Effects of Elevated Ozone on Stoichiometry and Nutrient Pools of Phoebe Bournei (Hemsl.) Yang and Phoebe Zhennan S. Lee et F. N. Wei Seedlings in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. O3 Fumigation Treatment
2.3. Measurements
2.4. Statistical Analysis
3. Results
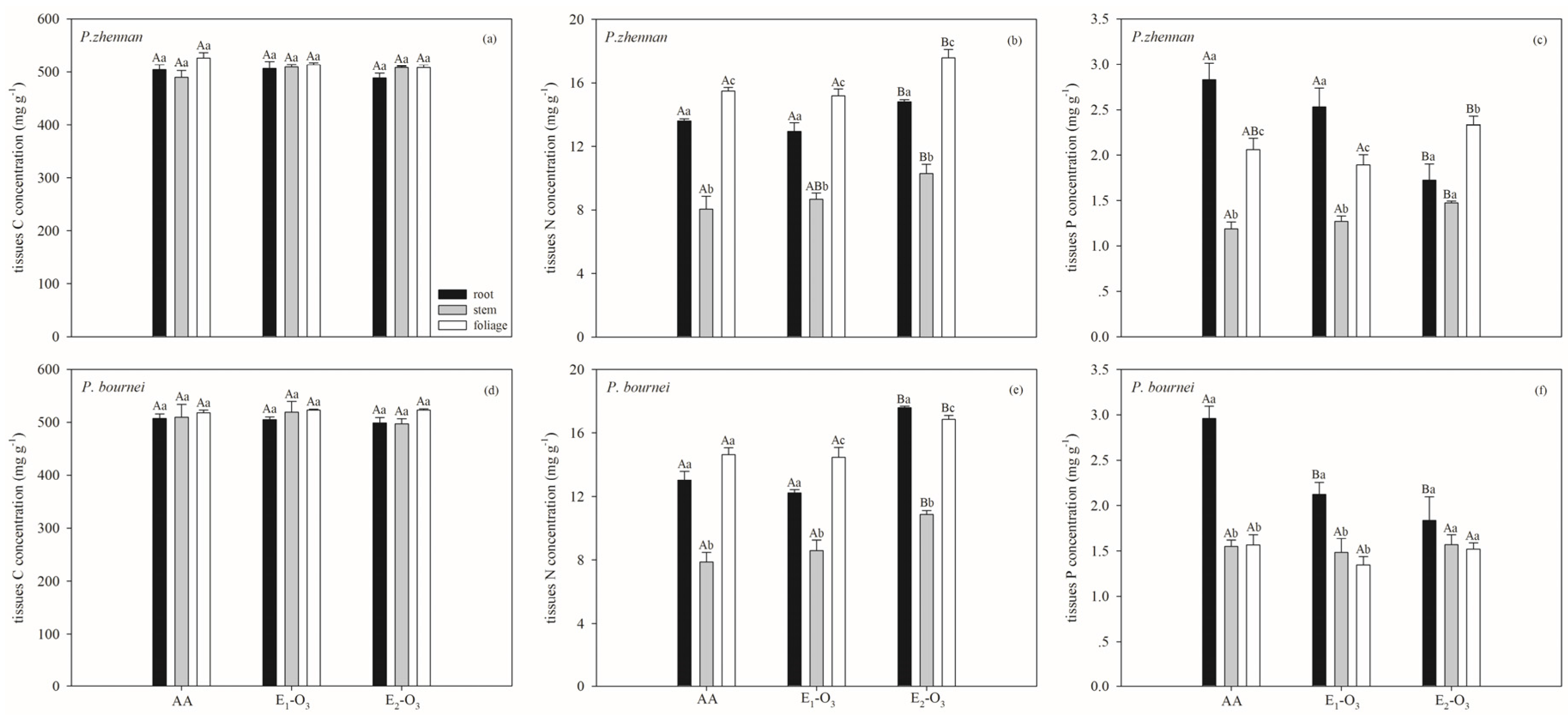
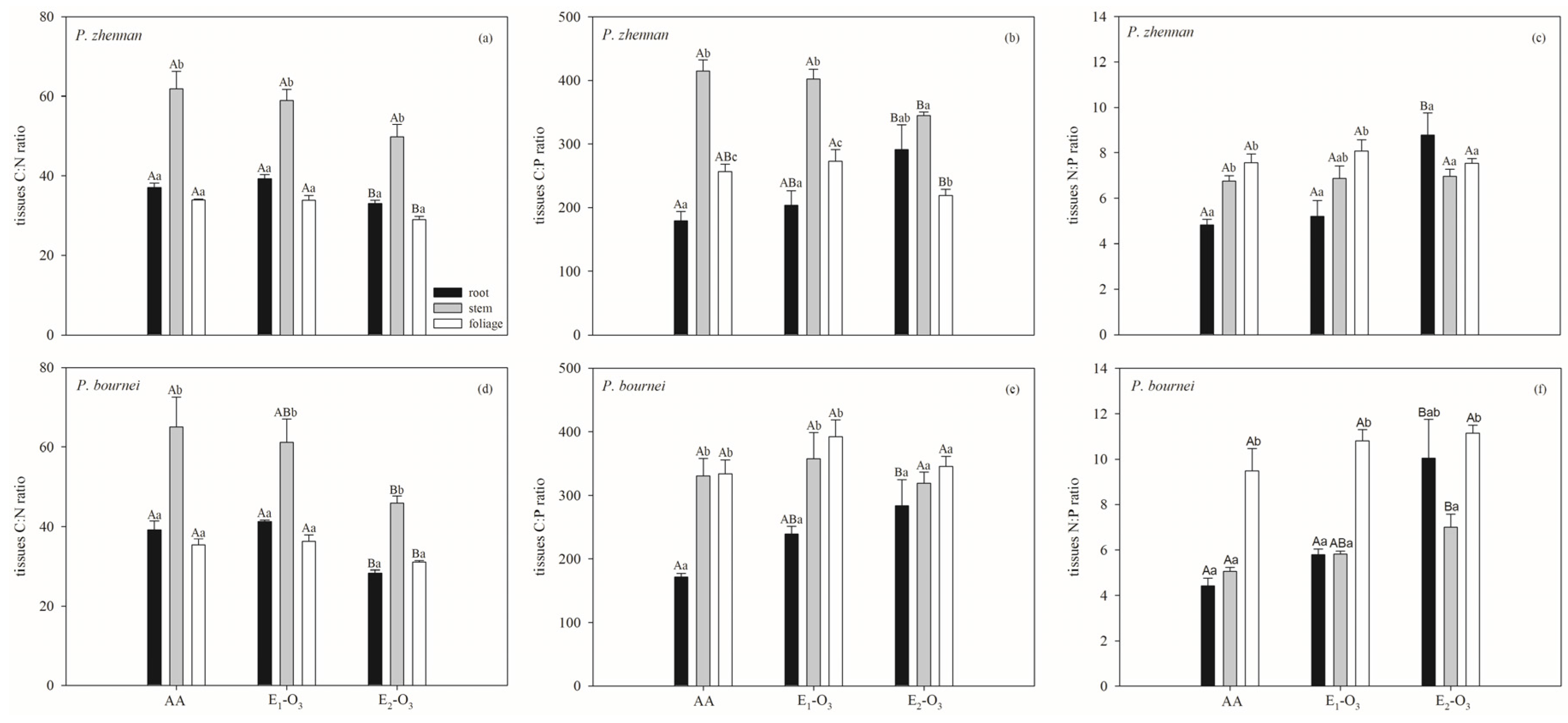
3.1. Effects of Elevated O3 on C, N, and P Concentrations and Stoichiometric Ratios
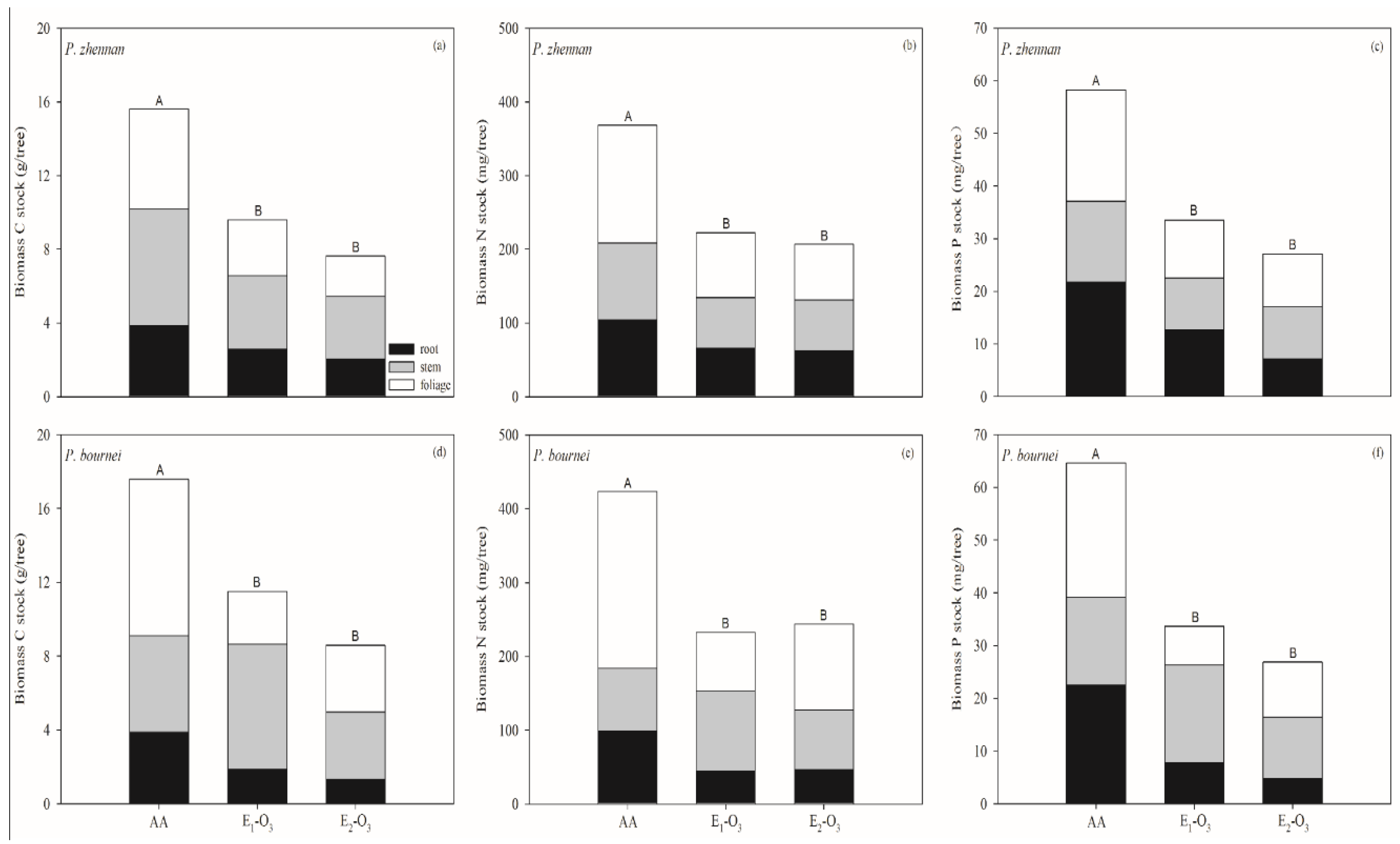
3.2. Response of C, N, and P Pools to Elevated O3
4. Discussion
5. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Feng, Z.; Pang, J.; Kobayashi, K.; Zhu, J.; Ort, D.R. Differential responses in two varieties of winter wheat to elevated ozone concentration under fully open-air field conditions. Glob. Chang. Biol. 2011, 17, 580–591. [Google Scholar] [CrossRef]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 38, 3431–3442. [Google Scholar] [CrossRef]
- Zhang, W. Effects of Elevated O3 Level on the Native Tree Species in Subtropical China. Ph.D. Thesis, Research center for eco-environmental sciences, Chinese Academy of Sciences, Beijing, China, 2011. [Google Scholar]
- Zhang, W.; Feng, Z.; Wang, X.; Niu, J. Elevated ozone negatively affects photosynthesis of current-year leaves but not previous-year leaves in evergreen Cyclobalanopsis glauca seedlings. Environ. Pollut. 2014, 184, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wittig, V.E.; Ainsworth, E.A.; Long, S.P. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ. 2007, 30, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Soba, M.; Dueck, T.A.; Puppi, G.; Kuiper, P.J. Interactions of elevated CO2, NH3 and O3 on mycorrhizal infection, gas exchange and N metabolism in saplings of Scots pine. Plant Soil. 1995, 176, 107–116. [Google Scholar] [CrossRef]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic damage under two scenarios of O3 pollution. Atmos. Environ. 2011, 45, 2297–2309. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Chen, T.; Zhang, D.; Sun, F.; Wei, Q.; Dong, X.; Sun, R.; Huan, N.; Pan, L. Ground-level ozone in urban Beijing over a 1-year period: Temporal variations and relationship to atmospheric oxidation. Atmos. Res. 2015, 164, 110–117. [Google Scholar] [CrossRef]
- Li, J.; Lu, K.; Lv, W.; Li, J.; Zhong, L.; Ou, Y.; Chen, D.; Huang, X.; Zhang, Y. Fast increasing of surface ozone concentrations in Pearl River Delta characterized by a regional air quality monitoring network during 2006–2011. J. Environ. Sci. 2014, 26, 23–36. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Wang, X.; Niu, J. Responses of native broadleaved woody species to elevated ozone in subtropical China. Environ. Pollut. 2012, 163, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New Phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Wright, L.M.; Lockaby, B.G.; Meldahl, R.; Thornton, F.; Chappelka, A.H. The influence of acid precipitation and ozone on nitrogen nutrition of young loblolly pine. Water Air Soil Pollut. 1990, 54, 135–142. [Google Scholar] [CrossRef]
- Weigt, R.B.; Häberle, K.H.; Rötzer, T.; Matyssek, R. Whole-tree seasonal nitrogen uptake and partitioning in adult Fagus sylvatica L. and Picea abies L. [Karst.] trees exposed to elevated ground-level ozone. Environ. Pollut. 2015, 196, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- González, A.L.; Fariña, J.M.; Pinto, R.; Pérez, C.; Weathers, K.C.; Armesto, J.J.; Marquet, P.A. Bromeliad growth and stoichiometry: Responses to atmospheric nutrient supply in fog-dependent ecosystems of the hyper-arid Atacama Desert, Chile. Oecologia 2011, 167, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, Y.; Lu, M.; Schädel, C.; Han, W. Terrestrial C: N stoichiometry in response to elevated CO2 and N addition: A synthesis of two meta-analyses. Plant Soil 2011, 343, 393–400. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, J.; Wang, J.M.; Wang, Y.; Lu, Y.X.; Zheng, Q.R. The response of photosynthetic characters and biomass allocation of P. bournei young trees to different light regimes. Acta Ecol. Sin. 2012, 32, 3841–3848. (In Chinese) [Google Scholar] [CrossRef]
- He, W.; Hu, T.; Wang, R.; Zhong, Y.; Zhou, X.; Jing, L. Effects of fertilization on photosynthetic physiology and growth characteristics of Phoebe zhannan seedlings. Acta Bot. Boreal.–Occident. Sin. 2014, 34, 1187–1197. (In Chinese) [Google Scholar]
- Dong, W.; Zhang, X.; Wang, H.; Dai, X.; Sun, X.; Qiu, W.; Yang, F. Effect of different fertilizer application on the soil fertility of paddy soils in red soil region of southern China. PLoS ONE 2012, 7, e44504. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S. The peculiarity of soil formation of the paddy soils derived from red earth in Kiangsi, China. Acta Pedol. Sin. 1964, 12, 155–163. (In Chinese) [Google Scholar]
- De Leeuw, F.A.A.M.; van Zantvoort, E.D.G. Mapping of exceedances of ozone critical levels for crops and forest trees in the Netherlands: Preliminary results. Environ. Pollut. 1997, 96, 89–98. [Google Scholar] [CrossRef]
- Liu, G.S. Soil Physical and Chemical Analysis and Description of Soil Profiles, 1st ed.; Standards Press of China: Beijing, China, 1996; pp. 166–167. (In Chinese) [Google Scholar]
- Güsewell, S.; Koerselman, W. Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect. Plant Ecol. 2002, 5, 37–61. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. Factors affecting nutrient concentration and stoichiometry of forest trees in Catalonia (NE Spain). For. Ecol. Manag. 2011, 262, 2024–2034. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Ni, L.; Zhang, X.; Zhu, G.; Xie, P. Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol. Eng. 2013, 61, 358–365. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Watanabe, M.; Iwasaki, M.; Tabe, C.; Matsumura, H.; Kohno, Y.; Izuta, T. Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads. Trees 2007, 21, 707–718. [Google Scholar] [CrossRef]
- Beyers, J.L.; Riechers, G.H.; Temple, P.J. Effects of long-term ozone exposure and drought on the photosynthetic capacity of ponderosa pine (Pinus ponderosa Laws.). New Phytol. 1992, 122, 81–90. [Google Scholar] [CrossRef]
- Niu, J. Effects of Elevated Ozone and Nitrogen Deposition on the Growth and Physiology of Cinnamomum Camphora Seedlings. Ph.D. Thesis, Research center for eco-environmental sciences, Chinese Academy of Sciences, Beijing, China, 2012. [Google Scholar]
- Zak, D.R.; Holmes, W.E.; Pregitzer, K.S.; King, J.S.; Ellsworth, D.S.; Kubiske, M.E. Belowground competition and the response of developing forest communities to atmospheric CO2 and O3. Glob. Chang. Biol. 2007, 13, 2230–2238. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.J. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein:RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.B.; Kim, N.Y.; Han, T.S.; Fang, J.Y.; Han, W.X. Effects of nitrogen and phosphorus fertilization on leaf carbon, nitrogen and phosphorus stoichiometry of Arabidopsis thaliana. Chin. J. Plant Ecol. 2013, 37, 551–557. (In Chinese) [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Cobb, E.D. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. Am. J. Bot. 2005, 92, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I.; Hyvönen, R.; Berglund, S.L.; Hobbie, S.E. Estimating the critical N:C from litter decomposition data and its relation to soil organic matter stoichiometry. Soil Biol. Biochem. 2013, 67, 312–318. [Google Scholar] [CrossRef]
- Paoletti, E. Impact of ozone on Mediterranean forests: A review. Environ. Pollut. 2006, 144, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.J.; Kozovits, A.R.; Reiter, I.M.; Heerdt, C.; Leuchner, M.; Lütz, C.; Liu, X.; Löw, M.; Winkler, J.B.; Grams, T.E.E.; et al. Comparison of ozone uptake and sensitivity between a phytotron study with young beech and a field experiment with adult beech (Fagus sylvatica). Environ. Pollut. 2005, 137, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.; Cape, J.N.; Coyle, M.; Flechard, C.; Kuylenstierna, J.; Hicks, K.; Derwent, D.; Johnson, C.; Stevenson, D. The gobal exposure of forests to air pollutants. Water Air Soil Pollut. 1999, 116, 5–32. [Google Scholar] [CrossRef]
- Ren, W.; Tian, H.; Tao, B.; Chapelka, A.; Sun, G.; Lu, C.; Liu, M.; Chen, G.; Xu, X. Impacts of tropospheric ozone and climate change on net primary productivity and net carbon exchange of China’s forest ecosystems. Glob. Ecol. Biogeogr. 2011, 20, 391–406. [Google Scholar] [CrossRef]
- Curtis, P.S.; Wang, X. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 1998, 113, 299–313. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; Pregitzer, K.S. Atmospheric CO2 and O3 alter the flow of 15N in developing forest ecosystems. Ecology 2007, 88, 2630–2639. [Google Scholar] [CrossRef] [PubMed]
| Treatments | 8 h Mean O3 Concentration (ppb) | AOT40 (ppm·h) |
|---|---|---|
| AA | 26.92 | 2.23 |
| E1–O3 | 99.66 | 54.44 |
| E2–O3 | 147.08 | 96.23 |
| Objects | Percentage of Total Sum of Squares (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| s | t | o | s × t | s × o | t × o | s × t × o | Error | |
| C | 1.99 | 15.89 * | 3.79 | 0.06 | 0.03 | 4.14 | 7.25 | 66.85 |
| N | 0.01 | 77.34 *** | 14.62 *** | 0.66 | 1.06 * | 1.00 | 1.14 | 4.17 |
| P | 1.90 * | 47.42 *** | 5.39 ** | 10.32 *** | 0.97 | 20.22 *** | 1.67 | 12.11 |
| C:N | 0.12 | 73.68 *** | 12.16 *** | 0.13 | 0.75 | 2.70 | 0.41 | 10.05 |
| C:P | 1.72 | 47.04 *** | 2.44 | 17.04 *** | 1.35 | 13.28 *** | 0.62 | 16.51 |
| N:P | 3.17 * | 32.67 *** | 18.09 *** | 11.88 *** | 2.51 | 14.95 *** | 0.03 | 16.70 |
| Tree Species | Tissues Biomass | C:N | C:P | N:P |
|---|---|---|---|---|
| P. zhennan | foliage biomass | 0.722 * | NS | NS |
| stem biomass | NS | 0.685 * | NS | |
| root biomass | NS | NS | NS | |
| total biomass | NS | NS | NS | |
| P. bournei | foliage biomass | NS | NS | NS |
| stem biomass | NS | NS | NS | |
| root biomass | NS | −770 * | −798 ** | |
| total biomass | NS | NS | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Shang, H.; Chen, Z.; Tian, Y.; Yu, H. Effects of Elevated Ozone on Stoichiometry and Nutrient Pools of Phoebe Bournei (Hemsl.) Yang and Phoebe Zhennan S. Lee et F. N. Wei Seedlings in Subtropical China. Forests 2016, 7, 78. https://doi.org/10.3390/f7040078
Cao J, Shang H, Chen Z, Tian Y, Yu H. Effects of Elevated Ozone on Stoichiometry and Nutrient Pools of Phoebe Bournei (Hemsl.) Yang and Phoebe Zhennan S. Lee et F. N. Wei Seedlings in Subtropical China. Forests. 2016; 7(4):78. https://doi.org/10.3390/f7040078
Chicago/Turabian StyleCao, Jixin, He Shang, Zhan Chen, Yun Tian, and Hao Yu. 2016. "Effects of Elevated Ozone on Stoichiometry and Nutrient Pools of Phoebe Bournei (Hemsl.) Yang and Phoebe Zhennan S. Lee et F. N. Wei Seedlings in Subtropical China" Forests 7, no. 4: 78. https://doi.org/10.3390/f7040078





