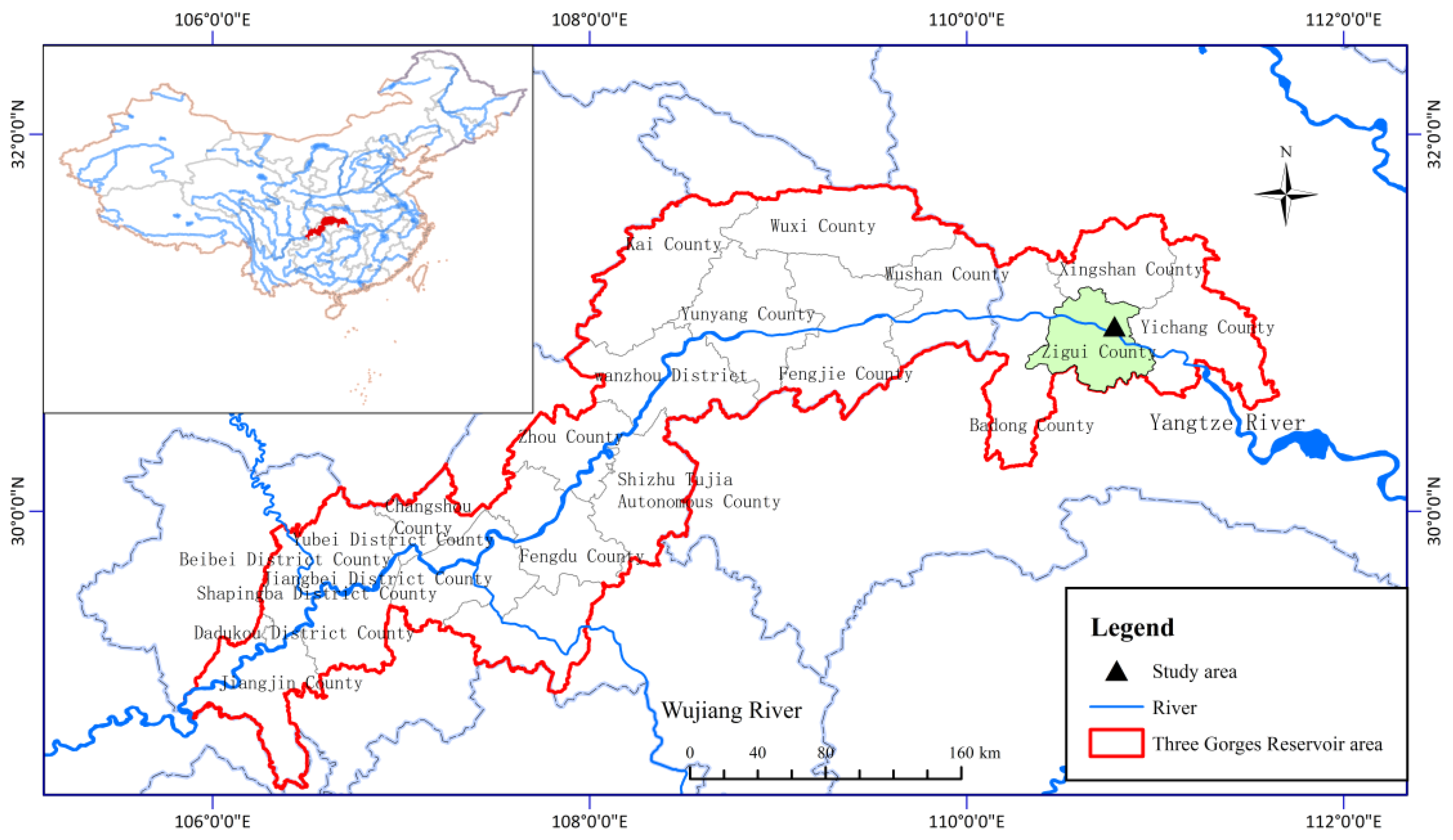
2.1. Study Site
2.2. Determination of Fine Root Standing Biomass (FRB) and Necromass
2.3. Fine Root Decomposition Experiment
2.4. Chemical Analysis of Litter and Soil Samples
2.5. Date Analyses
3. Results
3.1. Belowground Environment and Soil Chemical Properties
3.2. Fine Root Biomass, Necromass and Annual Production
3.3. The Initial C and N Content and Annual C and N Production in Fine Roots
3.4. Remaining Fine Root Mass after Decomposition Experiment
3.5. Changes in C and N Concentrations in Fine Roots during Decomposition
3.6. Relationships between Fine Root Indicators and Soil Characteristics
4. Discussion
4.1. Fine Roots Biomass and Production in Different Diameter Classes
4.2. The Decomposition Rate and C, N Dynamics of Fine Roots in Decomposition Experiment
4.3. Soil Factors Influences on Fine Root Traits
5. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Six, J.; Callewaert, P.; Lenders, S.; De Gryze, S.; Morris, S.; Gregorich, E.; Paul, E.; Paustian, K. Measuring and understanding carbon storage in afforested soils by physical fractionation. Soil Sci. Soc. Am. J. 2002, 66, 1981–1987. [Google Scholar] [CrossRef]
- Chapin, F.S.; Ruess, R.W. Carbon cycle: The roots of the matter. Nature 2001, 411, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S.E.; Gaudinski, J.B. The secret lives of roots. Science 2003, 302, 1344–1345. [Google Scholar] [CrossRef] [PubMed]
- Upson, M.; Burgess, P. Soil organic carbon and root distribution in a temperate arable agroforestry system. Plant Soil 2013, 373, 43–58. [Google Scholar] [CrossRef]
- Leppälammi-Kujansuu, J.; Aro, L.; Salemaa, M.; Hansson, K.; Dan, B.K.; Helmisaari, H.S. Fine root longevity and carbon input into soil from below- and above-ground litter in climatically contrasting forests. For. Ecol. Manag. 2014, 326, 79–90. [Google Scholar] [CrossRef]
- McCormack, M.; Eissenstat, D.; Prasad, A.; Smithwick, E. Regional scale patterns of fine root lifespan and turnover under current and future climate. Glob. Chang. Biol. 2013, 19, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Pregitzer, K.S.; DeForest, J.L.; Burton, A.J.; Allen, M.F.; Ruess, R.W.; Hendrick, R.L. Fine root architecture of nine North American trees. Ecol. Monogr. 2002, 72, 293–309. [Google Scholar] [CrossRef]
- Mccormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.S.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef] [PubMed]
- McClaugherty, C.A.; Aber, J.D.; Melillo, J.M. The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 1982, 63, 1481–1490. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornwell, W.K.; Wardle, D.A.; Elumeeva, T.G.; Liu, W.; Jackson, B.G.; Onipchenko, V.G.; Soudzilovskaia, N.A.; Tao, J.; Cornelissen, J.H.C. Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol. 2013, 101, 943–952. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.Y. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: Literature review and meta-analyses. Crit. Rev. Plant Sci. 2010, 29, 204–221. [Google Scholar] [CrossRef]
- Lin, C.F.; Yang, Y.S.; Guo, J.F.; Chen, G.S.; Xie, J.S. Fine root decomposition of evergreen broadleaved and coniferous tree species in mid-subtropical China: Dynamics of dry mass, nutrient and organic fractions. Plant Soil 2011, 338, 311–327. [Google Scholar] [CrossRef]
- Sun, T.; Mao, Z.J.; Dong, L.L.; Hou, L.L.; Song, Y.; Wang, X.W. Further evidence for slow decomposition of very fine roots using two methods: Litterbags and intact cores. Plant Soil 2013, 366, 633–646. [Google Scholar] [CrossRef]
- Jackson, R.B.; Mooney, H.; Schulze, E.-D. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl. Acad. Sci. USA 1997, 94, 7362–7366. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Talhelm, A.F.; Pregitzer, K.S. Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol. 2015, 208, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Helmisaari, H.S.; Makkonen, K.; Kellomäki, S.; Valtonen, E.; Mälkönen, E. Below- and above-ground biomass, production and nitrogen use in Scots pine stands in eastern Finland. For. Ecol. Manag. 2002, 165, 317–326. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey oak stand in relation to seasonal changes in soil moisture in the Southern Apennines. Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef]
- Björk, R.G.; Majdi, M.; Klemedtsson, L.; Jonsson, L.L.; Molau, U. Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plan communities in northern Sweden. New Phytol. 2007, 176, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Park, B.B.; Yanai, R.D.; Fahey, T.J.; Bailey, S.W.; Siccama, T.G.; Shanley, J.B.; Cleavitt, N.L. Fine root dynamics and forest production across a calcium gradient in northern hardwood and conifer ecosystems. Ecosystems 2008, 11, 325–341. [Google Scholar] [CrossRef]
- Jackson, R.B.; Schenk, H.J.; Jobbágy, E.G.; Canadell, J.; Colello, G.D.; Dickinson, R.E.; Field, C.B.; Friedlingstein, P.; Heimann, M.; Hibbard, K.; et al. Belowground consequences of vegetation change and their treatment in models. Ecol. Appl. 2000, 10, 470–483. [Google Scholar] [CrossRef]
- Joslin, J.D.; Gaudinski, J.B.; Torn, M.S.; Riley, W.J.; Hanson, P.J. Fine-root turnover patterns and their relationship to root diameter and soil depth in a C14 labeled hardwood forest. New Phytol. 2006, 172, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Finér, L.; Helmisaari, H.S.; Lohmus, K.; Majdi, H.; Brunner, I.; Børja, I.; Eldhuset, T.; Godbold, D.; Grebenc, T.; Konopka, B.; et al. Variation in fine root biomass of three European tree species: Beech (Fagus sylvatica L.), Norway spruce (Picea abies L. Karst.), and Scots pine (Pinus sylvestris L.). Plant Biosyst. 2007, 141, 394–405. [Google Scholar] [CrossRef]
- Noguchi, K.; Konopka, B.; Satomura, T.; Kaneko, S.; Takahashi, M. Biomass and production of fine roots in Japanese forests. J. For. Res. 2007, 12, 83–95. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Hishi, T. Heterogeneity of individual roots within the fine root architecture: Causal links between physiological and ecosystem functions. J. For. Res. 2007, 12, 126–133. [Google Scholar] [CrossRef]
- Noguchi, K.; Matsuura, Y.; Sparrow, S.D.; Hinzman, L.D. Fine root biomass in two black spruce stands in interior Alaska: Effects of different permafrost conditions. Trees 2016, 30, 441–449. [Google Scholar] [CrossRef]
- Makita, N.; Hirano, Y.; Dannoura, M.; Kominami, Y.; Mizoguchi, T.; Ishii, H.; Kanazawa, Y. Fine root morphological traits determine variation in root respiration of Quercus serrata. Tree physiol. 2009, 29, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.L.; Mitchell, R.J.; Hendricks, J.J. Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 2004, 140, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Hishi, T.; Takeda, H. Dynamics of heterorhizic root systems: Protoxylem groups within the fine-root system of Chamaecyparis obtusa. New Phytol. 2005, 167, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Rewald, B.; Ephrath, J.E.; Rachmilevitch, S. A root is a root is a root? Water uptake rates of Citrus root orders. Plant Cell Environ. 2011, 34, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.P.; Hendricks, J.J. Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: Root branch order predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, J.; Vogt, K.A.; Wargo, P.M. Tree root turnover and senescence. In Plant Roots:The Hidden Half, 2nd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Dekker: New York, NY, USA, 1996; pp. 363–381. [Google Scholar]
- Rustad, L.E. The response of terrestrial ecosystems to global climate change: Towards an integrated approach. Sci. Total Environ. 2008, 404, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Feinstein, L.M.; Kershner, M.W.; Blackwood, C.B. Aggregated and complementary: Symmetric proliferation, overyielding, and mass effects explain fine-root biomass in soil patches in a diverse temperate deciduous forest landscape. New Phytol. 2015, 205, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.; Clarke, N.; Sigurdsson, B.D.; Gundersen, P. Do tree species influence soil carbon stocks in temperate and boreal forests? For. Ecol. Manag. 2013, 309, 4–18. [Google Scholar] [CrossRef]
- Wang, R.L.; Cheng, R.M.; Xiao, W.F.; Feng, X.H.; Liu, Z.B.; Ge, X.G.; Wang, X.R.; Zhang, W.Y. Fine root production and turnover in Pinus massoniana plantation in Three Gorges Reservoir area of China. Chin. J. Appl. Ecol. 2012, 23, 2346–2352. [Google Scholar]
- Ge, X.G.; Xiao, W.F.; Zeng, L.X.; Huang, Z.L.; Fu, T.; Feng, X.H. Relationships between litter substrate quality and soil nutrients in different-aged Pinus massoniana stands. Acta Ecol. Sin. 2012, 32, 852–862. [Google Scholar]
- Hansson, K.; Helmisaari, H.S.; Sah, S.P.; Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manag. 2013, 309, 58–65. [Google Scholar] [CrossRef]
- Hirano, Y.; Tanikawa, T.; Makita, N. Biomass and morphology of fine roots in eight Cryptomeria japonica stands in soils with different acid-buffering capacities. For. Ecol. Manag. 2017, 384, 122–131. [Google Scholar] [CrossRef]
- Gong, Z.T. Chinese Soil Taxonomy; China Science Press: Beijing, China, 2003. [Google Scholar]
- Brassard, B.W.; Chen, H.Y.; Cavard, X.; Laganiere, J.o.; Reich, P.B.; Bergeron, Y.; Pare, D.; Yuan, Z. Tree species diversity increases fine root productivity through increased soil volume filling. J. Ecol. 2013, 101, 210–219. [Google Scholar] [CrossRef]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Crossley, D.; Hoglund, M.P. A litter-bag method for the study of microarthropods inhabiting leaf litter. Ecology 1962, 43, 571–573. [Google Scholar] [CrossRef]
- Ostertag, R.; Marín-Spiotta, E.; Silver, W.L.; Schulten, J. Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems 2008, 11, 701–714. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; ASA-SSSA: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Zhu, T.B.; Zhang, J.B.; Meng, T.Z.; Zhang, Y.C.; Yang, J.J.; Müller, C.; Cai, Z.C. Tea plantation destroys soil retention of NO3—And increases N2O emissions in subtropical China. Soil Biol. Biochem. 2014, 73, 106–114. [Google Scholar] [CrossRef]
- Zhang, W.R.; Yang, G.C.; Tu, X.N. Adiministration Forestry Standard of People’s Republic of China—Method of Forest Soil Analysis; Chinese Standard Press: Beijing, China, 1999. [Google Scholar]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Mei, L.; Wang, Z.Q.; Zhang, X.J.; Yu, L.Z.; Du, Y. Effects of nitrogen fertilization on fine root biomass production and turnover of Fraxinus mandshurica plantation. Chin. J. Ecol. 2008, 27, 1663–1668. [Google Scholar]
- Vogt, K.A.; Vogt, D.J.; Bloomfield, J. Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 1998, 200, 71–89. [Google Scholar] [CrossRef]
- Finér, L.; Ohashi, M.; Noguchi, K.; Hirano, Y. Factors causing variation in fine root biomass in forest ecosystems. For. Ecol. Manag. 2011, 261, 265–277. [Google Scholar] [CrossRef]
- Ostonen, I.; Lõhmus, K.; Pajuste, K. Fine root biomass, production and its proportion of NPP in a fertile middle-aged Norway spruce forest: Comparison of soil core and ingrowth core methods. For. Ecol. Manag. 2005, 212, 264–277. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Mo, J. Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 2010, 335, 289–298. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant litter. In Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Berlin, Germany, 2008. [Google Scholar]
- Lin, C.F.; Yang, Y.S.; Chen, G.S.; Guo, J.F.; Han, Z.G. Decomposition dynamics of fine roots of Cunninghamia lanceolata in mid-subtropics. J. Subtrop. Resour. Environ. 2008, 3, 15–23. [Google Scholar]
- Wang, C.G.; Chen, Z.X.; Ma, C.E.; Lin, G.G.; Han, S.J. Three potential pathways influencing contrasting decomposition rates of fine roots. J. Beijing For. Univ. 2016, 38, 123–128. [Google Scholar]
- Goebel, M.; Hobbie, S.E.; Bulaj, B.; Zadworny, M.; Archibald, D.D.; Oleksyn, J.; Reich, P.B.; Eissenstat, D.M. Decomposition of the finest root branching orders: Linking belowground dynamics to fine-root function and structure. Ecol. Monogr. 2011, 81, 89–102. [Google Scholar] [CrossRef]
- Cusack, D.F.; Chou, W.W.; Yang, W.H.; Harmon, M.E.; Silver, W.L. Controls on long-term root and leaf litter decomposition in neotropical forests. Glob. Chang. Biol. 2009, 15, 1339–1355. [Google Scholar] [CrossRef]
- Gimbel, K.F.; Felsmann, K.; Baudis, M.; Puhlmann, H.; Gessler, A.; Bruelheide, H.; Kayler, Z.; Ellerbrock, R.H.; Ulrich, A.; Welk, E. Drought in forest understory ecosystems—A novel rainfall reduction experiment. Biogeosciences 2015, 12, 14319–14358. [Google Scholar] [CrossRef]
- Steinberger, Y.; Degani, R.; Barness, G. Decomposition of root litter and related microbial population dynamics of a Negev Desert shrub, Zygophyllum dumosum. J. Arid Environ. 1995, 31, 383–399. [Google Scholar] [CrossRef]
- Wieder, R.K.; Wright, S.J. Tropical forest litter dynamics and dry season irrigation on Barro Colorado Island, Panama. Ecology 1995, 76, 1971–1979. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, W. The decomposition of fine and coarse roots: Their global patterns and controlling factors. Sci. Rep. 2014, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, S.E.; Oleksyn, J.; Eissenstat, D.M.; Reich, P.B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 2010, 162, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Q.; Hui, D.F.; Luo, Y.Q.; Zhou, G.Y. Rates of litter decomposition in terrestrial ecosystems: Global patterns and controlling factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef]
- Violita, T.; Triadiati, T.; Anas, I.; Miftahudin, M. Fine root production and decomposition in lowland rainforest and oil palm plantations in Sumatra, Indonesia. Hayati J. Biosci. 2016, 23, 7–12. [Google Scholar] [CrossRef]
- Berg, B.; Berg, M.P.; Bottner, P.; Box, E.; Breymeyer, A.; Ca de Anta, R.; Couteaux, M.; Escudero, A.; Gallardo, A.; Kratz, W.; et al. Litter mass loss rates in pine forests of Europe and Eastern United States: Some relationships with climate and litter quality. Biogeochemistry 1993, 20, 127–159. [Google Scholar] [CrossRef]
- Ruess, R.W.; Hendrick, R.L.; Burton, A.J.; Pregitzter, K.S.; Sveinbjornsson, B.; Allen, M.F.; Maurer, G.E. Coupling fine root dynamics with ecosystem carbon cycling in black spruce forests of interior Alaska. Ecol. Monogr. 2003, 73, 643–662. [Google Scholar] [CrossRef]
- Yang, Y.S.; Chen, G.S.; Guo, J.F.; Lin, P. Decomposition dynamic of fine roots in a mixed forest of Cunninghamia lanceolata and Tsoongiodendron odorum in midsubtropics. Ann. For. Sci. 2004, 61, 65–72. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Carol Adair, E.; Brandt, L.A.; Hart, S.C.; et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The global stoichiometry of litter nitrogen mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Fahey, T.J.; Hughes, J.W.; Pu, M.; Arthur, M.A. Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. For. Sci. 1988, 34, 744–768. [Google Scholar]
- Fan, P.; Jiang, Y. Nitrogen dynamics differed among the first six root branch orders of Fraxinus mandshurica and Larix gmelinii during short-term decomposition. J. Plant Res. 2010, 123, 433–438. [Google Scholar] [CrossRef] [PubMed]



| Size Classes | C Concentration (g·kg−1) | N Concentration (g·kg−1) | C/N Ratio | Annual C Production (g·m−2·a−1) | Annual N Production (g·m−2·a−1) |
|---|---|---|---|---|---|
| 0–0.5 mm | 42.72 ± 0.39 A | 1.17 ± 0.02 a | 36.66 ± 0.39 A | 6.99 ± 0.38 A | 0.19 ± 0.02 A |
| 0.5–1 mm | 45.04 ± 0.12 B | 1.00 ± 0.01 b | 45.04 ± 0.57 B | 7.63 ± 0.07 A | 0.17 ± 0.01 A |
| 1–2 mm | 45.95 ± 0.16 B | 0.90 ± 0.03 c | 51.06 ± 1.54 C | 12.88 ± 0.16 B | 0.25 ± 0.04 B |
| Incubation Time (Days) | 0–0.5 mm | 0.5–1 mm | 1–2 mm |
|---|---|---|---|
| Mass Remaining (%) | Mass Remaining (%) | Mass Remaining (%) | |
| 69 day | 85.5 ± 0.01 a | 89.8 ± 0.01 b | 91.1 ± 0.02 c |
| 136 day | 74.3 ± 0.02 a | 78.4 ± 0.01 b | 81.1 ± 0.02 c |
| 169 day | 71.5 ± 0.02 a | 75.5 ± 0.01 a | 75.6 ± 0.02 a |
| 245 day | 69.1 ± 0.02 a | 73.2 ± 0.01 a | 76.2 ± 0.01 a |
| 368 day | 66.2 ± 0.01 a | 72.8 ± 0.01 a | 74.4 ± 0.03 a |
| k (a−1) | 0.52 ± 0.02 a | 0.31 ± 0.01 b | 0.31 ± 0.01 b |
| Size Classes | Fine Root Characteristic | Fine Root Initial Indicators | Soil Indicators | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N | C/N | Moisture | Temp. | Total N | NO3−-N | NH4+-N | Total Ca | ||
| 0–0.5 mm | Production | 0.213 | 0.416 | 0.119 | 0.168 | 0.572 * | −0.051 | −0.004 | 0.810 ** | 0.810 ** |
| k | −0.687 * | 0.717 * | −0.701 * | 0.206 | 0.617 * | 0.241 | 0.438 | −0.585 * | −0.585 * | |
| 0.5–1 mm | Production | 0.337 | −0.290 | 0.321 | 0.227 | 0.258 | −0.267 | 0.207 | 0.855 ** | 0.855 ** |
| k | −0.669 * | 0.227 | −0.786 * | 0.356 | 0.815 ** | 0.032 | 0.497 | −0.720 * | −0.720 * | |
| 1–2 mm | Production | 0.326 | 0.417 | 0.232 | 0.339 | 0.186 | 0.111 | −0.353 | 0.054 | 0.054 |
| k | −0.671 * | 0.439 * | −0.727 * | 0.738 ** | 0.968 ** | −0.015 | 0.556 * | −0.835 ** | −0.835 ** | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Wang, N.; Cheng, R.; Xiao, W.; Yang, S.; Guo, Y.; Lei, L.; Zeng, L.; Wang, X. Characteristics of Fine Roots of Pinus massoniana in the Three Gorges Reservoir Area, China. Forests 2017, 8, 183. https://doi.org/10.3390/f8060183
Shen Y, Wang N, Cheng R, Xiao W, Yang S, Guo Y, Lei L, Zeng L, Wang X. Characteristics of Fine Roots of Pinus massoniana in the Three Gorges Reservoir Area, China. Forests. 2017; 8(6):183. https://doi.org/10.3390/f8060183
Chicago/Turabian StyleShen, Yafei, Na Wang, Ruimei Cheng, Wenfa Xiao, Shao Yang, Yan Guo, Lei Lei, Lixiong Zeng, and Xiaorong Wang. 2017. "Characteristics of Fine Roots of Pinus massoniana in the Three Gorges Reservoir Area, China" Forests 8, no. 6: 183. https://doi.org/10.3390/f8060183





