Effects of Temporal and Interspecific Variation of Specific Leaf Area on Leaf Area Index Estimation of Temperate Broadleaved Forests in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Leaf-Litter Collection and SLA Measurements
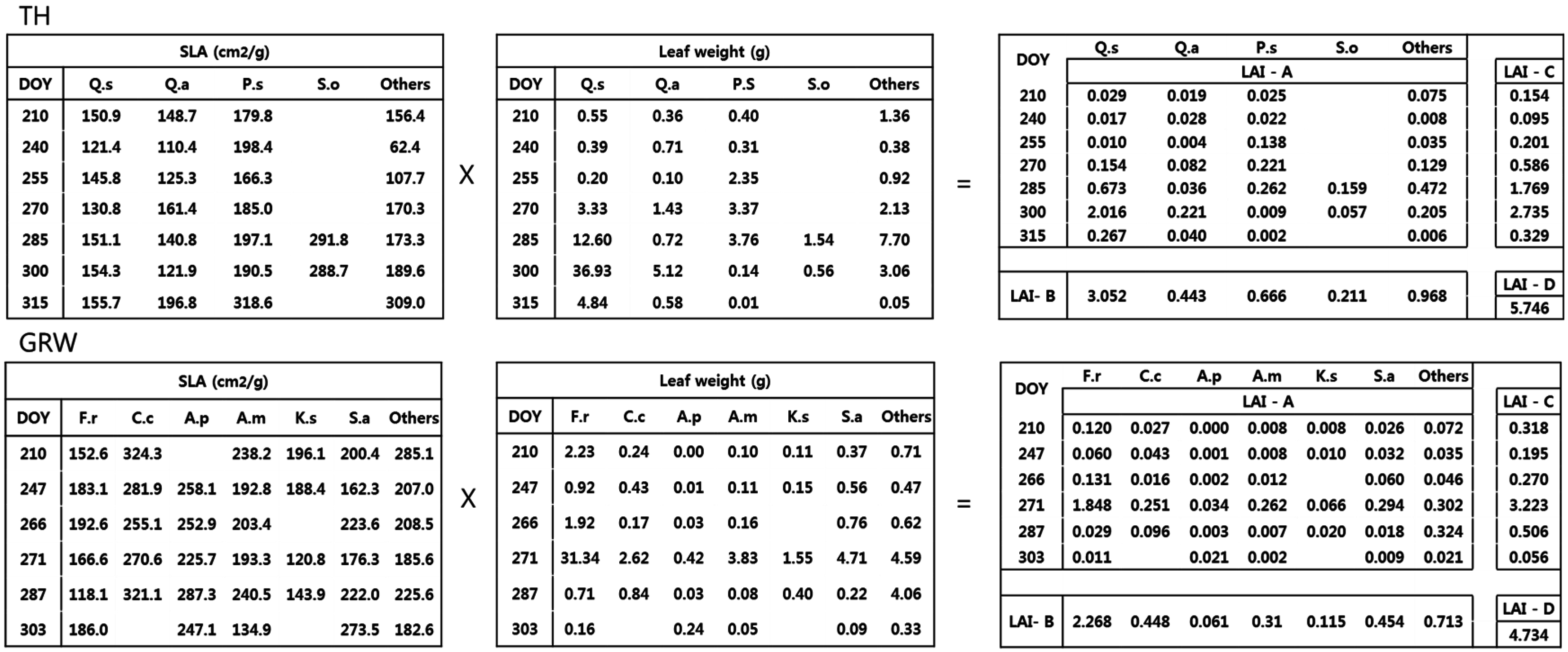
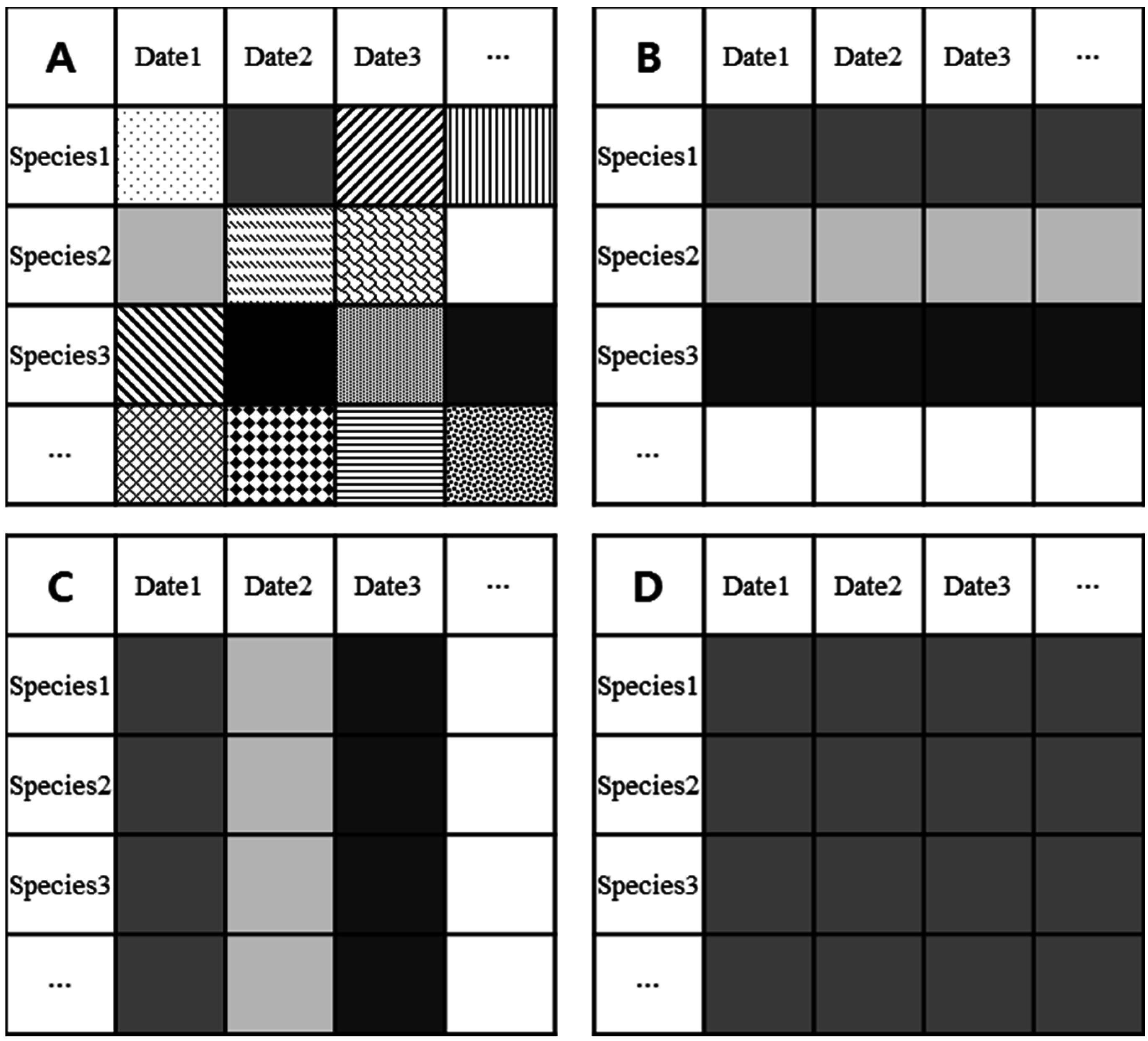
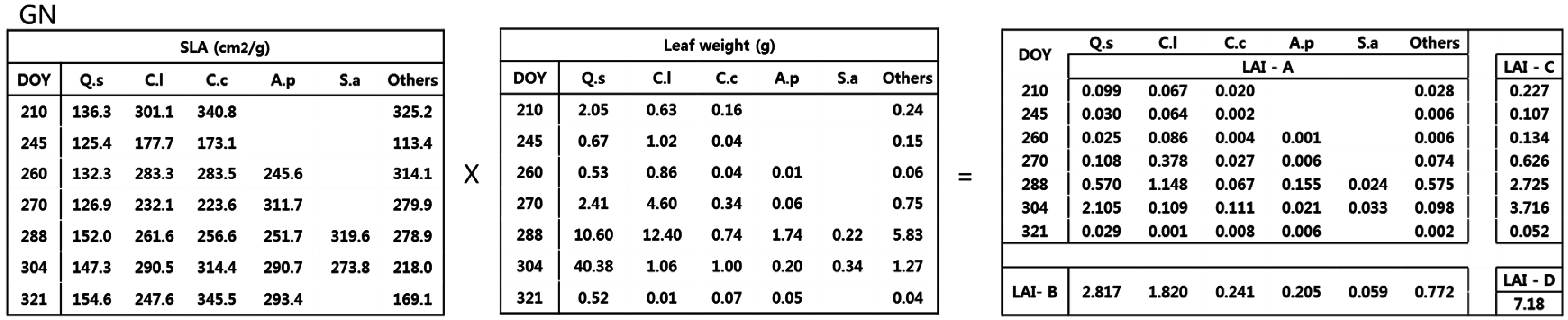
2.3. LAI Estimation
- Method A used the species specific SLA and dry weight from each collection date. This is considered as the true or reference LAI since it accounted for species and temporal variations.
- Method B used the average species specific SLA from all collection dates and the total dry weight of each species, ignoring seasonal variation.
- Method C used the average period specific SLA, ignoring interspecific variation, and the total dry weight of each period.
- Method D used the average SLA, ignoring temporal and interspecific variation, and the total dry weight of all species.
2.4. Statistical Analysis
3. Results
3.1. Temporal and Interspecific SLA Variation
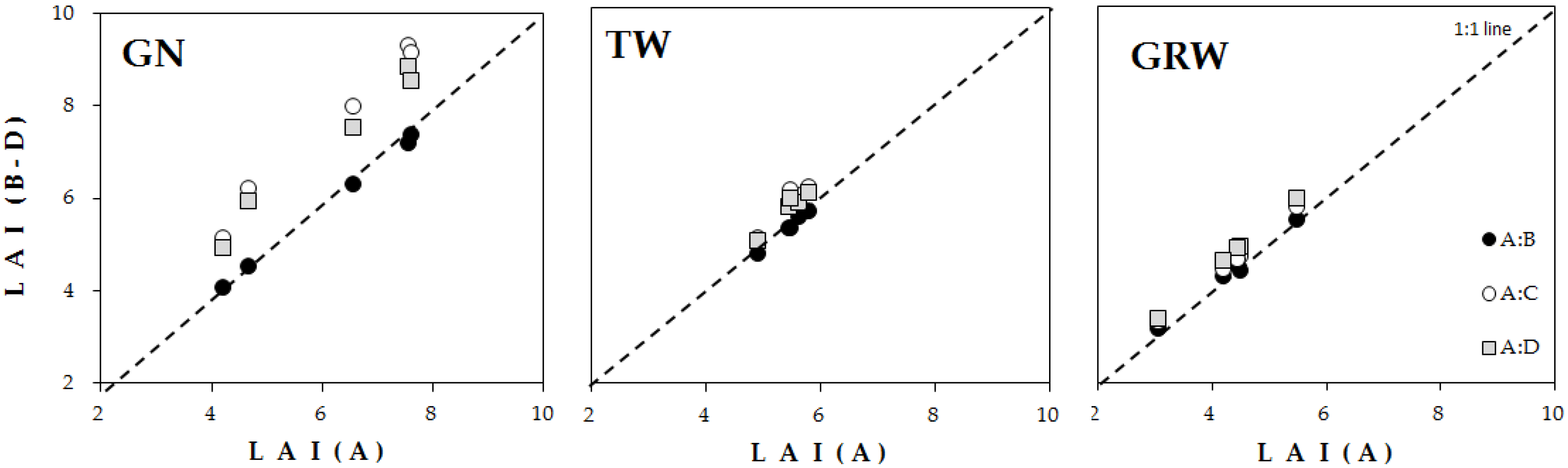
3.2. Estimation of LAI Based on Calculation Method
4. Discussion
4.1. Temporal and Interspecific Variation of SLA
4.2. LAI Estimation Based on Four Calculation Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix
References
- Leverenz, J.W.; Hinckley, T.M. Shoot Structure, Leaf Area Index and Productivity of Evergreen Conifer Stands. Tree Physiol. 1990, 6, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M. Optically-Based Methods for Measuring Seasonal Variation of Leaf Area Index in Boreal Conifer Stands. Agric. For. Meteorol. 1996, 80, 135–163. [Google Scholar] [CrossRef]
- Cutini, A.; Matteucci, G.; Mugnozza, G.S. Estimation of Leaf Area Index with the Li-Cor LAI 2000 in Deciduous Forests. For. Ecol. Manag. 1998, 105, 55–65. [Google Scholar] [CrossRef]
- Clark, D.B.; Olivas, P.C.; Oberbauer, S.F.; Clark, D.A.; Ryan, M.G. First Direct Landscape-scale Measurement of Tropical Rain Forest Leaf Area Index, a Key Driver of Global Primary Productivity. Ecol. Lett. 2008, 11, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, H.; Eklundh, L.; Hall, K.; Lindroth, A. Estimating LAI in Deciduous Forest Stands. Agric. For. Meteorol. 2005, 129, 27–37. [Google Scholar] [CrossRef]
- Osada, N.; Takeda, H.; Kawaguchi, H.; Furukawa, A.; Awang, M. Estimation of Crown Characters and Leaf Biomass from Leaf Litter in a Malaysian Canopy Species, Elateriospermum Tapos (Euphorbiaceae). For. Ecol. Manag. 2003, 177, 379–386. [Google Scholar] [CrossRef]
- Le Dantec, V.; Dufrêne, E.; Saugier, B. Interannual and Spatial Variation in Maximum Leaf Area Index of Temperate Deciduous Stands. For. Ecol. Manag. 2000, 134, 71–81. [Google Scholar] [CrossRef]
- Chen, J.; Black, T. Measuring Leaf Area Index of Plant Canopies with Branch Architecture. Agric. For. Meteorol. 1991, 57, 1–12. [Google Scholar] [CrossRef]
- Bouriaud, O.; Soudani, K.; Bréda, N. Leaf Area Index from Litter Collection: Impact of Specific Leaf Area Variability within a Beech Stand. Can. J. Remote Sens. 2003, 29, 371–380. [Google Scholar] [CrossRef]
- Chason, J.W.; Baldocchi, D.D.; Huston, M.A. A Comparison of Direct and Indirect Methods for Estimating Forest Canopy Leaf Area. Agric. For. Meteorol. 1991, 57, 107–128. [Google Scholar] [CrossRef]
- Breda, N.J. Ground-Based Measurements of Leaf Area Index: A Review of Methods, Instruments and Current Controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of Methods for in Situ Leaf Area Index Determination: Part I. Theories, Sensors and Hemispherical Photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Gower, S.T.; Kucharik, C.J.; Norman, J.M. Direct and Indirect Estimation of Leaf Area Index, F APAR, and Net Primary Production of Terrestrial Ecosystems. Remote Sens. Environ. 1999, 70, 29–51. [Google Scholar] [CrossRef]
- Weiss, M.; Baret, F.; Smith, G.; Jonckheere, I.; Coppin, P. Review of Methods for in Situ Leaf Area Index (LAI) Determination: Part II. Estimation of LAI, Errors and Sampling. Agric. For. Meteorol. 2004, 121, 37–53. [Google Scholar] [CrossRef]
- Watanabe, T.; Yokozawa, M.; Emori, S.; Takata, K.; Sumida, A.; Hara, T. Developing a Multilayered Integrated Numerical Model of Surface Physics-Growing Plants Interaction (MINoSGI). Glob. Chang. Biol. 2004, 10, 963–982. [Google Scholar] [CrossRef]
- Walcroft, A.; Brown, K.; Schuster, W.; Tissue, D.; Turnbull, M.; Griffin, K.; Whitehead, D. Radiative Transfer and Carbon Assimilation in Relation to Canopy Architecture, Foliage Area Distribution and Clumping in a Mature Temperate Rainforest Canopy in New Zealand. Agric. For. Meteorol. 2005, 135, 326–339. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Yan, E. Leaf Nutrient Concentration, Nutrient Resorption and Litter Decomposition in an Evergreen Broad-Leaved Forest in Eastern China. For. Ecol. Manag. 2007, 239, 150–158. [Google Scholar]
- Bondeau, A.; Kicklighter, D.W.; Kaduk, J.; Intercomparison, T. Participants of the Potsdam NpP. Model Intercomparison. Comparing Global Models of Terrestrial Net Primary Productivity (NPP): Importance of Vegetation Structure on Seasonal NPP Estimates. Glob. Chang. Biol. 1999, 5, 35–45. [Google Scholar] [CrossRef]
- Song, Y.; Ryu, Y. Seasonal Changes in Vertical Canopy Structure in a Temperate Broadleaved Forest in Korea. Ecol. Res. 2015, 30, 821–831. [Google Scholar] [CrossRef]
- Dufrêne, E.; Bréda, N. Estimation of Deciduous Forest Leaf Area Index using Direct and Indirect Methods. Oecologia 1995, 104, 156–162. [Google Scholar] [CrossRef]
- McCarthy, H.R.; Oren, R.; Finzi, A.C.; Ellsworth, D.S.; Kim, H.; Johnsen, K.H.; Millar, B. Temporal Dynamics and Spatial Variability in the Enhancement of Canopy Leaf Area Under Elevated Atmospheric CO2. Glob. Chang. Biol. 2007, 13, 2479–2497. [Google Scholar] [CrossRef]
- Garcia, R.L.; Norman, J.M.; McDermitt, D.K. Measurements of Canopy Gas Exchange using an Open Chamber System. Remote Sens. Rev. 1990, 5, 141–162. [Google Scholar] [CrossRef]
- Ellsworth, D.; Reich, P. Canopy Structure and Vertical Patterns of Photosynthesis and Related Leaf Traits in a Deciduous Forest. Oecologia 1993, 96, 169–178. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and Consequences of Variation in Leaf Mass per Area (LMA): A Meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jin, G.; Chen, J.M.; Qi, Y. Evaluating Optical Measurements of Leaf Area Index Against Litter Collection in a Mixed Broadleaved-Korean Pine Forest in China. Trees 2015, 29, 59–73. [Google Scholar] [CrossRef]
- Clough, B.; Tan, D.T.; Buu, D.C. Canopy Leaf Area Index and Litter Fall in Stands of the Mangrove Rhizophora Apiculata of Different Age in the Mekong Delta, Vietnam. Aquat. Bot. 2000, 66, 311–320. [Google Scholar] [CrossRef]
- Braun, E.L. Deciduous Forests of Eastern North America; Blakiston Co.; Blakiston Co.: Philadelphia, PA, USA, 1950. [Google Scholar]
- Kim, H.; Lee, M.; Kim, S.; Guenther, A.B.; Park, J.P.; Cho, G.; Kim, H. Measurements of Isoprene and Monoterpenes at Mt. Taewha and Estimation of their Emissions. Korean J. Agric. For. Meteorol. 2015, 17, 217–226. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, G.; Jeon, S.; Song, Y.; Kim, H. Monitoring Multi-Layer Canopy Spring Phenology of Temperate Deciduous and Evergreen Forests using Low-Cost Spectral Sensors. Remote Sens. Environ. 2014, 149, 227–238. [Google Scholar] [CrossRef]
- Kang, M.; Kwon, H.; Lim, J.; Kim, J. Understory Evapotranspiration Measured by Eddy-Covariance in Gwangneung Deciduous and Coniferous Forests. Korean J. Agric. For. Meteorol. 2009, 11, 233–246. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll Fluorescence Imaging of Photosynthetic Activity in Sun and Shade Leaves of Trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Anten, N.P.; Hirose, T. Interspecific Differences in Above-ground Growth Patterns Result in Spatial and Temporal Partitioning of Light among Species in a Tall-grass Meadow. J. Ecol. 1999, 87, 583–597. [Google Scholar] [CrossRef]
- Niinemets, U. Photosynthesis and Resource Distribution through Plant Canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, A.; Clerkx, A. Shading and Root–Shoot Relations in Saplings of Silver Birch, Pedunculate Oak and Beech. For. Ecol. Manag. 2003, 176, 439–448. [Google Scholar] [CrossRef]
- Dewar, R.C.; Tarvainen, L.; Parker, K.; Wallin, G.; McMurtrie, R.E. Why does Leaf Nitrogen Decline within Tree Canopies Less Rapidly than Light? An Explanation from Optimization Subject to a Lower Bound on Leaf Mass Per Area. Tree Physiol. 2012, 32, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Peltoniemi, M.S.; Duursma, R.A.; Medlyn, B.E. Co-Optimal Distribution of Leaf Nitrogen and Hydraulic Conductance in Plant Canopies. Tree Physiol. 2012, 32, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Santa Regina, I.; Leonardi, S.; Rapp, M. Foliar Nutrient Dynamics and Nutrient-use Efficiency in Castanea Sativa Coppice Stands of Southern Europe. Forestry 2001, 74, 1–10. [Google Scholar] [CrossRef]
- Koike, T. Autumn Coloring, Photosynthetic Performance and Leaf Development of Deciduous Broad-Leaved Trees in Relation to Forest Succession. Tree Physiol. 1990, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, K. Leaf Survival of Woody Plants in Deciduous Broad-Leaved Forests. I. Tall Trees. Can. J. Bot. 1983, 61, 2133–2139. [Google Scholar]
- Koike, T. Leaf Structure and Photosynthetic Performance as Related to the Forest Succession of Deciduous Broad-Leaved Trees. Plant Species Biol. 1988, 3, 77–87. [Google Scholar] [CrossRef]
- Kitaoka, S.; Koike, T. Seasonal and Yearly Variations in Light use and Nitrogen use by Seedlings of Four Deciduous Broad-Leaved Tree Species Invading Larch Plantations. Tree Physiol. 2005, 25, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Couteaux, M.; Bottner, P.; Berg, B. Litter Decomposition, Climate and Liter Quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- Kalacska, M.; Calvo-Alvarado, J.C.; Sanchez-Azofeifa, G.A. Calibration and Assessment of Seasonal Changes in Leaf Area Index of a Tropical Dry Forest in Different Stages of Succession. Tree Physiol. 2005, 25, 733–744. [Google Scholar] [CrossRef] [PubMed]
| Site * | GN * | TH † | GRW ‡ |
|---|---|---|---|
| Elevation (m) | 256 | 242 | 1199 |
| Slope steepness (%) | 18 | 8 | 33 |
| Aspect (°) | 110 | 10 | 355 |
| Species (density (number of stems ha−1), relative basal area (%)) | Quercus mongolica (128, 75.0), Carpinus laxiflora (96, 15.5), Others (752, 9.5) | Q. mongolica (550, 72.2), Q. aliena (75, 10.9), Others (625, 17.0) | Fraxinus rhynchophylla (224, 44.2), Kalopanax septemlobus (48, 22.9), Sorbus alnifolia (48, 13.7) Others (672, 19.2) |
| Site | Source | Type III Sum of Squares | Df | Mean Square | F | p-Value |
|---|---|---|---|---|---|---|
| GN * | Corrected Model | 4,208,254.826 * | 34 | 123,772.201 | 30.237 | 0.000 |
| Intercept | 13,156,498.857 | 1 | 13,156,498.857 | 3214.127 | 0.000 | |
| Species | 1,654,488.762 | 5 | 330,897.752 | 80.838 | 0.000 | |
| Collection Dates | 240,640.423 | 6 | 40,106.737 | 9.798 | 0.000 | |
| Species × Collection Dates | 556,636.615 | 23 | 24,201.592 | 5.912 | 0.000 | |
| Error | 3,610,321.280 | 882 | 4093.335 | |||
| Total | 53,337,330.131 | 917 | ||||
| Corrected Total | 7,818,576.106 | 916 | ||||
| TH † | Corrected Model | 992,829.674 † | 30 | 33,094.322 | 6.976 | 0.000 |
| Intercept | 4,842,026.866 | 1 | 4,842,026.866 | 1020.605 | 0.000 | |
| Species | 222,195.659 | 4 | 55,548.915 | 11.709 | 0.000 | |
| Collection Dates | 132,159.957 | 6 | 22,026.659 | 4.643 | 0.000 | |
| Species × Collection Dates | 164,700.465 | 20 | 8235.023 | 1.736 | 0.024 | |
| Error | 3,420,620.353 | 721 | 4744.272 | |||
| Total | 25,972,407.402 | 752 | ||||
| Corrected Total | 4,413,450.026 | 751 | ||||
| GRW ‡ | Corrected Model | 2,267,049.395 ‡ | 37 | 61,271.605 | 15.249 | 0.000 |
| Intercept | 7,261,444.895 | 1 | 7,261,444.895 | 1807.224 | 0.000 | |
| Species | 780,273.620 | 6 | 130,045.603 | 32.366 | 0.000 | |
| Collection Dates | 117,973.814 | 5 | 23,594.763 | 5.872 | 0.000 | |
| Species × Collection Dates | 476,833.782 | 26 | 18,339.761 | 4.564 | 0.000 | |
| Error | 3,439,416.999 | 856 | 4018.011 | |||
| Total | 42,054,738.045 | 894 | ||||
| Corrected Total | 5,706,466.394 | 893 |
| Site | Species | SLA (cm2·g−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| September | September | October | October | November | November | December | Avg. | ||
| -Early | -Mid | -Early | -Mid | -Early | -Mid | -Early | |||
| GN | Quercus serrata | 136.3 ± 4.3Aabc | 125.4 ± 7.3Aa | 132.3 ± 9.8Aab | 126.9 ± 3.8Aa | 152.0 ± 4.6Ac | 147.3 ± 5.6Abc | 154.6 ± 7.0Ac | 139.3 ± 2.1A |
| (n = 72) | (n = 23) | (n = 17) | (n = 55) | (n = 49) | (n = 51) | (n = 21) | (n = 288) | ||
| Carpinus laxflora | 301.1 ± 14.5Bc | 177.7 ± 6.8Aa | 283.3 ± 14.0Bbc | 232.1 ± 11.1BCb | 261.6 ± 7.4Bbc | 290.5 ± 8.7CDbc | 247.6 ± 23.6Bbc | 249.9 ± 4.8B | |
| (n = 38) | (n = 70) | (n = 50) | (n = 58) | (n = 54) | (n = 45) | (n = 3) | (n = 318) | ||
| Carpinus cordata | 340.8 ± 18.8Bd | 173.1 ± 72.3Aa | 283.5 ± 47.4Bbcd | 223.6 ± 12.9Bab | 256.6 ± 10.7Bbc | 314.4 ± 11.6Dcd | 345.5 ± 10.1Cd | 286.2 ± 8.4D | |
| (n = 10) | (n = 2) | (n = 4) | (n = 14) | (n = 13) | (n = 19) | (n = 7) | (n = 69) | ||
| Acer pesudosieboldianum | - | - | 245.6 ± 11.2Ba | 311.7 ± 35.7Da | 251.7 ± 17.0Ba | 290.7 ± 13.3CDa | 293.4 ± 36.9BCa | 279.9 ± 9.7CD | |
| (n = 2) | (n = 5) | (n = 12) | (n = 18) | (n = 4) | (n = 41) | ||||
| Sorbus alnifola | - | - | - | - | 319.6 ± 8.6C* | 273.8 ± 21.7C * | - | 297.7 ± 12.1D | |
| (n = 12) | (n = 11) | (n = 23) | |||||||
| Others † | 325.2 ± 41.1Bd | 113.4 ± 28.1Aa | 314.1 ± 36.2Bcd | 279.9 ± 11.4CDcd | 278.9 ± 10.3Bcd | 218.0 ± 13.2Bbc | 169.1 ± 27.1Aab | 262.1 ± 6.9BC | |
| (n = 3) | (n = 7) | (n = 7) | (n = 56) | (n = 70) | (n = 32) | (n = 3) | (n = 178) | ||
| Total | 208.5 ± 9.5b | 161.4 ± 5.9a | 253.0 ± 12.0c | 217.0 ± 6.8b | 244.2 ± 5.6c | 237.4 ± 6.5c | 212.9 ± 14.1b | 222.8 ± 3.1 | |
| (n = 123) | (n = 102) | (n = 80) | (n = 188) | (n = 210) | (n = 176) | (n = 38) | (n = 917) | ||
| TH | Quercus serrata | 150.9 ± 5.5Aa | 121.4 ± 10.3Aa | 145.8 ± 10.7Aa | 130.8 ± 6.5Aa | 151.1 ± 6.9Aa | 154.3 ± 19.3Aa | 155.7 ± 6.0Aa | 146.6 ± 4.3A |
| (n = 22) | (n = 13) | (n = 12) | (n = 45) | (n = 39) | (n = 41) | (n = 52) | (n = 224) | ||
| Quercus aliena | 148.7 ± 14.1Aab | 110.4 ± 8.6Aa | 125.3 ± 10.9Aa | 161.4 ± 28.8ABab | 140.8 ± 9.0Aab | 121.9 ± 7.1Aa | 196.8 ± 17.1Ab | 138.8 ± 6.6A | |
| (n = 8) | (n = 16) | (n = 2) | (n = 16) | (n = 9) | (n = 33) | (n = 10) | (n = 94) | ||
| Prunus sargentii | 179.8 ± 12.2Aa | 198.4 ± 11.2Ba | 166.3 ± 6.3Aa | 185.0 ± 9.0Ba | 197.1 ± 9.8Ba | 190.5 ± 26.6Aa | 318.6 ** | 182.0 ± 4.1B | |
| (n = 27) | (n = 22) | (n = 78) | (n = 43) | (n = 41) | (n = 5) | (n = 1) | (n = 217) | ||
| Styrax obassia | - | - | 162.0 ± 37.5Aa | - | 291.8 ± 11.1Cb | 288.7 ± 15.5Bb | - | 284.0 ± 10.2C | |
| (n = 2) | (n = 27) | (n = 7) | (n = 36) | ||||||
| Others †† | 156.4 ± 17.6Aa | 62.4 ** | 107.7 ± 4.7Aa | 170.3 ± 13.7Ba | 173.3 ± 11.4ABa | 189.6 ± 12.9Aa | 309.0 ± 33.3Bb | 175.2 ± 6.7B | |
| (n = 10) | (n = 1) | (n = 7) | (n = 39) | (n = 65) | (n = 57) | (n = 2) | (n = 181) | ||
| Total | 163.1 ± 6.2a | 149.4 ± 8.4a | 158.9 ± 5.3a | 161.3 ± 6.2a | 190.0 ± 6.2b | 168.7 ± 8.4bc | 169.2 ± 7.0bc | 169.3 ± 2.8 | |
| (n = 67) | (n = 52) | (n = 101) | (n = 143) | (n = 181) | (n = 143) | (n = 65) | (n = 752) | ||
| GRW | Fraxinus rhynchophylla | 152.6 ± 12.5Aab | 183.1 ± 14.3Aab | 192.6 ± 8.0Ab | 166.6 ± 4.0Bab | 118.1 ± 6.1Aa | 186.0 ± 49.1ABb | - | 171.9 ± 3.4B |
| (n = 24) | (n = 13) | (n = 59) | (n = 157) | (n = 2) | (n = 3) | (n = 258) | |||
| Kalopanax septemlobus | 196.1 ± 31.4ABa | 188.4 ± 15.1Abc | - | 120.8 ± 9.5Aa | 143.9 ± 10.9Aab | - | 146.9 ± 8.9A | ||
| (n = 3) | (n = 5) | (n = 13) | (n = 5) | (n = 26) | |||||
| Sorbus alnifola | 200.4 ± 16.7ABa | 162.3 ± 14.4Aa | 223.6 ± 18.8Aab | 176.3 ± 8.6Ba | 222.0 ± 12.1Bab | 273.5 ± 46.4Bb | - | 191.2 ± 6.4BC | |
| (n = 12) | (n = 15) | (n = 15) | (n = 44) | (n = 8) | (n = 3) | (n = 97) | |||
| Carpinus cordata | 324.3 ± 22.7Cb | 281.9 ± 15.9Bab | 255.1 ± 20.2Aa | 270.6 ± 9.3Da | 321.1 ± 10.3Ca | - | - | 294.1 ± 6.5E | |
| (n = 20) | (n = 21) | (n = 9) | (n = 46) | (n = 40) | (n = 136) | ||||
| Acer pesudosieboldianum | - | 258.1 ± 43.6Ba | 252.9 ± 60.0Aa | 225.7 ± 5.8Ca | 287.3 ± 17.6BCa | 247.1 ** | - | 237.2 ± 6.5D | |
| (n = 2) | (n = 2) | (n = 29) | (n = 5) | (n = 1) | (n = 39) | ||||
| Acer mono | 238.2 ± 16.5ABCa | 192.8 ± 18.5Aa | 203.4 ± 12.3Aa | 193.3 ± 8.5Ba | 240.5 ± 16.6Ba | 134.9 ** | - | 202.3 ± 6.1C | |
| (n = 8) | (n = 7) | (n = 11) | (n = 44) | (n = 6) | (n = 1) | (n = 77) | |||
| Others ‡ | 285.1 ± 30.5BCd | 190.1 ± 8.6Ac | 212.8 ± 11.3Ac | 174.8 ± 6.2Bbc | 129.2 ± 7.5Aab | 107.7 ± 15.4Aa | - | 186.7 ± 5.2BC | |
| (n = 21) | (n = 30) | (n = 60) | (n = 109) | (n = 37) | (n = 4) | (n = 261) | |||
| Total | 239.0 ± 12.1c | 207.0 ± 7.2ab | 208.5 ± 5.9ab | 185.6 ± 3.1a | 225.6 ± 10.0bc | 182.6 ± 25.2a | - | 201.6 ± 2.7 | |
| (n = 88) | (n = 93) | (n = 156) | (n = 442) | (n = 103) | (n = 12) | (n = 894) | |||
| A | B | C | D | A vs. B | A vs. C | A vs. D | |
|---|---|---|---|---|---|---|---|
| GN | 6.09 ± 0.72 | 5.91 ± 0.68 | 7.59 ± 0.82 | 7.18 ± 0.75 | 0.03 | 0.25 | 0.18 |
| TH | 5.42 ± 0.15 | 5.34 ± 0.15 | 5.87 ± 0.20 | 5.75 ± 0.18 | 0.02 | 0.08 | 0.06 |
| GRW | 4.33 ± 0.39 | 4.37 ± 0.37 | 4.57 ± 0.39 | 4.73 ± 0.41 | 0.01 | 0.06 | 0.09 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, B.; Kim, H.S.; Jeon, J.; Yi, M.J. Effects of Temporal and Interspecific Variation of Specific Leaf Area on Leaf Area Index Estimation of Temperate Broadleaved Forests in Korea. Forests 2016, 7, 215. https://doi.org/10.3390/f7100215
Kwon B, Kim HS, Jeon J, Yi MJ. Effects of Temporal and Interspecific Variation of Specific Leaf Area on Leaf Area Index Estimation of Temperate Broadleaved Forests in Korea. Forests. 2016; 7(10):215. https://doi.org/10.3390/f7100215
Chicago/Turabian StyleKwon, Boram, Hyun Seok Kim, Jihyeon Jeon, and Myong Jong Yi. 2016. "Effects of Temporal and Interspecific Variation of Specific Leaf Area on Leaf Area Index Estimation of Temperate Broadleaved Forests in Korea" Forests 7, no. 10: 215. https://doi.org/10.3390/f7100215






