1. Introduction
The forestry sector in Northern Spain and Northern Portugal is based on species belonging to the genera
Pinus L.,
Quercus,
Fagus L., and
Eucalyptus L'Hér. The latter genus, with over 600 species, mostly from Australia, has undergone a spectacular development over the last 50 years [
1].
Eucalyptus globulus Labill. is the main hardwood species grown in pulpwood plantations in temperate regions of the World [
2,
3,
4,
5]. In Cantabria (Northern Spain), the bioclimatic and geographical characteristics are optimal for the development of this forest species.
Eucalyptus globulus was introduced into Northern Spain and Portugal in 1880 [
6]. Development and improvement of this species began in the 1940s with the establishment of the Sniace group. This company, an industrial chemicals group, owns more than 1500 ha and over 25,000 ha in consortia, all of them used for the cultivation of
E. globulus. Bosques 2000 S.L. belongs to this industrial group and deals with the task of research and forest management.
Eucalyptus globulus is clearly better than native species in terms of wood produced and pulp yield [
7,
8,
9]. There are alternative
Eucalyptus species for cooler regions such as
E. nitens H. Deane & Maiden, but its pulp performance is much lower [
10]. This species has proved itself to be highly adaptable to the climatic and soil conditions of the region, with average growth around 20 cubic meters per hectare per year. Regeneration is by coppice, with successful results found even in four rotations.
The genus
Eucalyptus is affected by a large number of fungal leaf diseases [
11], although
Mycosphaerella leaf disease (MLD), which includes species within
Mycospaherella Johanson and
Teratosphaeria Syd. & P. Syd. [
12], is seen as one of the most important [
13,
14,
15]. Infection of leaves appears when the acospores germinating on the leaf surface produce germ tubes which enter the leaf via stomata [
16]. MLD is the major biotic limitation for development of this species in this region.
Over the past fifteen years, this disease has caused extensive defoliation to
E. globulus plantations in Cantabria, resulting in a marked growth reduction, stem deformation and, in combination with frost, tree mortality. The disease affects over 41,000 ha in Cantabria and bordering regions, including Asturias and the Basque Country, accounting for an affected area in Northern Spain of approximately 200,000 ha. World-wide, this problem affects countries such as Australia, New Zealand, Chile, Spain, South Africa, Uruguay, and Portugal [
17,
18,
19,
20,
21].
In the scientific literature on the subject there are studies that identify the species of the genus
Mycosphaerella affecting
Eucalyptus [
22,
23,
24,
25,
26]. At this point, it must be said that in Northern Spain the species
Mycosphaerella nubilosa (Cooke) Hansford,
Mycosphaerella marksii Carnegie & Keane,
Mycosphaerella parva R.F. Park & Keane,
Mycosphaerella molleriana (Thüm.) Lindau,
Mycosphaerella madeira Crous & Denman,
Mycosphaerella readeriellophora Crous & J.P. Mansilla,
Mycosphaerella communis Crous & J.P. Mansilla,
Mycosphaerella lateralis Crous & M. J. Wingfield, and
Mycosphaerella aurantia A. Maxwell have all been identified, although 90% corresponds to
Mycosphaerella nubilosa [
27].
Mycosphaerella nubilosa infects preferentially juvenile foliage, which explains the fact that the vast majority of trees showing symptoms of the disease are young or else young shoots with leaves, while trees with adult foliage are not damaged to such an extent. This is observed in other studies that have found the infection of MLD in young leaves of
E. globulus [
16,
24,
28]. Thus, it is the young stage of the species which is more relevant and on which this study is carried out.
In the literature, there are studies that show how to evaluate the severity of the disease [
15,
24,
29,
30]. This issue is of vital importance when managing industrial
E. globulus stands since it could lead to failure.
Eucalyptus globulus is a widely used species because of its potential as an energy crop in short rotations [
31,
32]. The management of MLD is especially important in this case, due to the short rotation length in which the young stage of this species predominates.
This article analyzes experimental Eucalyptus globulus stands in areas where MLD has high prevalence rates
The main objective of this study was to evaluate the impact of MLD on growth. Apart from this, other interesting points have been observed. The influence of fertilization in severity to MLD could offer an important information to forest managers in order to minimize the impact of MLD. It was also essential to check the impact of MLD in different origins, families, and subspecies. The other two issues that have been considered in this article were the influence of the type of foliage and the effect of clonal propagation. All this previous work could help to identify if there are families and individuals who present disease tolerance.
2. Material and Methods
Four trials or series were performed in 2004, 2007 (2), and 2010 (
Table 1). The “seedlots” used in the trials have a range of provenances and subspecies within the natural range of
E. globulus, as well as open-pollinated individual families, clones, and controlled-pollinated families. The seed from the Australian source, provided by CSIRO Forestry, belongs to three subspecies:
E. globulus ssp.
globulus,
E. globulus ssp.
Bicostata Maiden, and
E. globulus ssp.
Pseudoglobulus Naudin ex Maiden. Other provenances, open-pollinated individual families, clones, and controlled-pollinated families are local selections made by the Forest Research Center Bosques 2000 SL from natural forest in Northern Spain. The trials were planted in a completely randomized block design, with one plant material per block and 25 replicates. The planting framework was 2.5 m × 2.5 m. A temperature control-release fertilizer (formula 11-22-9+6 MgO) was added to the soil at a ratio of 20–30 g per plant at planting.
Site characteristics of each trial, including soil type and nutritional status, are provided in
Table 2.
For all trials, the severity of MLD in the whole crown was assessed based on disease assessment diagrams from references [
29,
30], whereby a branch was chosen at random and assessed for severity of affected leaf area based on disease assessment diagrams. This branch was used to represent severity over the whole juvenile crown. The intensity of defoliation was also assessed, based on the proportion of juvenile crown defoliation. A Crown Damage Index (CDI) was then calculated (Equation (1)), which combined the amount of foliage defoliated with the amount of leaf area damaged on leaves (i.e., MLD severity).
Diameter at Breast Height over Bark (DBHOB) and height were measured at different ages, and height was measured at the adult-juvenile crown transition zone (
Table 3). Percentage of adult crown was calculated based on the ratio between total height of tree and height of adult crown. Volume over bark was calculated using the volume equations developed by reference [
33].
The percentage of adult leaf is estimated by the ratio between the height where adult leaf is found and the total height of the tree.
Each series tested different plant material, except for a number of control codes that were repeated. These codes were plant material whose tolerance to MLD was better than its counterparts in previous series and about 20 codes of control families (which serve as a reference for comparison between different series). These control codes fall into three groups: (1) control families without any genetic improvement; (2) control codes improved with form and force criteria from open pollination, and (3) control codes from controlled crosses. None of these control families was selected with the criterion of tolerance to MLD. As the trials ran, the number of controlled crosses and clones of the best families, increased (
Table 1).
2.1. Trial 1: Campos Forest (2004 Series)
The 2004 series is represented by the tests in Campos (210 m.a.s.l.) with an area of 2.5 ha. The plant material in this series was the genetic starting point. The starting plant material was considered sufficiently variable and representative for
Eucalyptus globulus (
Table 1). Only in this trial were subspecies of
Eucalyptus globulus (
globulus,
pseudoglobulus and
bicostata) incorporated. The objectives were:
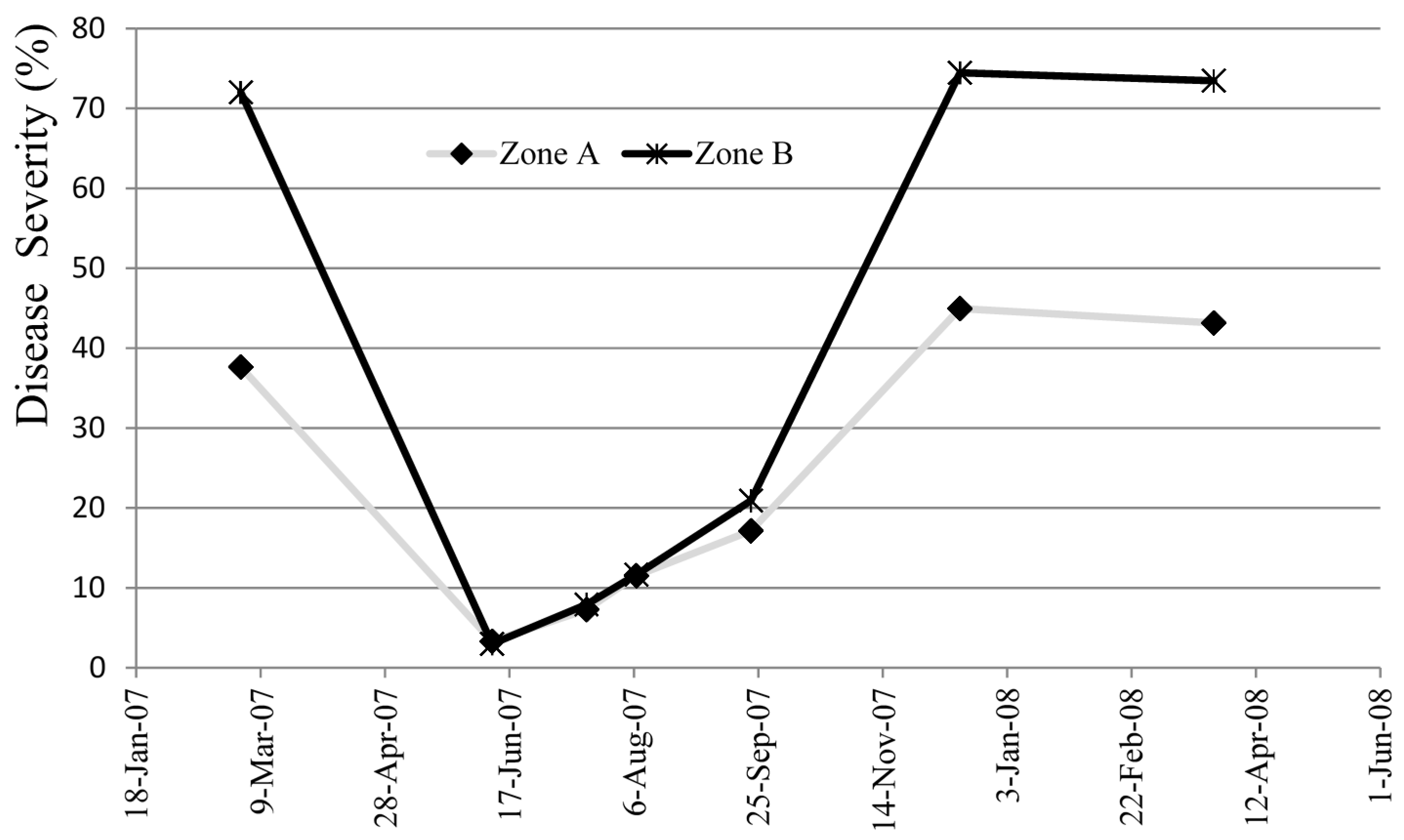
To analyze the evolution of MLD severity over a year in two zones (A and B) of approximately 1 ha, with different conditions in terms of topography, fog, sunshine, dew, and ventilation. Area A is located in the upper forest, with more natural ventilation, while Area B corresponds to a valley with more dew, fewer hours of sunshine, frequent accumulation of fog, and poor natural ventilation.
To observe if there are significant differences in the severity of the MLD between subspecies of E globulus.
To check the wood volume at 39 months, by category of disease severity for seedlings at 25 months of E. globulus.
2.2. Trial 2: Cabanzón Forest (2007 Series)
The 2007 series is represented by two tests: Cabanzón forest (220 m.a.s.l.) and Arenal forest (270 m.a.s.l.). This series incorporates new genetic material, material from the previous series and from the control families.
The objectives of the study performed in Cabanzón forest were four. First, the authors pretended to correlate the average MLD severity with the percentage of adult leaf. Another important point was to observe the change in average volume depending on severity categories. The relation of the Crown Damage Index (CDI) with other variables such as height, DBHOB and volume, was also matter of interest. Finally, the differences in volume between clones and seedlings will be analyzed.
2.3. Trial 3: Arenal Forest (2007 Series)
In Arenal forest the fundamental objective was to observe the effect of fertilization on MLD severity in families non-selected for their tolerance to MLD. In this trial, 12 months after planting, treatments with commercial fertilizers were used in order to favor the recovery of the plant after the attacks and to compare the evolution of MLD and growth to control (only fertilized at planting). Nine months after the treatment the severity of MLD was evaluated, in families from seed non-selected for their tolerance to MLD and 15 months after, height and percentage of mature foliage. The treatment followed these steps:
the nitrogen treatments were the soluble complex N-P-K (15-15-15) at a ratio of 100 g per plant.
the control-release fertilizer (11-22-9+6 MgO) at a ratio of 30 g per plant.
the treatment without any nitrogen (superphosphate 18% P2O5) at a ratio of 150 g per plant.
2.4. Trial 4: La Cavada Forest (2010 Series)
The 2010 series is represented by a test located in La Cavada forest (320 m). This series incorporates new material plant tolerant from the disease. The objective was to compare between selected and non-selected material by their tolerance to MLD. Clones of both groups were also considered in this analysis. Clones obtained by own massal selection came from a selection whose main criterion was resistance to MLD. This selection was based on previous reforestation work performed in the region, on trees between 8 and 24 months, also taking into account shape and strength of trees. These trees were mobilized by grafting and cuttings.
The plant material selected for this series includes:
MLD-tolerant families selected in previous series (Cabanzón forest (2007 series)).
Families non-selected by their tolerance to MLD.
Clones from one of these families (CI 105).
Clones selected by external genetic testing (selected by form and force, not by their tolerance to MLD).
Clones from own massal selection.
2.5. Statistical Analysis
In the statistical analysis, correlations between average severities of open-pollinated families were conducted in order to study the response to infection in the different trials. An analysis of variance (ANOVA) was also carried out in order to compare the severity averages [
34]. Post hoc analysis for comparison of averages was undertaken using post hoc Tukey HSD test (α = 0.05). The percentage data were treated using arc-sine square root transformation prior to analyses, in order to stabilize the variance error [
35]. In the forest Cabanzón, the volume vs. CDI and adult leaves percentage vs. severity, were adjusted with linear and nonlinear equations, respectively. All tests were performed using IBM SPSS 21.0 statistical software program (SPSS Inc, Chicago IL, USA).
4. Discussion
In all trials, there was a similarity between the families and provenances that tolerate the disease better. For the same material plant, a severity correlation of
r = 0.95 was found between two different places [
36]. Similar results were described in reference [
37], with correlation between 0.98 and 0.95. These high correlations were possible because the trials were close and started at the same time and with the same plant material. In this study, although the correlation between sites is lower, this is positive and significant. This correlation indicates that tolerance to the disease is linked to genetic factors, and that families are tolerant regardless of the place. This genetic variation on the tolerance to MLD was also observed by other authors [
15,
38]. This indicates that the same selection of resistant genotypes on one site can be used at other sites.
Some studies relate the virulence of the MLD with rainy summers with high relative moisture [
16,
22]. Genotypes originating in areas with prevailing warm temperatures and high humidity have greater MLD tolerance than those originating in drier and cooler places [
29,
30,
38]. A highly significant negative correlation between MLD damage and altitude was found by Jordan et al. [
39]. In studies performed in Australia [
30] showing MLD severity throughout the year it can be observed that the MLD impact is lower in winter than in summer. The results of this study show the opposite: it is specifically in summer that the severity is low. This may be due to the fact that because in Northern Spain during autumn and winter the combination of mild temperatures with frequent rainfall favors the development of MLD. The opposite is the case in summer, when higher temperatures and low rainfall reduce the development of MLD. The fact that there are great variations in terms of disease severity among stands in different microclimates (ventilation conditions, mist, duration of foggy periods, dew, etc.), is shown in
Figure 1 for one year. Area A of the trial is less affected, recovering the spring following the attack, with the leaf changing to adulthood. In area B, with less ventilation and high accumulation of mist, the attack is very strong. This area does not recover after the attack, with most of the trees affected dying during the second year.
No significant differences can be observed between both areas in severity from May to October. During autumn and winter the severity of the disease reached the maximum annual in area B. These results corroborate the fact that small variations in microclimate variables, such as exposure to prevailing winds, altitude, relief, etc. are sufficient to produce significant oscillations in MLD incidence between areas close to each other.
Reductions in
Eucalyptus nitens volume have been reported when more than 25% of the juvenile crown is defoliated [
29]; below this value the tree would have a similar response to that of trees not infected by MLD (response type 1).
Several studies describe differences between provenances of
E. globulus mainly associated with the subspecies [
11,
30,
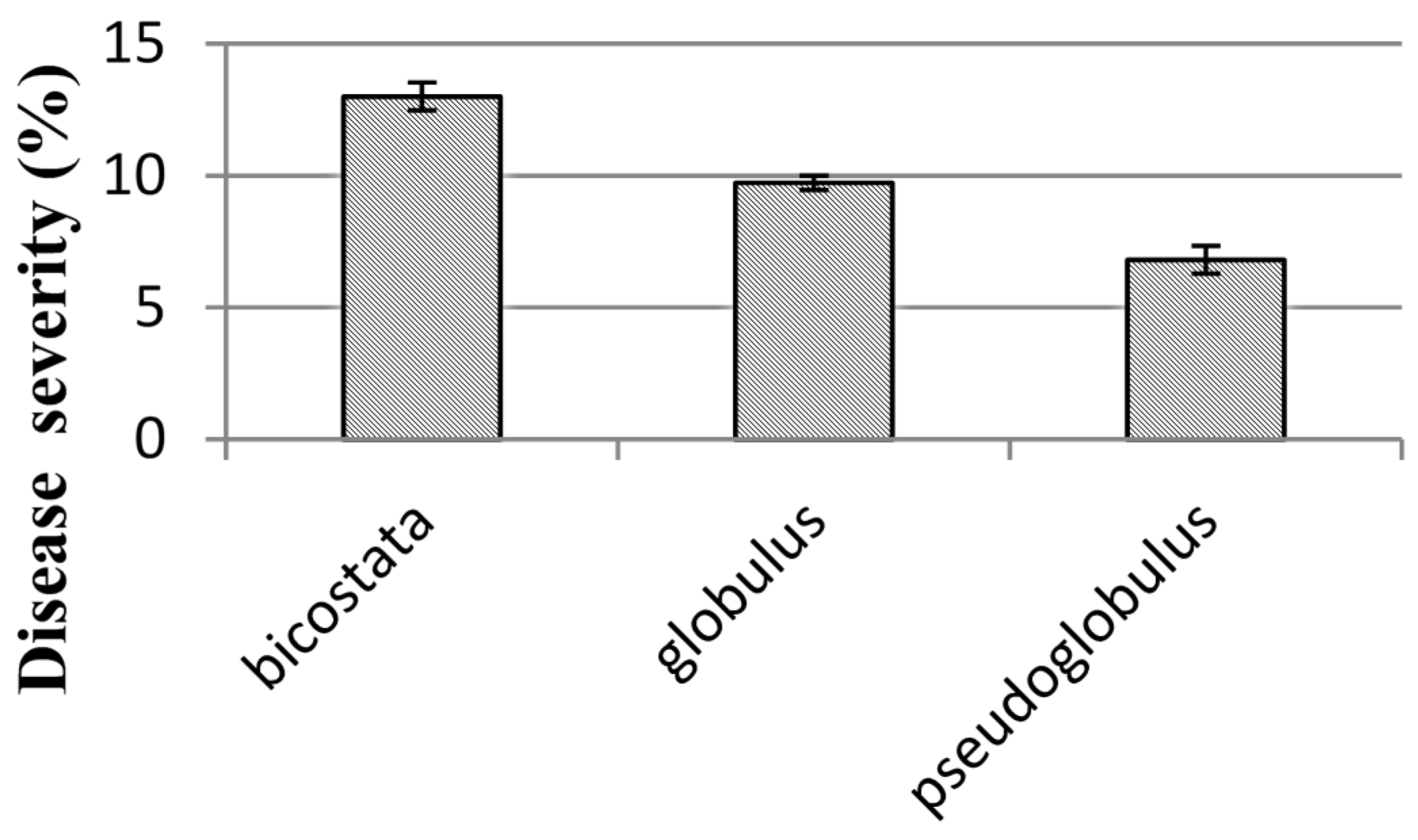
38]. In Campos forest the three provenances of
E. globulus ssp.
bicostata were the most affected (
Figure 2). The eight remaining origins of the subspecies
E. globulus spp
. globulus show intermediate severity with no significant differences between them.
E. globulus spp.
pseudo globulus showed the lowest severity. Our results are similar to those obtained by reference [
30] and different from reference [
38] where the subspecies
bicostata had the lowest disease severity in juvenile foliage. These differences may be explained by the fact that provenances with rainy summers may have more natural resistance to MLD.
MLD mainly affects juvenile foliage [
16]. There is genetic variation among individuals regarding height of transition from juvenile to adult leaves [
40,
41]. The heritability of the age at which leaf changes and its influence on MLD tolerance has been studied by Dungey et al. [
36]. In Cabanzón forest, the severity measured at 25 months of age was lower for those families with higher proportion of adult leaf (
Figure 3). This could be a way to select those families that show leaf change at younger ages, since they would be more tolerant of MLD. This hypothesis was presented by Freeman et al. [
41] and could be valid for areas with a high prevalence of MLD. However, it would be necessary to check whether the age of phase change affects the volume of the tree at a certain age. The observations made by the authors conclude that the families of trees that are first to change from young to adult stage generate lower volumes than their congeners, at the same age. In Cabanzón forest the disease incidence was very high. Similarly, in Campos forest, there were no significant differences in the volume measured at 27 months among families with severities below 6% at 25 months (
Table 4). Those families most susceptible to the disease (50% severity) show volume reductions. In Cabanzón forest, (September 2009) levels of defoliation are very high: 60.6%, which is much higher than previously described—34% [
15]. Volume losses of 64% in
E. nitens have been associated with defoliation levels between 63%–75% [
29]. This value is similar to that found in this study for
E. globulus (61.5%) for a CDI equal to 60.6%.This loss of growth is not recovered over the life of the tree [
42].
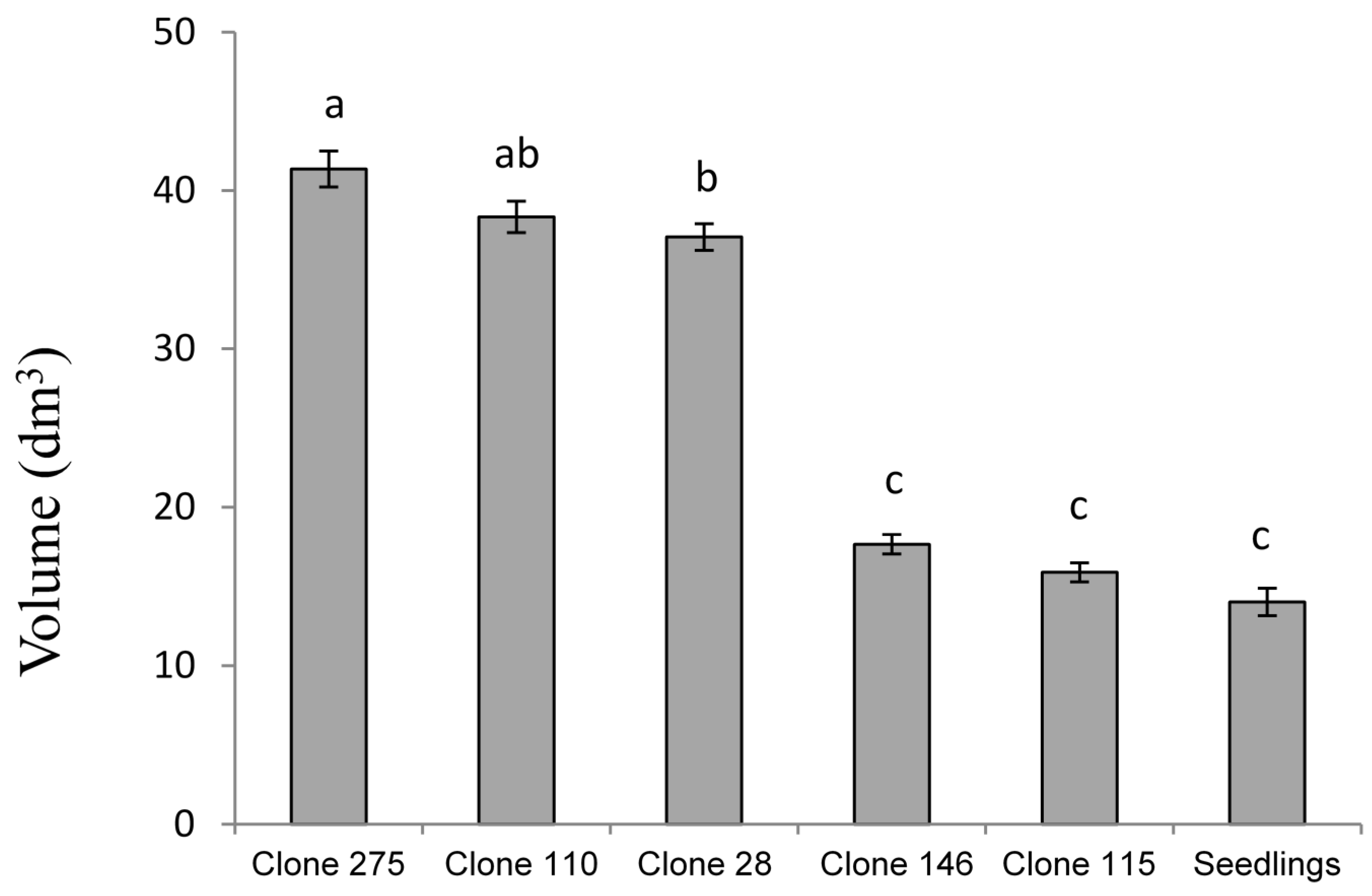
The clones from adult trees present a premature change from young to adult leaf (
Figure 5). This feature makes these trees less sensitive to MLD so that the average severity for adult clones is lower than for seedlings. This lower sensitivity to MLD results in a lower loss of growth and, so, the clonal stands seem to offer a possible solution. In our experiment, the clones with high genetic value for growth, planted in areas with high prevalence of MLD and with an age of leaf change similar to that of seedlings show a growth loss similar to these (
Figure 5: clone 146, clone 115). This shows that the cloning of a tree itself does not increase its tolerance to MLD and that the clones that do not have a premature leaf change do not present better growth in areas where there is high prevalence of MLD. As in other studies [
38], in the stands of this work the infection occurred mainly during the second year, so strategies to advance leaf exchange seemed to mitigate the effects of MLD.
The fertility level of soil is a key factor in recovery after attack [
42]. Nutritional requirements change with the age of the tree; for instance, in younger age groups the need for nitrogen is higher. According to reference [
43], a N/P ratio around 10 seems to be stable in the first three years of life. The concentration of N in leaves is four times higher than in the trunk. This requirement may increase as there is a loss of leaf mass caused by the disease, and may also explain the differences in volume found in most fertilized stands. With the aim of reducing the juvenile stage of the species and achieving recovery of the plant after the attack, fertilization programs were used (
Table 6). The change from young to adult leaf in
E. globulus is under strong genetic control [
41]. The environment influences the transition to adult foliage in
E. nitens [
44]. There is an environmental component that influences the transition from juvenile to adult leaf in
E. globulus [
45]. Nitrogen treatments help to reduce this transition while phosphorus has no influence. The application of phosphorus to
E. nitens promoted leaf change in two places [
44]. Levels of phosphorous were correlated with increased susceptibility of
E. globulus to infection by
Mycosphaerella cryptica in a 6-year-old fertilizer trial [
46]. There is no unanimity among the authors, indicating that more experiments should be performed to better understand the role played by fertilization in the change to adult foliage. In our tests, the use of phosphorous decreased the severity 8% with regard to control (
Table 6). The proportion of crown length in adult foliage is increased by applications of nitrogen [
47]. In Arenal forest, fertilization treatments with NPK showed fewer severities, higher growth, and higher percentage of mature leaf than the reference at 27 months of age. In most of the trials, as the percentage of mature leaf increased the recovery was more remarkable. At present, stands are beginning to be seen where the infection is advanced and starts during the first year of life, so this strategy based on reducing the juvenile stage with fertilization is less effective. This coincides with reference [
28], where it was noted that the MLD incidence was higher during the second year. The application of nitrogen fertilization in Arenal forest after heavy defoliation caused by MLD may have led to tree instability due to the size of the crown regenerated. This showed that nitrogen fertilization must be provided before defoliation starts. This fact was already observed in other places [
15,
47]. Fertilization benefits are not enough to dampen the damage caused by MLD on growth of young plantations of
E. globulus. Severities where less than 10% of leaf is affected have negative effects on growth of young plantations of
E. globulus [
48]. This study shows that severities lower than 6% do not significantly affect the volume at 39 months of age (
Table 4 and
Table 5). In Arenal forest, severity in the three scenarios is above 25%, so fertilization helps to reduce the damage caused by MLD, but is far from a definitive tool.
Resistence to
Mycosphaerella is highly heritable, meaning that the deployment of resistant genotypes is a management option in the long term [
15] for producing healthier plantations. The selections that do not consider tolerance to the disease confer only vigor but no greater tolerance to MLD (
Table 7: non mature cuttings, severity 32.9%). The greater tolerance of mature cuttings due to early change to adult leaf is sometimes not enough in places with high prevalence. In La Cavada forest the families selected for their tolerance to MLD have lower severities than adult cuttings with premature leaf change (
Table 7). These families obtained from seed selected by their tolerance presented severities of around 6.3% at 25 months, significantly lower than the non-tolerant plant material (seedlings and clones). The average severity of seedlings from these families was not significantly different from that of their clones. This again proves that cloning has no effect on resistance to MLD. Between these families, there is enough variability to find individuals that are exceptionally resistant to MLD (
Table 8, clone CI 105). These individuals present average severities of around 1.2%, i.e., they suffer practically no damage in areas of high prevalence of the disease. From our point of view, the way forward must be in the direction of the reproduction of individuals exceptionally tolerant to MLD. This reproduction can be through cloning or through controlled crossing between them. This is, like resistance to MLD, another extremely rare feature under strong genetic control.