Allometry of Sapwood Depth in Five Boreal Trees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites and Data Collection
2.2. Measurement of Sapwood Depth and Estimation of Sapwood Area
3. Results
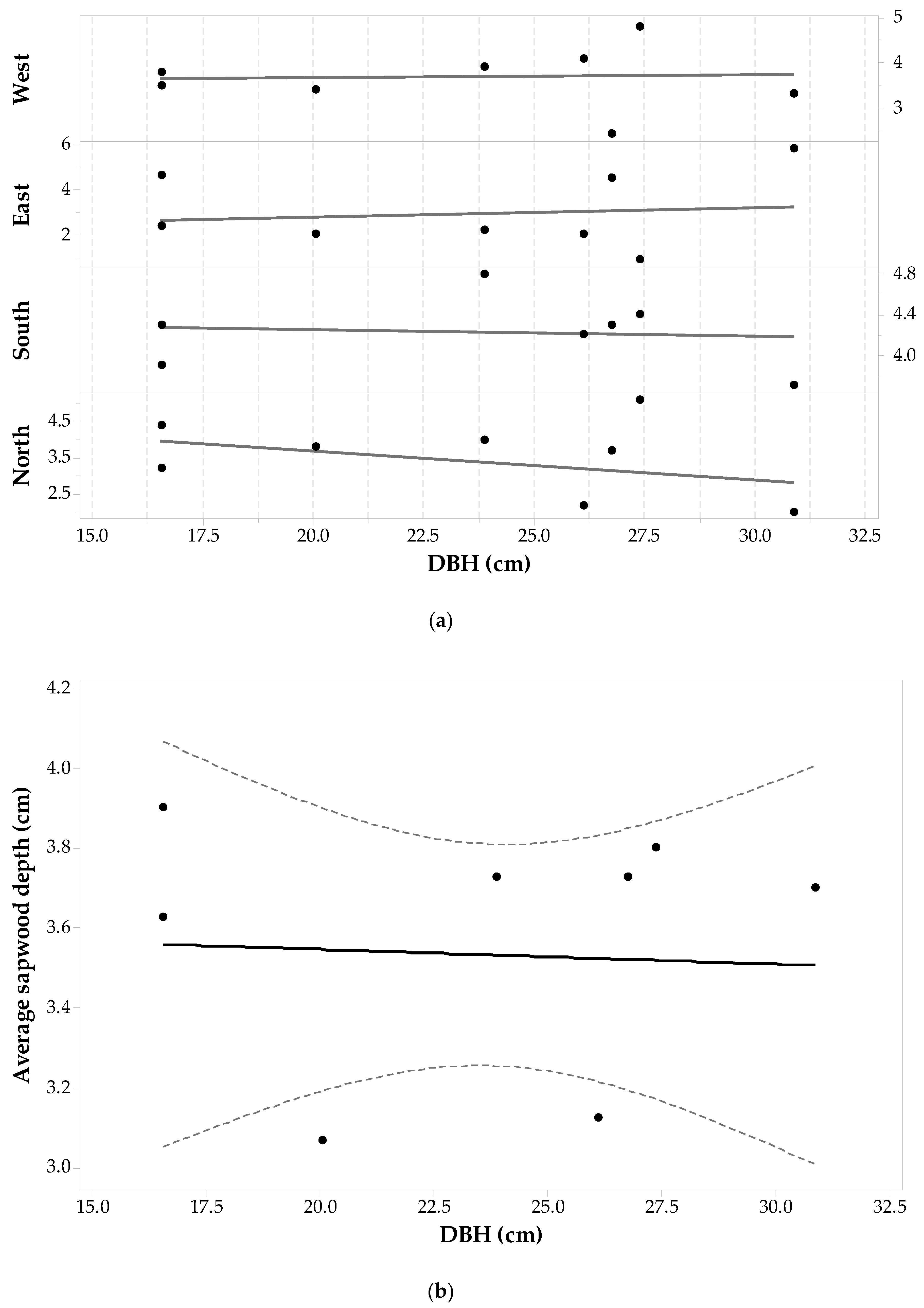
3.1. Pinus banksiana Allometry
3.2. Pinus contorta Allometry
3.3. Populus tremuloides Allometry
3.4. Picea mariana Allometry
3.5. Picea glauca Allometry
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grier, C.C.; Waring, R.H. Conifer Foliage Mass Related to Sapwood Area. For. Sci. 1974, 20, 205–206. [Google Scholar]
- Kaufmann, M.R.; Troendle, C.A. The relationship of leaf area and foliage biomass to sapwood conducting area in four Subalpine forest tree species. For. Sci. 1981, 27, 477–482. [Google Scholar]
- Marchand, J.P. Sapwood area as an estimator of foliage biomass and projected leaf area for Abies balsamea and Picea rubens. Can. J. For. Res. 1984, 14, 85–87. [Google Scholar] [CrossRef]
- Dean, T.J.; Long, J.N. Variation in sapwood area-leaf area relations within two stands of Lodgepole pine. For. Sci. 1986, 32, 749–758. [Google Scholar]
- Gilmore, W.; Seymour, S.; Maguire, A. Foliage-sapwood area relationships for Abies balsamea in Central Maine, USA. Can. J. For. Res. 1996, 26, 2071–2079. [Google Scholar] [CrossRef]
- Sievänen, R.; Nikinmaa, E.; Perttunen, J. Evaluation of importance of sapwood senescence on tree growth using the model LIGNUM. Silva Fenn. 1997, 31, 329–340. [Google Scholar] [CrossRef]
- Mokany, K.; McMurtrie, R.E.; Atwell, B.J.; Keith, H. Interaction between sapwood and foliage area in alpine ash (Eucalyptus delegatensis) trees of different heights. Tree Physiol. 2003, 23, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Delzon, S.; Sartore, M.; Burlett, R.; Dewar, R.; Loustau, D. Hydraulic responses to height growth in maritime pine trees. Plant Cell Environ. 2004, 27, 1077–1087. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Morris, J.D. Transpiration along an age series of Eucalyptus globulus plantations in southeastern Australia. For. Ecol. Manag. 2010, 259, 1754–1760. [Google Scholar] [CrossRef]
- Jones, D.A.; O’Hara, K.L.; Battles, J.J.; Gersonde, R.F. Leaf Area Prediction Using Three Alternative Sampling Methods for Seven Sierra Nevada Conifer Species. Forests 2015, 6, 2631–2654. [Google Scholar] [CrossRef]
- Vertessy, R.A.; Benyon, R.G.; O’Sullivan, S.K.; Gribben, P.R. Relationships between stem diameter, sapwood area, leaf area and transpiration in a young mountain ash forest. Tree Phys. 1995, 15, 559–567. [Google Scholar] [CrossRef]
- Cienciala, E.; Kucera, J.; Malmer, A. Tree sap flow and stand transpiration of two Acacia mangium plantations in Sabah, Borneo. J. Hydrol. 2000, 236, 109–120. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Norby, T.J. Sap velocity and canopy transpiration in a sweetgum stand exposed to free-air CO2 enrichment (FACE). New Phytol. 2001, 150, 489–498. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Hanson, P.; Todd, D. Transpiration from a multi-species deciduous forest as estimated by xylem sap flow techniques. For. Ecol. Manag. 2001, 143, 205–213. [Google Scholar] [CrossRef]
- Roberts, S.; Vertessy, R.; Grayson, R. Transpiration from Eucalyptus sieberi (L. Johnson) forests of different age. For. Ecol. Manag. 2001, 143, 153–161. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Above ground and below ground biomass and sapwood area allometric equations for six boreal tree species of northern Manitoba. Can. J. For. Res. 2002, 32, 1441–1450. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Bond, B.J.; Warren, J.M.; Woodruff, D.R. Does water transport scale universally with tree size? Funct. Ecol. 2005, 19, 558–565. [Google Scholar] [CrossRef]
- Pfautsch, S.; Keitel, C.; Turnbull, T.L.; Braimbridge, M.J.; Wright, T.E.; Simpson, R.R.; O’Brien, J.; Adams, M.A. Diurnal patterns of water use in Eucalyptus victrix indicate pronounced desiccation-rehydration cycles despite unlimited water supply. Tree Physiol. 2011, 31, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Pfautsch, S.; Macfarlane, C.; Ebdon, N.; Meder, R. Assessing sapwood depth and wood properties in Eucalyptus and Corymbia spp. using visual methods and near infrared spectroscopy (NIR). Trees 2012, 26, 963–974. [Google Scholar] [CrossRef]
- Hart, J.H. Morphological differences between sapwood, discolored sapwood and heartwood in Black locust and Osage orange. For. Sci. 1968, 14, 334–338. [Google Scholar]
- James, S.A.; Clearwater, M.J.; Meinzer, F.C.; Goldstein, G. Heat dissipation sensors of variable length for the measurement of sap flow in trees with deep sapwood. Tree Physiol. 2002, 22, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Nadezhdina, N.; Nadezhdin, V.; Ferreira, M.A.; Pitacco, A. Variability with xylem depth in sap flow in trunks and branches of mature olive trees. Tree Physiol. 2006, 27, 105–113. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Childs, K.W.; King, A.W.; Hanson, P.J. A model of heat transfer in sapwood and implications for sap flux density measurements using thermal dissipation probes. Tree Physiol. 2011, 31, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Gholz, H.L.; Grier, C.C.; Plummer, M.L. Evaluating stem conducting tissue as an estimator of leaf area in four woody angiosperms. Can. J. Bot. 1977, 55, 1474–1477. [Google Scholar] [CrossRef]
- Whitehead, D. The estimation of foliage area from sapwood basal area in Scots pine. Forestry 1978, 51, 137–149. [Google Scholar] [CrossRef]
- Blanche, C.A.; Hodges, J.D.; Nebeker, T.E. A leaf area-sapwood area ratio developed to rate loblolly pine tree vigor. Can. J. For. Res. 1985, 15, 1181–1184. [Google Scholar] [CrossRef]
- Keane, M.G.; Weetman, G.F. Leaf area-sapwood cross-sectional area relationships in repressed stands of lodgepole pine. Can. J. For. Res. 1987, 17, 205–209. [Google Scholar] [CrossRef]
- Dean, T.J.; Long, J.N.; Smith, F.W. Bias in leaf area-sapwood area ratios and its impact on growth analysis in Pinus contorta. Trees 1988, 2, 104–109. [Google Scholar] [CrossRef]
- Coyea, M.R.; Margolis, H.A. Factors affecting the relationship between sapwood area and leaf area of balsam fir. Can. J. For. Res. 1992, 22, 1684–1693. [Google Scholar] [CrossRef]
- Shelburne, V.B.; Hedden, R.L.; Allen, R.M. The effects of site, stand density and sapwood permeability on the relationship between leaf area and sapwood area in loblolly pine (Pinus taeda L.). For. Ecol. Manag. 1993, 58, 193–209. [Google Scholar] [CrossRef]
- Gartner, B. Sapwood and inner bark quantities in relation to leaf area and wood density in Douglas fir. IAWA J. 2002, 23, 267–285. [Google Scholar] [CrossRef]
- Meadows, S.J.; Hodges, D.J. Sapwood area as an estimator of leaf area and foliar weight in cherrybark oak and green ash. For. Sci. 2002, 48, 69–76. [Google Scholar]
- Kumagai, T.; Nagasawaa, H.; Mabuchia, T.; Ohsakia, S.; Kubotaa, K.; Kogia, K.; Utsumia, Y.; Kogaa, S.; Otsuki, K. Sources of error in estimating stand transpiration using allometric relationships between stem diameter and sapwood area for Criptomeria japonica and Chamaecyparis obtusa. For. Ecol. Manag. 2005, 206, 191–195. [Google Scholar] [CrossRef]
- Medhurst, J.L.; Beadle, C.L. Sapwood hydraulic conductivity and leaf area sapwood area relationships following thinning of a Eucalyptus nitens plantation. Plant Cell Environ. 2002, 25, 1011–1019. [Google Scholar] [CrossRef]
- Coletta, V.; Menguzzato, G.; Pellicone, G.; Veltri, A.; Marziliano, P.A. Effect of thinning on above-ground biomass accumulation in a Douglas-fir plantation in southern Italy. J. For. Res. 2016, 27, 1313–1320. [Google Scholar] [CrossRef]
- Marziliano, P.A.; Coletta, V.; Menguzzato, G.; Nicolaci, A.; Pellicone, G.; Veltri, A. Effects of planting density on the distribution of biomass in a douglas-fir plantation in southern Italy. iForest—Biogeosci. For. 2015, 8, 368–376. [Google Scholar] [CrossRef]
- Peet, R.K. Forests and Meadows of the Rocky Mountains. In North American Terrestrial Vegetation, 1st ed.; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; ISBN 13-978-0-52-126198-2. [Google Scholar]
- Rowe, J.S. Forest Regions of Canada; Department of the Environment Canadian Forestry Service: Ottawa, ON, Canada, 1972; p. 177.
- Strong, W.L.; Leggat, K.R. Ecoregions of Alberta; Forestry, Lands, and Wildlife (1986–1993): Edmonton, AB, Canada, 1992; p. 59. [Google Scholar]
- Government of Canada Activities and Initiatives. Available online: climate.weather.gc.ca/climate_normals/results_1981_2010_e.html (accessed on 9 October 2017).
- Chapter 16: Correlation of Canadian Soil Taxonomy with Other Systems. Available online: sis.agr.gc.ca/cansis/taxa/cssc3/chpt16.html (accessed on 9 October 2017).
- Strong, W.L. Ecoregions and Ecodistricts of Alberta; Alberta Forestry, Lands and Wildlife, Land Information Services Division, Resource Information Branch: Edmonton, AB, Canada, 1992; Volume 1. [Google Scholar]
- Elliot-Frisk, D.L. The taiga and boreal forest. In North American Terrestrial Vegetation, 1st ed.; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: New York, NY, USA, 1988; ISBN 13-978-0-52-126198-2. [Google Scholar]
- Soil Map of Saskatchewan. Available online: sis.agr.gc.ca/cansis/publications/surveys/sk/sk_1967/index.html (accessed on 9 October 2017).
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Quiñonez-Piñón, M.R. Improved Techniques for Measuring and Estimating Scaling Factors Used to Aggregate Forest Transpiration. Ph.D. Thesis, University of Calgary, Schulich School of Engineering, Geomatics Engineering Department, Calgary, AB, Canada, 2007. [Google Scholar]
- Jeremic, D.; Cooper, P.; Srinivasan, U. Comparative analysis of Balsam fir wetwood, heartwood and sapwood properties. Can. J. For. Res. 2004, 34, 1241–1250. [Google Scholar] [CrossRef]
- Ward, J.C.; Pong, W.Y. Wetwood in Trees: A Timber Resource Problem; Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1980. [Google Scholar]
- Kozlowski, T.T.; Pallardy, S.G. Physiology of Woody Plants, 2nd ed.; Academic Press, Inc.: Burlington, MA, USA, 1997; p. 411. ISBN 978-0-12-424162-6. [Google Scholar]
- Tyree, M.T.; Zimmermann, M.H. Xylem Structure and the Ascent of Sap, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2002; p. 257. ISBN 978-3-66-204931-0. [Google Scholar]
- Granier, A.; Huc, R.; Barigah, S.T. Transpiration of natural rain forest and its dependence on climate factors. Agric. For. Meteorol. 1996, 78, 19–29. [Google Scholar] [CrossRef]
- Philipson, W.R.; Ward, J.M.; Butterfield, B.G. Reaction of the cambium to gravity and to the displacement of branches. In The Vascular Cambium, Its Development and Activity, 1st ed.; Philipson, W.R., Ward, J.M., Butterfield, B.G., Eds.; Chapman and Hall LTD: Bungay, UK, 1971; pp. 159–169. [Google Scholar]
- Philipson, W.R.; Ward, J.M.; Butterfield, B.G. Variations in the size of fusiform cambial initials. In The Vascular Cambium, Its Development and Activity, 1st ed.; Philipson, W.R., Ward, J.M., Butterfield, B.G., Eds.; Chapman and Hall LTD: Bungay, UK, 1971. [Google Scholar]
- Auslander, M.; Nevo, E.; Inbar, M. The effects of slope orientation on plant growth, developmental instability and susceptibility to herbivores. J. Arid Environ. 2003, 55, 402–416. [Google Scholar] [CrossRef]
- Cienciala, E.; Mellander, P.E.; Kucera, J.; Oplustilova, M.; Ottosson-Lofvenius, M.; Bishop, K. The effect of a north-facing forest edge on tree water use in a boreal Scots pine stand. Can. J. For. Res. 2002, 32, 693–702. [Google Scholar] [CrossRef]
- Burns, R.M.; Honkala, B.H. Silvics of North America: Hardwoods; Agriculture handbook 654; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; Volume 2, p. 1711.
- Andersson, F. (Ed.) Ecosystems of the World 6. Coniferous Forests, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; p. 646. ISBN 978-0-08-093313-9. [Google Scholar]
- Bechtold, W.A. Crown position and Light Exposure Classification—An Alternative to field-assigned Crown Class. North. J. Appl. For. 2003, 20, 154–160. [Google Scholar]
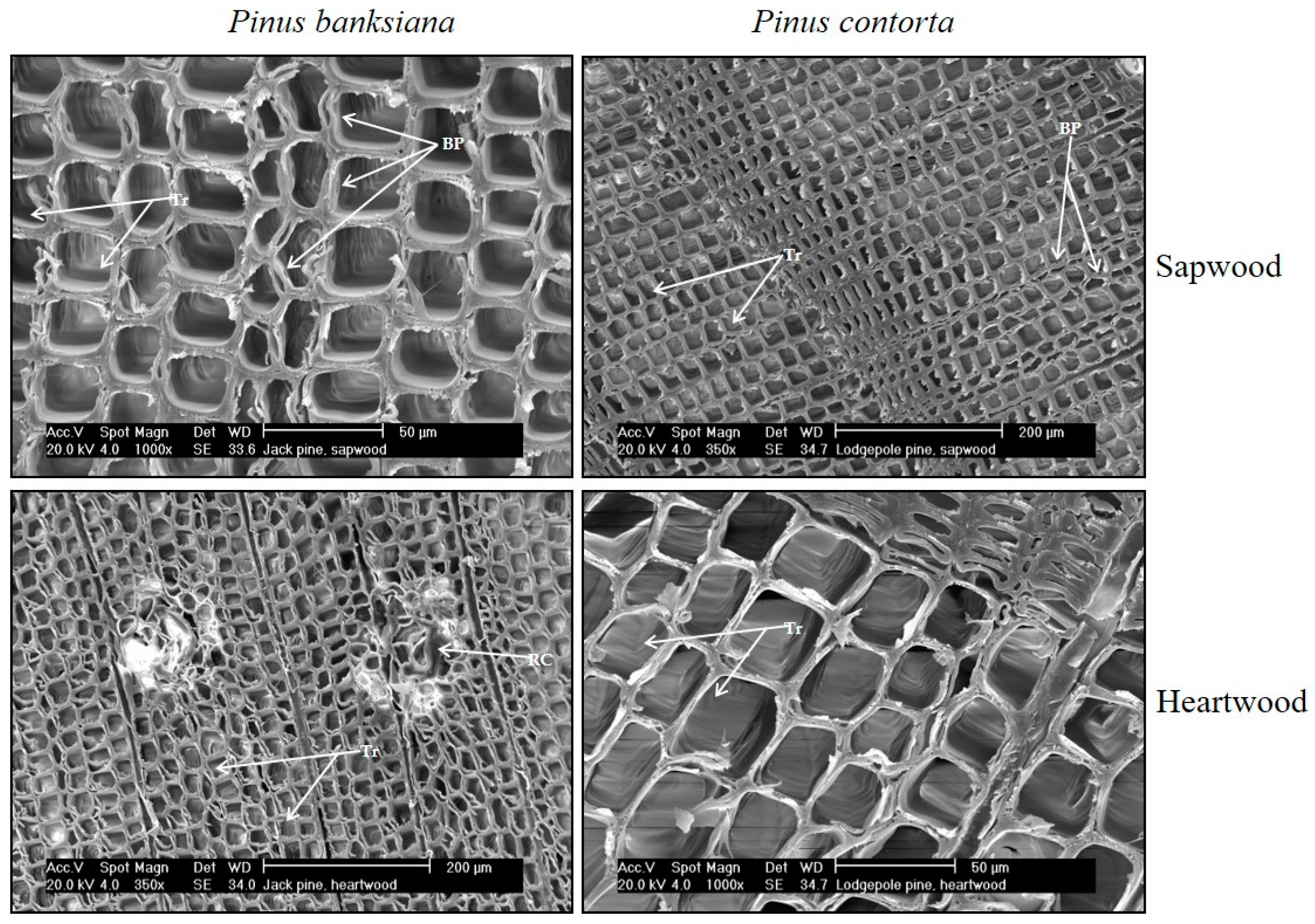
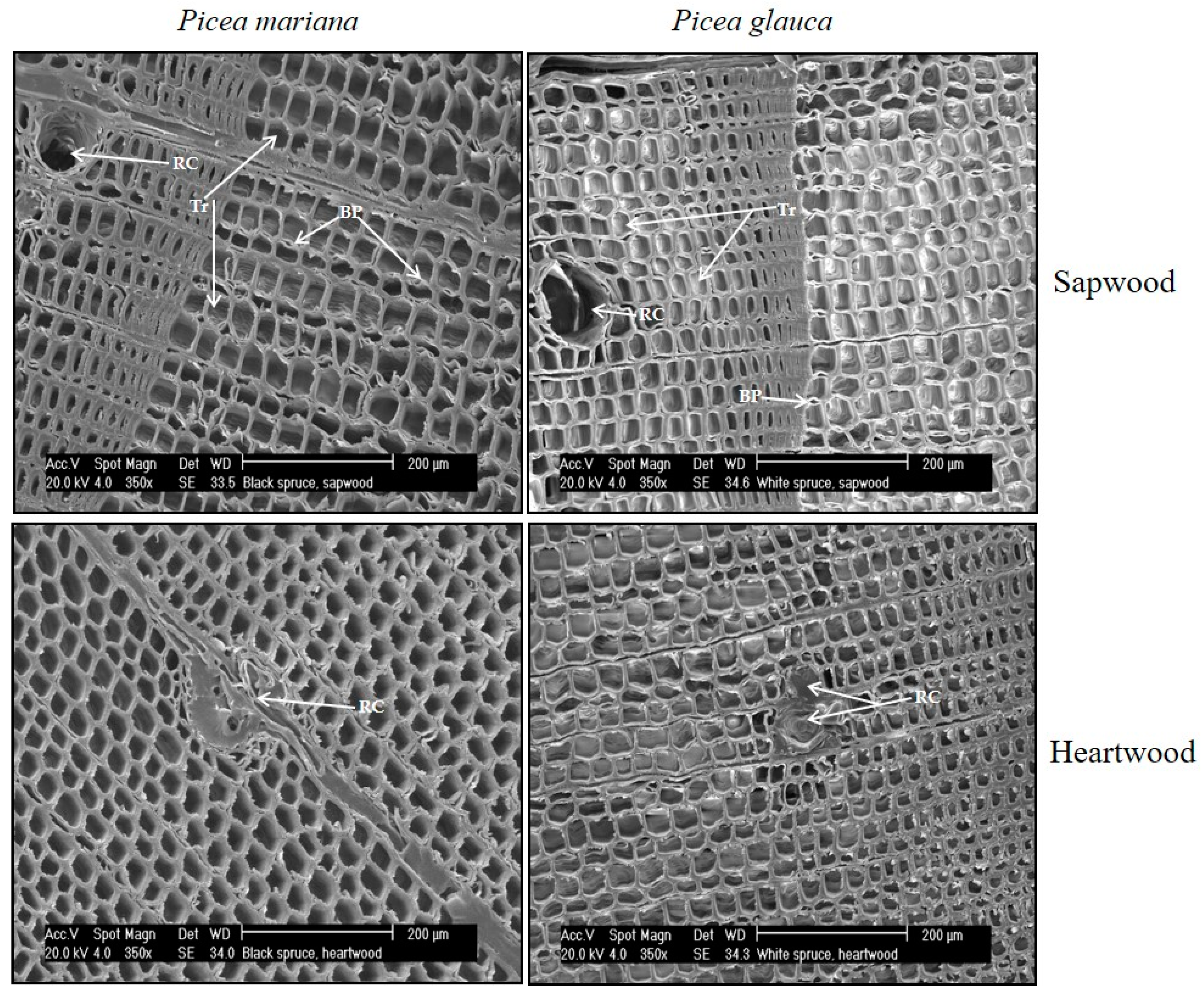
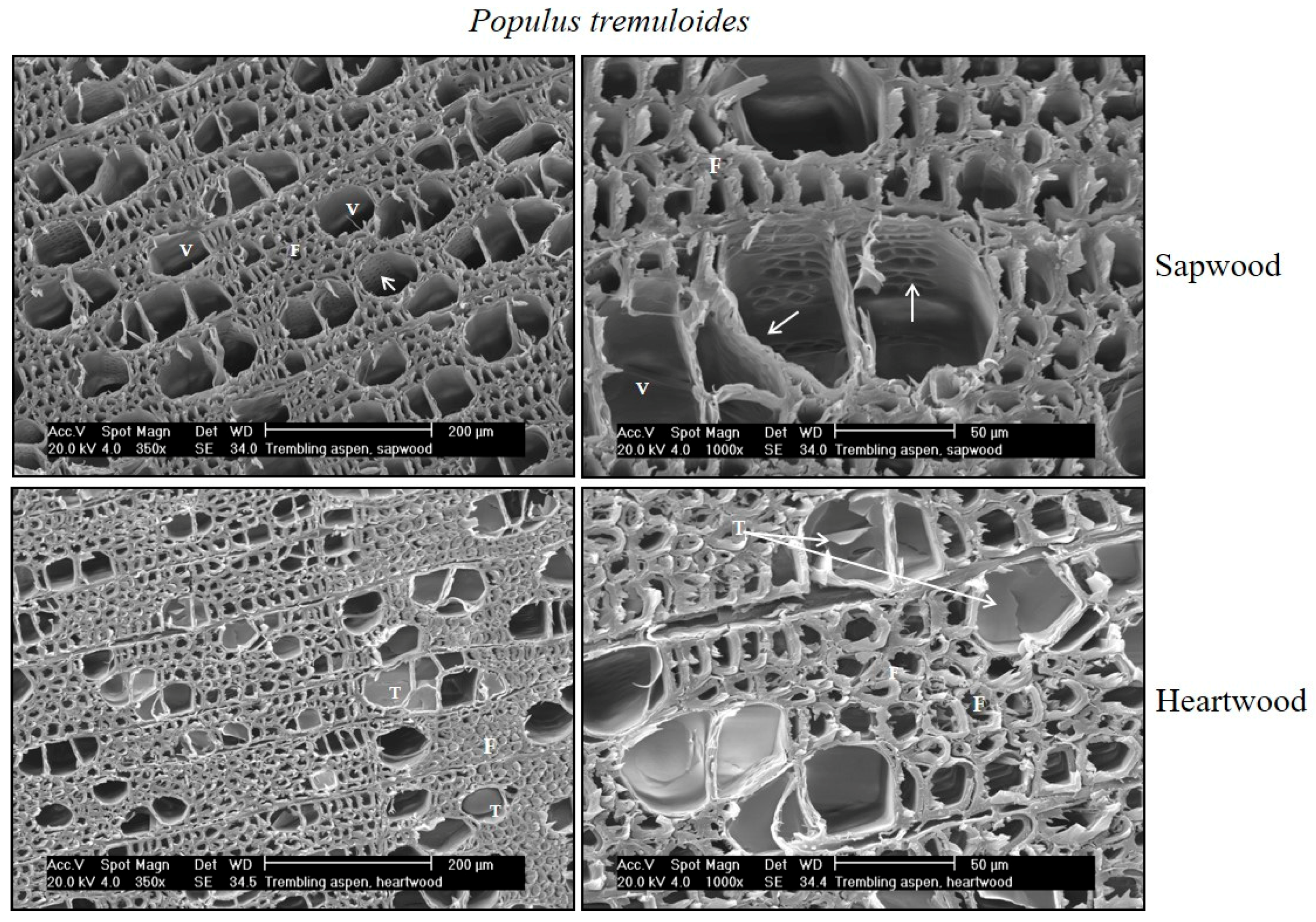

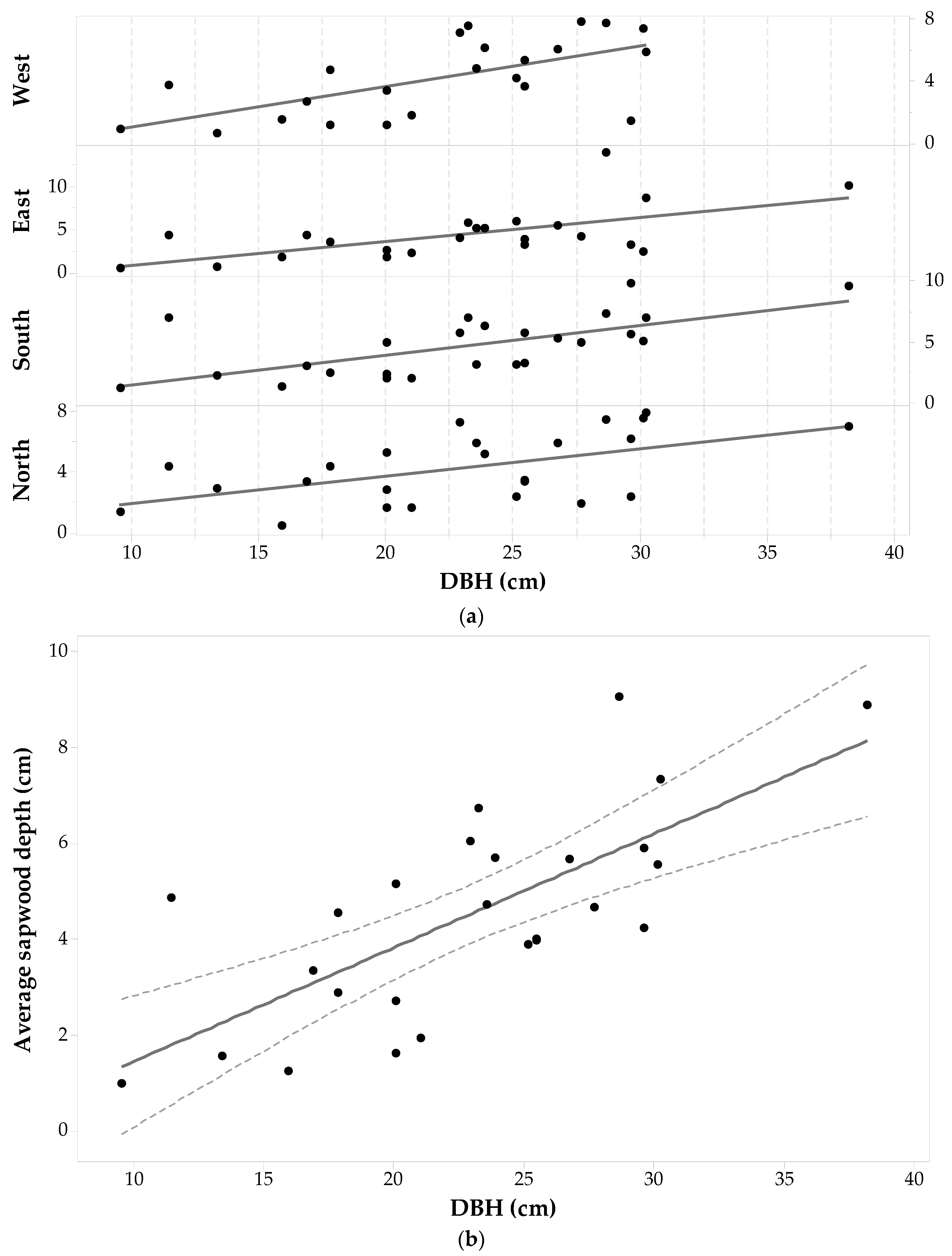
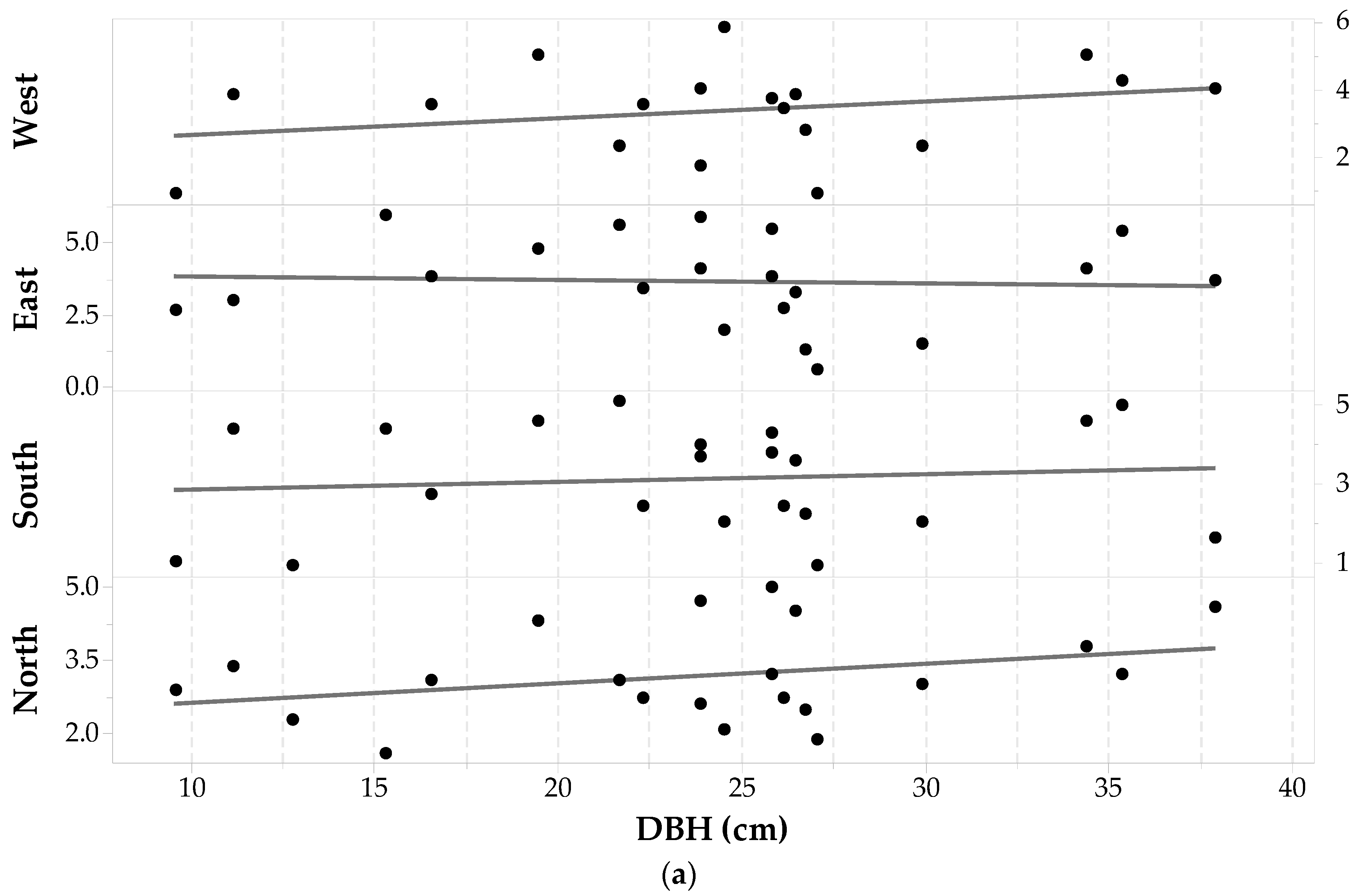
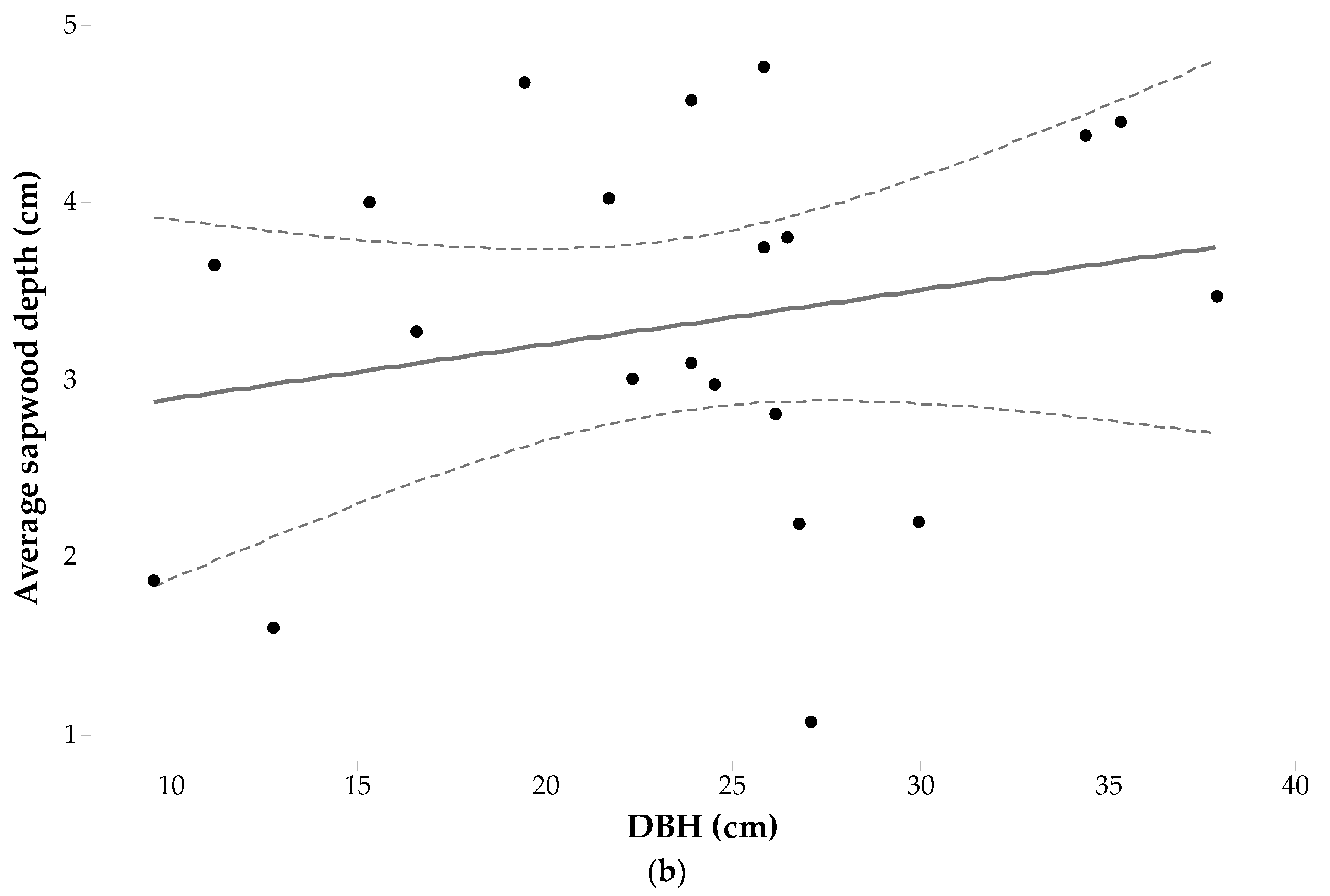
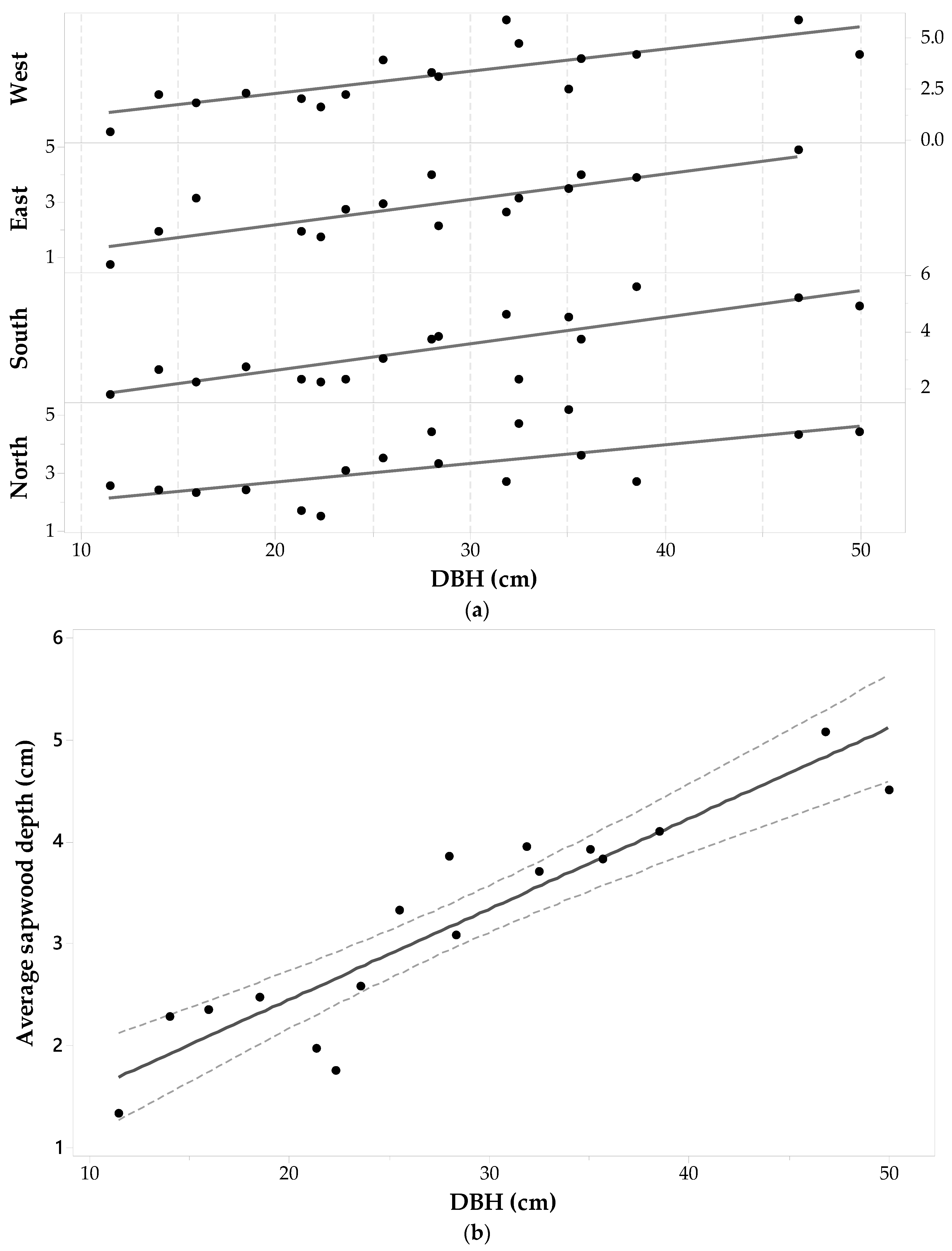
| Cardinal Point | N | Sapwood Depth | |||||
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | Mean | Mode | Standard Deviation | |||
| Pinus bansksiana | North | 24 | 5.20 | 1.34 | 3.37 | 3.30 | 0.94 |
| South | 24 | 5.06 | 1.25 | 3.05 | 2.10 | 0.95 | |
| East | 23 | 5.90 | 1.90 | 4.00 | 4.00 | 1.01 | |
| West | 23 | 5.20 | 2.00 | 3.54 | 3.00 | 0.79 | |
| Pinus contorta | North | 9 | 5.10 | 2.00 | 3.40 | 2.20 | 1.08 |
| South | 8 | 4.80 | 3.70 | 4.22 | 4.30 | 0.33 | |
| East | 9 | 5.80 | 0.90 | 2.93 | 2.00 | 1.16 | |
| West | 9 | 4.80 | 2.40 | 3.70 | 4.10 | 0.67 | |
| Populus tremuloides | North | 25 | 7.90 | 0.50 | 4.27 | 4.40 | 2.19 |
| South | 25 | 9.90 | 1.20 | 4.70 | 7.00 | 2.46 | |
| East | 23 | 13.90 | 0.50 | 4.45 | 4.40 | 3.07 | |
| West | 23 | 7.80 | 0.60 | 4.16 | 1.10 | 2.47 | |
| Picea mariana | North | 21 | 5.00 | 1.60 | 3.20 | 2.72 | 0.96 |
| South | 21 | 5.10 | 0.90 | 3.12 | 0.90 | 1.40 | |
| East | 20 | 6.00 | 0.60 | 3.66 | 4.10 | 1.58 | |
| West | 18 | 5.80 | 0.90 | 3.36 | 0.90 | 1.35 | |
| Picea glauca | North | 17 | 5.20 | 1.50 | 3.22 | 4.40 | 1.08 |
| South | 17 | 5.60 | 1.72 | 3.37 | 2.30 | 1.22 | |
| East | 15 | 4.90 | 0.69 | 2.87 | 3.10 | 1.10 | |
| West | 17 | 5.90 | 0.35 | 3.19 | 4.20 | 1.53 | |
| Cardinal Point | N | Standard Deviation (cm) | Difference (Power = 0.8) (cm) | Difference (Power) (cm) | |
|---|---|---|---|---|---|
| Pinus bansksiana | North | 24 | 0.94 | 0.56 | |
| South | 24 | 0.95 | 0.57 | ||
| East | 23 | 1.01 | 0.62 | ||
| West | 23 | 0.79 | 0.48 | ||
| Pinus contorta | North | 9 | 1.08 | 1.15 | 1.08 (0.75) |
| South | 8 | 0.33 | 0.38 | 0.34 (0.70) | |
| East | 9 | 1.16 | 1.24 | 1.16 (0.75) | |
| West | 9 | 0.67 | 0.71 | 0.67 (0.75) | |
| Populus tremuloides | North | 25 | 2.19 | 1.28 | |
| South | 25 | 2.46 | 1.44 | ||
| East | 23 | 3.14 | 1.92 | ||
| West | 23 | 2.56 | 1.56 | ||
| Picea mariana | North | 21 | 0.96 | 0.62 | |
| South | 21 | 1.40 | 0.90 | ||
| East | 20 | 1.58 | 1.04 | ||
| West | 18 | 1.35 | 0.95 | ||
| Picea glauca | North | 17 | 1.08 | 0.78 | |
| South | 17 | 1.22 | 0.88 | ||
| East | 15 | 1.10 | 0.86 | ||
| West | 17 | 1.53 | 1.11 |
| sdcp | SA | ||
|---|---|---|---|
| Picea mariana | North | 4.60 | 480.94 |
| East | 1.60 | 182.36 | |
| South | 3.70 | 397.30 | |
| West | 4.00 | 425.75 | |
| Populus tremuloides | North | 7.50 | 532.74 |
| East | 5.10 | 400.71 | |
| South | 2.35 | 204.94 | |
| West | 7.30 | 523.12 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñonez-Piñón, M.R.; Valeo, C. Allometry of Sapwood Depth in Five Boreal Trees. Forests 2017, 8, 457. https://doi.org/10.3390/f8110457
Quiñonez-Piñón MR, Valeo C. Allometry of Sapwood Depth in Five Boreal Trees. Forests. 2017; 8(11):457. https://doi.org/10.3390/f8110457
Chicago/Turabian StyleQuiñonez-Piñón, M. Rebeca, and Caterina Valeo. 2017. "Allometry of Sapwood Depth in Five Boreal Trees" Forests 8, no. 11: 457. https://doi.org/10.3390/f8110457






