Buffer-Mediated Effects of Clearcutting on In-Pool Amphibian Productivity: Can Aquatic Processes Compensate for Terrestrial Habitat Disturbance?
Abstract
:1. Introduction
- Eggmass abundance would increase with buffer width because eggmass number is positively correlated with adult abundance and wider buffers provide forested habitat for more adults.
- The number of metamorphs produced and metamorph snout-vent length would be negatively correlated with buffer width because these metamorphic traits are strongly shaped by negative density dependence in the larval stage. Thus, if eggmass abundance increased with buffer width (due to increased adult abundance), larval density should increase, and metamorph productivity decrease, with buffer width.
- Any treatment effects on eggmass and metamorph abundance and metamorph length would diminish over time as clearcuts regenerated. However, wood frogs would respond faster to regeneration than spotted salamanders, since wood frogs breed every year, whereas individual salamanders often skip years between breeding. Further, wood frogs can generate many times more metamorphs than spotted salamanders, during productive years [48].
2. Materials and Methods
2.1. Statistical Analyses
2.2. Compliance with Ethical Standards
3. Results
3.1. Spotted Salamanders
3.2. Wood Frogs
4. Discussion
4.1. Eggmass Abundance
4.2. Metamorph Productivity
4.2.1. Productivity over Time
4.2.2. Productivity and Hydroperiod
5. Conclusions and Conservation Implications
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Forum on Forests. Conclusions and recommendations for addressing key challenges of forests and economic development. In Proceedings of the Tenth Session of the United Nations Forum on Forests, Istanbul, Turkey, 8–19 April 2013; p. 9.
- Wondolleck, J. Public Lands Conflict and Resolution: Managing National Forest Disputes, 2nd ed.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Winkel, G. When the pendulum doesn’t find its center: Environmental narratives, strategies, and forest policy change in the US Pacific Northwest. Glob. Environ. Chang. 2014, 27, 84–95. [Google Scholar] [CrossRef]
- Ringold, P.L.; Mulder, B.; Alegria, J.; Czaplewski, R.L.; Tolle, T.; Burnett, K. Design of an ecological monitoring strategy for the Forest Plan0020in the Pacific Northwest. In Monitoring Ecosystems: Interdisciplinary Approaches for Evaluating Ecoregional Initiatives; Busch, D.E., Trexwell, J.C., Eds.; Island Press: Washington, DC, USA, 2003; pp. 73–99. [Google Scholar]
- Tilghman, J.M.; Ramee, S.W.; Marsh, D.M. Meta-analysis of the effects of canopy removal on terrestrial salamander populations in North America. Biol. Conserv. 2012, 152, 1–9. [Google Scholar] [CrossRef]
- Morris, D.L.A.; Porneluzi, P.; Haslerig, J.; Clawson, R.L.; Faaborg, J. Results of 20 years of experimental forest management on breeding birds in Ozark forests of Missouri, USA. For. Ecol. Manag. 2013, 310, 747–760. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Todd, B.D.; Blomquist, S.M.; Calhoun, A.J.K.; Gibbons, J.W.; Gibbs, J.P.; Graeter, G.J.; Harper, E.B.; Hocking, D.J.; Hunter, M.L.; et al. Effects of timber harvest on amphibian populations: Understanding mechanisms from forest experiments. BioScience 2009, 59, 853–862. [Google Scholar] [CrossRef]
- Broadmeadow, S.; Nisbet, T.R. The effects of riparian forest management on the freshwater environment: A literature review of best management practice. Hydrol. Earth Syst. Sci. 2004, 8, 286–305. [Google Scholar] [CrossRef]
- Mayer, P.M.; Reynolds, S.K.; McCutchen, M.D.; Canfield, T.J. Meta-analysis of nitrogen removal in riparian buffers. J. Environ. Qual. 2007, 36, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Castelle, A.J.; Johnson, A.W.; Conolly, C. Wetland and stream buffer size requirements—A review. J. Environ. Qual. 1994, 23, 878–882. [Google Scholar] [CrossRef]
- Dupuis, L.; Steventon, D. Riparian management and the tailed frog in northern coastal forests. For. Ecol. Manag. 1999, 124, 35–43. [Google Scholar] [CrossRef]
- Johnston, B.; Frid, L. Clearcut logging restricts the movements of terrestrial Pacific giant salamanders (Dicamptodon tenebrosus Good). Can. J. Zool. 2002, 80, 2170–2177. [Google Scholar] [CrossRef]
- Vesely, D.G.; McComb, W.C. Salamander abundance and amphibian species richness in riparian buffer strips in the Oregon coast range. For. Sci. 2002, 48, 291–297. [Google Scholar]
- Veysey Powell, J.S.; Babbitt, K.J. An experimental test of buffer utility as a technique for managing pool-breeding amphibians. PLoS ONE 2015, 10, e0133642. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.S.V.; Babbitt, K.J. Despite buffers, experimental forest clearcuts impact amphibian body size and biomass. PLoS ONE 2015, 10, e0143505. [Google Scholar]
- Semlitsch, R.D.; Bodie, J.R. Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conserv. Biol. 2003, 17, 1219–1228. [Google Scholar] [CrossRef]
- Olson, D.H.; Anderson, P.D.; Frissell, C.A.; Welsh, H.H., Jr.; Bradford, D.F. Biodiversity management approaches for stream-riparian areas: Perspectives for Pacific Northwest headwater forests, microclimates, and amphibians. For. Ecol. Manag. 2007, 246, 81–107. [Google Scholar] [CrossRef]
- Veysey, J.S.; Babbitt, K.J.; Cooper, A. An experimental assessment of buffer width: Implications for salamander migratory behavior. Biol. Conserv. 2009, 142, 2227–2239. [Google Scholar] [CrossRef]
- Wilbur, H.M. Propagule size, number, and dispersion pattern in Ambystoma and Asclepias. Am. Nat. 1977, 111, 43–68. [Google Scholar] [CrossRef]
- Kinkead, K.E.; Otis, D.L. Estimating superpopulation size and annual probability of breeding for pond-breeding salamanders. Herpetologica 2007, 63, 151–162. [Google Scholar] [CrossRef]
- Flageole, S.; Leclair, R., Jr. Etude démographique d’une population de salamandres (Ambystoma maculatum) à l’aide de la méthode squeletto-chronologique. Can. J. Zool. 1992, 70, 740–749. [Google Scholar] [CrossRef]
- Redmer, M.; Trauth, S.E. Rana sylvatica LeConte, 1825, Wood Frog. In Amphibian Declines: The Conservation Status of United States Species; Lannoo, M.J., Ed.; University of California Press: Berkeley, CA, USA, 2005. [Google Scholar]
- Berven, K.A. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 1990, 71, 1599–1608. [Google Scholar] [CrossRef]
- Berven, K.A. Population regulation in the wood frog, Rana sylvatica, from three diverse geographic localities. Aust. J. Ecol. 1995, 20, 385–392. [Google Scholar] [CrossRef]
- Semlitsch, R.D. Differentiating migration and dispersal processes for pond-breeding amphibians. J. Wildl. Manag. 2008, 72, 260–267. [Google Scholar] [CrossRef]
- Harper, E.B.; Patrick, D.A.; Gibbs, J.P. Impact of forestry practices at a landscape scale on the dynamics of amphibian populations. Ecol. Appl. 2015, 25, 2271–2284. [Google Scholar] [CrossRef] [PubMed]
- Peterman, W.E.; Rittenhouse, T.A.G.; Earl, J.E.; Semlitsch, R.D. Demographic network and multi-season occupancy modeling of Rana sylvatica reveal spatial and temporal patterns of population connectivity and persistence. Landsc. Ecol. 2013, 28, 1601–1613. [Google Scholar] [CrossRef]
- Regester, K.J.; Whiles, M.R.; Lips, K.R. Variation in the trophic basis of production and energy flow associated with emergence of larval salamander assemblages from forest ponds. Freshw. Biol. 2008, 53, 1754–1767. [Google Scholar] [CrossRef]
- Regester, K.J.; Lips, K.R.; Whiles, M.R. Energy flow and subsidies associated with the complex life cycle of ambystomatid salamanders in ponds and adjacent forest in Southern Illinois. Oecologia 2006, 147, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Capps, K.A.; Berven, K.A.; Tiegs, S.D. Modelling nutrient transport and transformation by pool-breeding amphibians in forested landscapes using a 21-year dataset. Freshw. Biol. 2015, 60, 500–511. [Google Scholar] [CrossRef]
- Pittman, S.E.; Semlitsch, R.D. Habitat type and distance to edge affect movement behavior of juvenile pond-breeding salamanders. J. Zool. 2013, 291, 154–162. [Google Scholar] [CrossRef]
- Osbourn, M.S.; Connette, G.M.; Semlitsch, R.D. Effects of fine-scale forest habitat quality on movement and settling decisions in juvenile pond-breeding salamanders. Ecol. Appl. 2014, 24, 1719–1729. [Google Scholar] [CrossRef]
- Wilbur, H.M. Experimental ecology of food webs: Complex systems in temporary ponds. Ecology 1997, 78, 2279–2302. [Google Scholar] [CrossRef]
- Schriever, T.A.; Williams, D.D. Ontogenetic and individual diet variation in amphibian larvae across an environmental gradient. Freshw. Biol. 2013, 58, 223–236. [Google Scholar] [CrossRef]
- Burley, L.A.; Moyer, A.T.; Petranka, J.W. Density of an intraguild predator mediates feeding group size, intraguild egg predation, and intra- and interspecific competition. Oecologia 2006, 148, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A.; Auld, J.R. Predator- and competitor-induced plasticity: How changes in foraging morphology affect phenotypic trade-offs. Ecology 2005, 86, 1723–1729. [Google Scholar] [CrossRef]
- Mott, C.L.; Maret, T.J. Species-specific patterns of agonistic behavior among larvae of three syntopic species of Ambystomatid salamanders. Copeia 2011, 2011, 9–17. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: The problem of separating human impacts from natural fluctuations. Science 1991, 253, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Brodman, R. Intraguild predation on congeners affects size, aggression, and survival among Ambystoma Salamander larvae. J. Herpetol. 2004, 38, 21–26. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Shea, K.; Kiesecker, J.M. The influence of multi-stage predation on population growth and the distribution of the pond-breeding salamander, Ambystoma jeffersonianum. Can. J. Zool. 2006, 84, 449–458. [Google Scholar] [CrossRef]
- Brodman, R. Effects of intraguild interactions on fitness and microhabitat use of larval Ambystoma salamanders. Copeia 1996, 1996, 372–378. [Google Scholar] [CrossRef]
- Davis, A.K.; Maerz, J.C. Effects of larval density on hematological stress indices in salamanders. J. Exp. Zool. 2009, 311A, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Berven, K. The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 1982, 36, 962–983. [Google Scholar] [CrossRef]
- Werner, E.E.; Glennemeier, K.S. Influence of forest canopy cover on the breeding pond distributions of several amphibian species. Copeia 1999, 1999, 1–12. [Google Scholar] [CrossRef]
- Rowe, C.L.; Dunson, W.A. Impacts of hydroperiod on growth and survival of larval amphibians in temporary ponds of central Pennsylvania, USA. Oecologia 1995, 102, 397–403. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Walls, S.C. Competition in two species of larval salamanders—A test of geographic-variation in competitive ability. Copeia 1993, 1993, 587–595. [Google Scholar] [CrossRef]
- Anderson, T.L.; Whiteman, H.H. Non-additive effects of intra- and interspecific competition between two larval salamanders. J. Anim. Ecol. 2015, 84, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Popescu, V.D.; Patrick, D.A.; Hunter, M.L., Jr.; Calhoun, A.J.K. The role of forest harvesting and subsequent vegetative regrowth in determining patterns of amphibian habitat use. For. Ecol. Manag. 2012, 270, 163–174. [Google Scholar] [CrossRef]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Crespi, E.J.; Warne, R.W. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Homan, R.N.; Regosin, J.V.; Rodrigues, D.M.; Reed, J.M.; Windmiller, B.S.; Romero, L.M. Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim. Conserv. 2003, 6, 11–18. [Google Scholar] [CrossRef]
- Bull, J.J.; Shine, R. Iteroparous animals that skip opportunities for reproduction. Am. Nat. 1979, 114, 296–303. [Google Scholar] [CrossRef]
- Spotila, J.R. Role of temperature and water in the ecology of lungless salamanders. Ecol. Monogr. 1972, 42, 95–125. [Google Scholar] [CrossRef]
- Crouch, W.B.; Paton, P.W.C. Using egg-mass counts to monitor wood frog populations. Wildl. Soc. Bull. 2000, 28, 895–901. [Google Scholar]
- Savage, W.K.; Zamudio, K.R. Ambystoma maculatum (Shaw, 1802), Spotted Salamander. In Amphibian Declines: The Conservation Status of United States Species; Lannoo, M.J., Ed.; University of California Press: Berkeley, CA, USA, 2005; pp. 621–627. [Google Scholar]
- Veysey, J.S.; Mattfeldt, S.D.; Babbitt, K.J. Comparative influence of isolation, landscape, and wetland characteristics on egg-mass abundance of two pool-breeding amphibian species. Landsc. Ecol. 2011, 26, 661–672. [Google Scholar] [CrossRef]
- Scott, D.E. Effects of larval density in Ambystoma opacum: An experiment in large-scale field enclosures. Ecology 1990, 71, 296–306. [Google Scholar] [CrossRef]
- Taylor, B.E.; Scott, D.E.; Gibbons, J.W. Catastrophic reproductive failure, terrestrial survival, and persistence of the marbled salamander. Conserv. Biol. 2006, 20, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Berven, K.A.; Grudzien, T.A. Dispersal in the wood frog (Rana sylvatica): Implications for genetic population-structure. Evolution 1990, 44, 2047–2056. [Google Scholar] [CrossRef]
- Gamble, L.R.; McGarigal, K.; Compton, B.W. Fidelity and dispersal in the pond-breeding amphibian, Ambystoma opacum: Implications for spatio-temporal population dynamics and conservation. Biol. Conserv. 2007, 139, 247–257. [Google Scholar] [CrossRef]
- Harper, E.B.; Rittenhouse, T.A.G.; Semlitsch, R.D. Demographic consequences of terrestrial habitat loss for pool-breeding amphibians: Predicting extinction risks associated with inadequate size of buffer zones. Conserv. Biol. 2008, 22, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Berven, K.A. Density dependence in the terrestrial stage of wood frogs: Evidence from a 21-year population study. Copeia 2009, 2009, 328–338. [Google Scholar] [CrossRef]
- Wilbur, H.M. Competition, predation, and structure of Ambystoma-Rana-sylvatica community. Ecology 1972, 53, 3–21. [Google Scholar] [CrossRef]
- Bennett, A.M.; Murray, D.L. Maternal body condition influences magnitude of anti-predator response in offspring. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141806. [Google Scholar] [CrossRef] [PubMed]
- Wellborn, G.A.; Skelly, D.K.; Werner, E.E. Mechanisms creating community structure across a freshwater habitat gradient. Annu. Rev. Ecol. Evolut. Syst. 1996, 27, 337–563. [Google Scholar] [CrossRef]
- Scott, D.E.; Fore, M.R. The effect of food limitation on lipid levels, growth, and reproduction in the marbled salamander, Ambystoma opacum. Herpetologica 1995, 51, 462–471. [Google Scholar]
- Berven, K.A. Factors affecting variation in reproductive traits within a population of wood frogs (Rana sylvatica). Copeia 1988, 1988, 605–615. [Google Scholar] [CrossRef]
- Woodward, B.D. Local intraspecific variation in clutch parameters in the spotted salamander (Ambystoma maculatum). Copeia 1982, 1982, 157–160. [Google Scholar] [CrossRef]
- Smith-Gill, S.J.; Berven, K.A. In vitro fertilization and assessment of male reproductive potential using mammalian gonadotropin-releasing hormone to induce spermiation in Rana sylvatica. Copeia 1980, 1980, 723–728. [Google Scholar] [CrossRef]
- Chandler, C.H.; Zamudio, K.R. Reproductive success by large, closely related males facilitated by sperm storage in an aggregate breeding amphibian. Mol. Ecol. 2008, 17, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Shoop, C.R. Yearly variation in larval survival of Ambystoma maculatum. Ecology 1974, 55, 440–444. [Google Scholar] [CrossRef]
- Batzer, D.P.; Wissinger, S.A. Ecology of insect communities in nontidal wetlands. Annu. Rev. Entomol. 1996, 41, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Tarr, T.; Baber, M.; Babbitt, K. Macroinvertebrate community structure across a wetland hydroperiod gradient in southern New Hampshire, USA. Wetl. Ecol. Manag. 2005, 13, 321–334. [Google Scholar] [CrossRef]
- Holbrook, C.T.; Petranka, J.W. Ecological interactions between Rana sylvatica and Ambystoma maculatum: Evidence of interspecific competition and facultative intraguild predation. Copeia 2004, 2004, 932–939. [Google Scholar] [CrossRef]
- Karraker, N.E.; Gibbs, J.P. Amphibian production in forested landscapes in relation to wetland hydroperiod: A case study of vernal pools and beaver ponds. Biol. Conserv. 2009, 142, 2293–2302. [Google Scholar] [CrossRef]
- Phillips, C.A.; Johnson, J.R.; Dreslik, M.J.; Petzing, J.E. Effects of hydroperiod on recruitment of mole salamanders (genus Ambystoma) at a temporary pond in Vermilion County, Illinois. Trans. Ill. State Acad. Sci. 2002, 95, 131–139. [Google Scholar]
- Semlitsch, R.D.; Conner, C.A.; Hocking, D.J.; Rittenhouse, T.A.G.; Harper, E.B. Effects of timber harvesting on pond-breeding amphibian persistence: Testing the evacuation hypothesis. Ecol. Appl. 2009, 18, 283–289. [Google Scholar] [CrossRef]
- Whitaker, D.M.; Carroll, A.L.; Montevecchi, W.A. Elevated numbers of flying insects and insectivorous birds in riparian buffer strips. Can. J. Zool. 2000, 78, 740–747. [Google Scholar] [CrossRef]
- Relyea, R.A. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology 2001, 82, 523–540. [Google Scholar] [CrossRef]
- Schoeppner, N.M.; Relyea, R.A. Phenotypic plasticity in response to fine-grained environmental variation in predation. Funct. Ecol. 2009, 23, 587–594. [Google Scholar] [CrossRef]
- Babbitt, K.J.; Baber, M.J.; Tarr, T.L. Patterns of larval amphibian distribution along a wetland hydroperiod gradient. Can. J. Zool. 2003, 81, 1539–1552. [Google Scholar] [CrossRef]
- Brooks, R.T. Annual and seasonal variation and the effects of hydroperiod on benthic macroinvertebrates of seasonal forest (“vernal”) ponds in central Massachusetts, USA. Wetlands 2000, 20, 707–715. [Google Scholar] [CrossRef]
- Hanson, M.A.; Church, J.O.; Miller, A.T.; Palik, B.J.; Butler, M.G. Testing the efficacy of harvest buffers on the invertebrate communities in seasonal forest wetlands. In Summaries of Wildlife Research Findings; Wingate, P.J., Kimmel, R.O., Lawrence, J.S., Lenarz, M.S., Eds.; Minnesota Department of Natural Resources, Division of Fish and Wildlife, Wildlife Populations and Research Unit: St. Paul, MN, USA, 2005; pp. 164–179. [Google Scholar]
- Miller, A.T. Aquatic Communities in Northern Forest Wetlands: Characteristics and Influences of Timber Harvest. Master’s Thesis, North Dakota State University of Agriculture and Applied Science, Fargo, ND, USA, 2001. [Google Scholar]
- Hanson, M.; Palik, B.; Church, J.; Miller, A. Influences of upland timber harvest on aquatic invertebrate communities in seasonal ponds: Efficacy of forested buffers. Wetl. Ecol. Manag. 2010, 18, 255–267. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- De Maynadier, P.G.; Hunter, M.L. Effects of silvicultural edges on the distribution and abundance of amphibians in Maine. Conserv. Biol. 1998, 12, 340–352. [Google Scholar] [CrossRef]
- Hocking, D.J.; Babbitt, K.J.; Yamasaki, M. Comparison of silvicultural and natural disturbance effects on terrestrial salamanders in northern hardwood forests. Biol. Conserv. 2013, 167, 194–202. [Google Scholar] [CrossRef]
- Halverson, M.A.; Skelly, D.K.; Kiesecker, J.M.; Freidenburg, L.K. Forest mediated light regime linked to amphibian distribution and performance. Oecologia 2003, 134, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.K.; Freidenburg, L.K.; Kiesecker, J.M. Forest canopy and the performance of larval amphibians. Ecology 2002, 83, 983–992. [Google Scholar] [CrossRef]
- Batzer, D.P.; George, B.M.; Braccia, A. Aquatic invertebrate responses to timber harvest in a bottomland hardwood wetland of South Carolina. For. Sci. 2005, 51, 284–291. [Google Scholar]
- Altwegg, R.; Reyer, H.-U. Patterns of natural selection on size at metamorphosis in water frogs. Evolution 2003, 57, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.E. The effect of larval density on adult demographic traits in Ambystoma opacum. Ecology 1994, 75, 1383–1396. [Google Scholar] [CrossRef]
- Herrmann, H.L.; Babbitt, K.J.; Baber, M.J.; Congalton, R.G. Effects of landscape characteristics on amphibian distribution in a forest-dominated landscape. Biol. Conserv. 2005, 123, 139–149. [Google Scholar] [CrossRef]
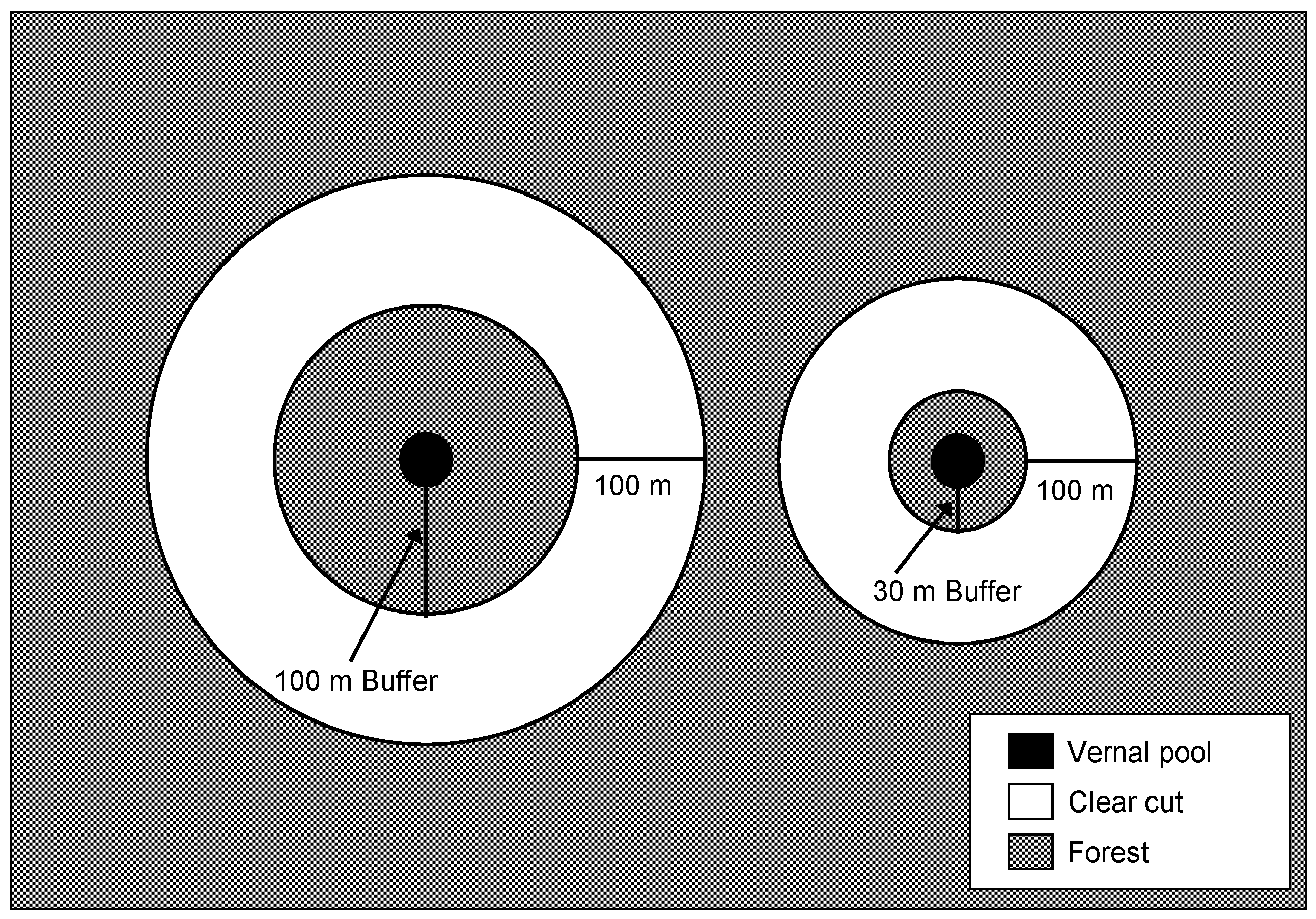
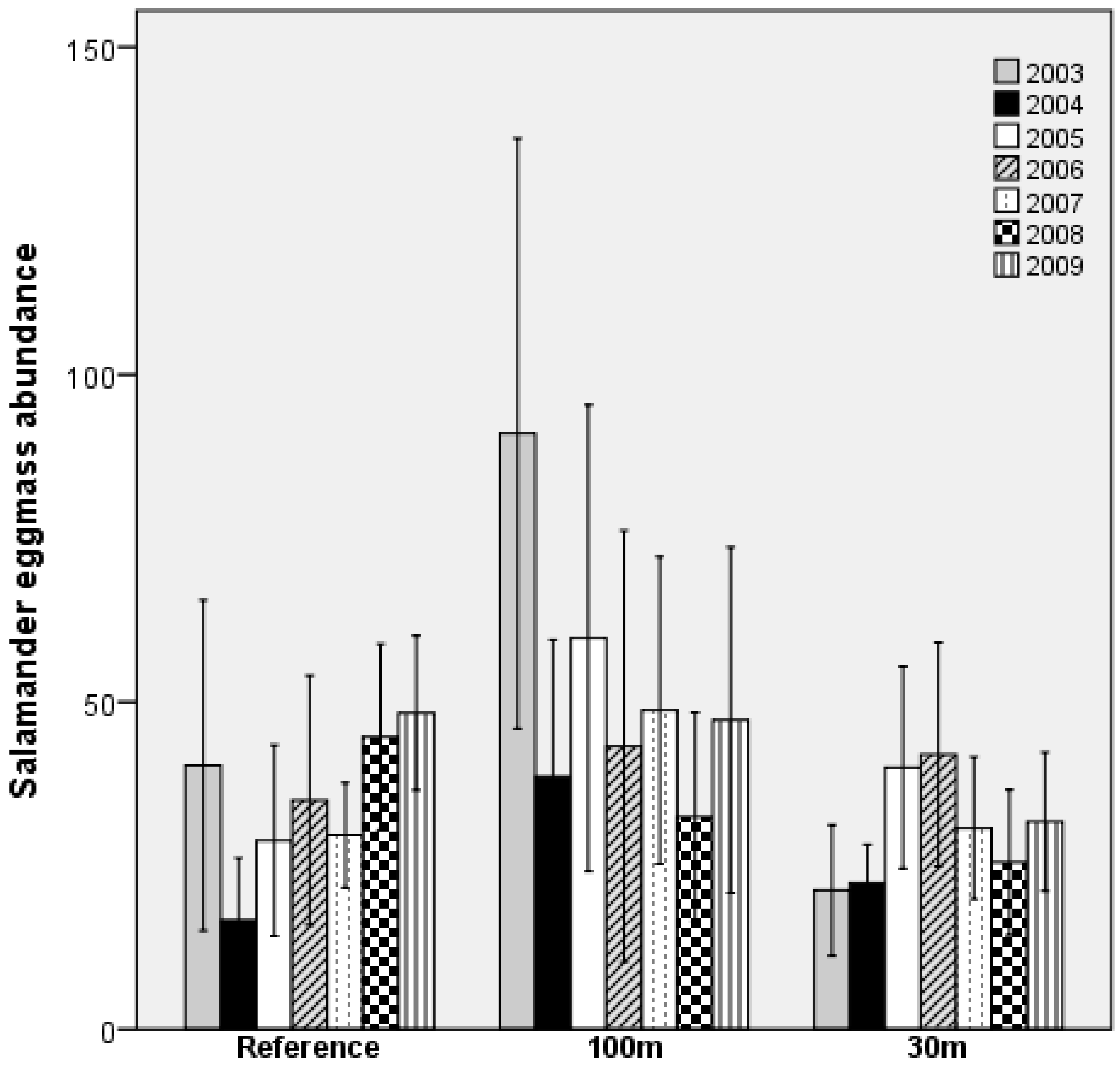
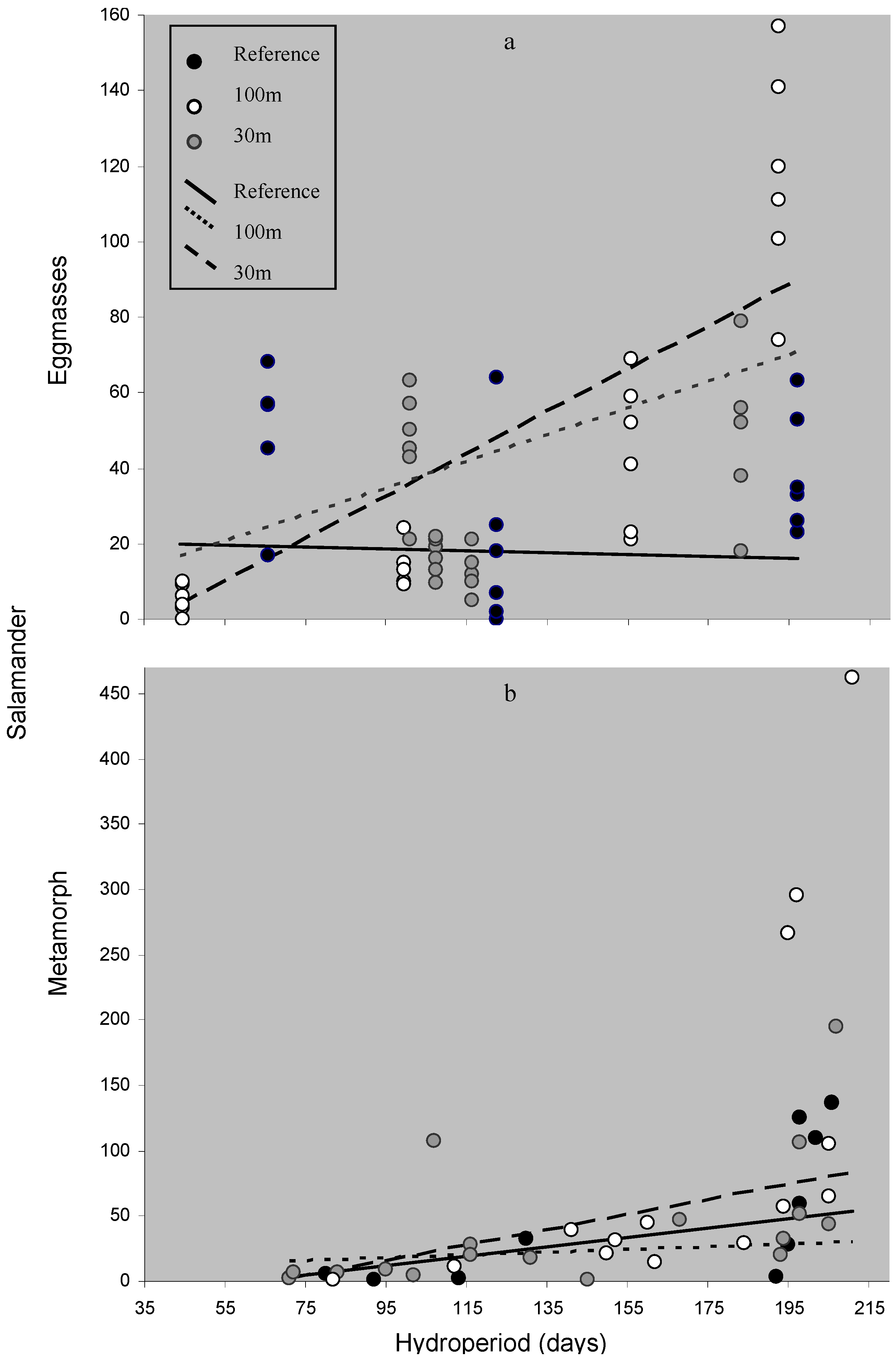
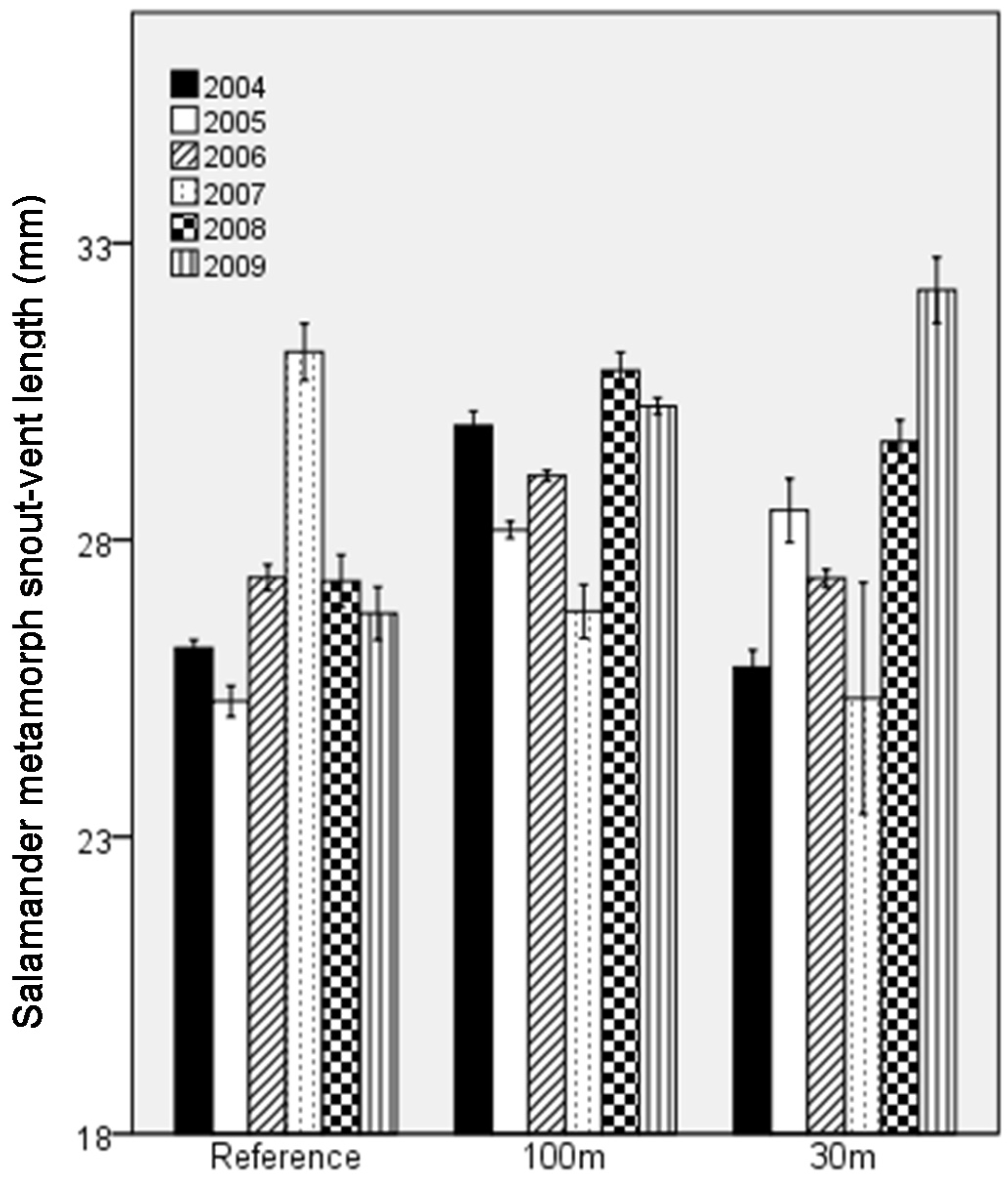
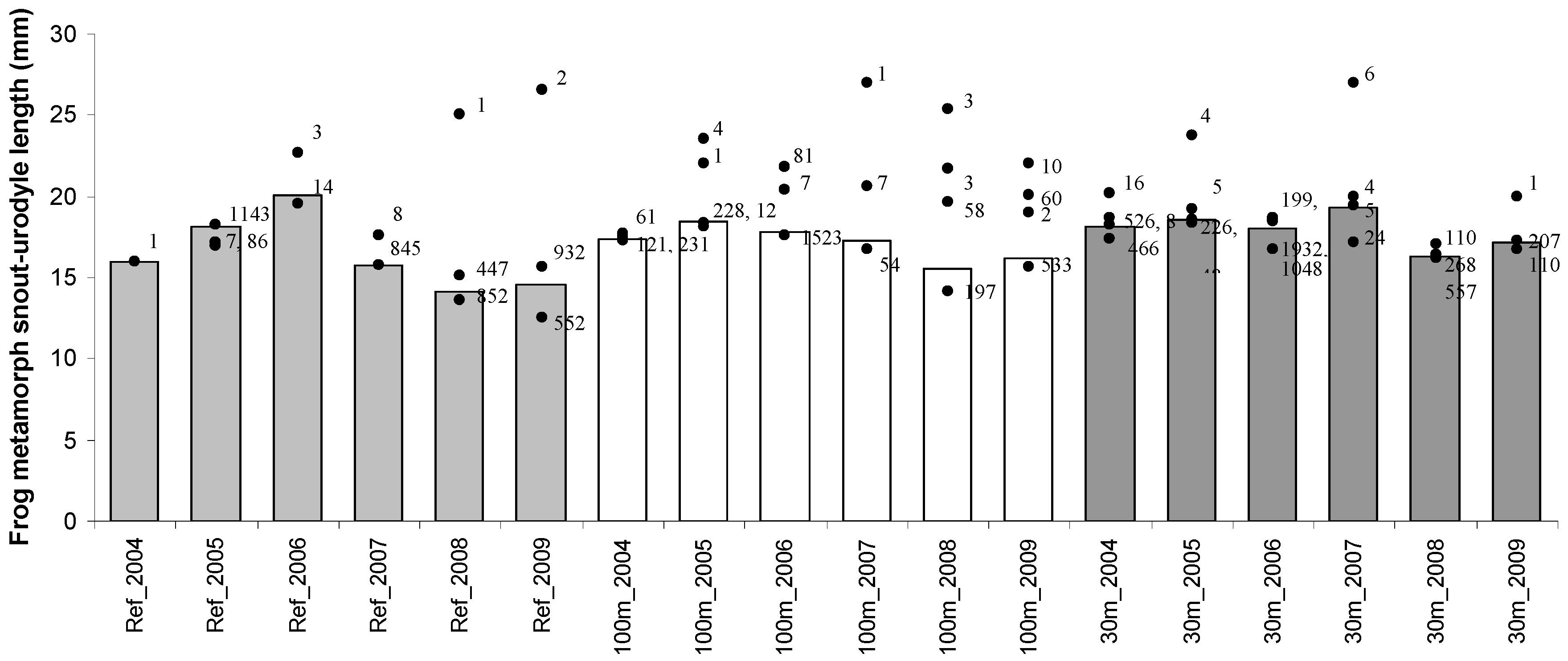
| Mean ± SE | Range | |||
| hydroperiod (days) | ||||
| current-year | 126 ± 7 | 0–211 | ||
| mean | 126 ± 6 | 45–197 | ||
| standard deviation | 32 ± 2 | 6–49 | ||
| Spotted Salamander | Wood Frog | |||
| Mean ± SE | Range | Mean ± SE | Range | |
| # Eggmasses | ||||
| Reference | 35 ± 5 | 0–90 | 56 ± 10 | 23–235 |
| 100 m | 52 ± 11 | 0–200 | 38 ± 6 | 0–105 |
| 30 m | 31 ± 4 | 4–79 | 58 ± 11 | 2–235 |
| # Metamorphs | ||||
| Reference | 28 ± 11 | 0–137 | 587 ± 331 | 0–5905 |
| 100 m | 60 ± 24 | 0–462 | 151 ± 73 | 0–1713 |
| 30 m | 30 ± 10 | 0–195 | 558 ± 331 | 0–7691 |
| SVL/SUL b (mm) | ||||
| Reference | 26.3 ± 0.14 | 20–42 | 15.6 ± 0.03 | 8–28 |
| 100 m | 29.3 ± 0.07 | 19–43 | 17.3 ± 0.04 | 10–32 |
| 30 m | 27.6 ± 0.14 | 18–45 | 17.8 ± 0.02 | 10–33 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veysey Powell, J.S.; Babbitt, K.J. Buffer-Mediated Effects of Clearcutting on In-Pool Amphibian Productivity: Can Aquatic Processes Compensate for Terrestrial Habitat Disturbance? Forests 2017, 8, 10. https://doi.org/10.3390/f8010010
Veysey Powell JS, Babbitt KJ. Buffer-Mediated Effects of Clearcutting on In-Pool Amphibian Productivity: Can Aquatic Processes Compensate for Terrestrial Habitat Disturbance? Forests. 2017; 8(1):10. https://doi.org/10.3390/f8010010
Chicago/Turabian StyleVeysey Powell, Jessica S., and Kimberly J. Babbitt. 2017. "Buffer-Mediated Effects of Clearcutting on In-Pool Amphibian Productivity: Can Aquatic Processes Compensate for Terrestrial Habitat Disturbance?" Forests 8, no. 1: 10. https://doi.org/10.3390/f8010010





