Labile and Non-Labile Fractions of Phosphorus and Its Transformations in Soil under Eucalyptus Plantations, Brazil
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Sites
2.2. Physical-Chemical Characterization of Soil
2.3. Soil P Fractions
| Site * | Species | Density (m) | Plot (m2) | Plants per Plot | Climate (1) | T (°C) | Precipitation (mm) | Soil (2) | Soil Texture | Age (Years) |
|---|---|---|---|---|---|---|---|---|---|---|
| AG | Eucalyptus grandis (G-232) | 3.0 × 2.0 | 144 | 24 | Aw | 21.6 | 1170 | LVd | loam | 3.0 |
| ANG | E. grandis × E. urophylla | 3.0 × 2.0 | 216 | 36 | Cwa | 20.6 | 1262 | RQ | sandy | 2.0 |
| BOT | E. grandis (G-232) | 3.0 × 2.0 | 144 | 24 | Cwa | 20.2 | 1302 | RQ | sandy | 3.0 |
| ITA | E. grandis (Suz.) | 3.0 × 2.0 | 144 | 24 | Cwa | 19.9 | 1308 | LVAd | loam | 4.0 |
| ALT (3) | E. grandis × E. urophylla | 3.0 × 2.5 | 180 | 24 | Aw | 20.7 | 1517 | RQ | sandy | 7.0 |
| CB1 (4) | E. grandis × E. urophylla | 3.0 × 3.0 | 360 | 40 | Cwa | 20.1 | 1210 | LAd | clayey | 9.0 |
| CB2 (5) | E. grandis × E. urophylla | 3.0 × 2.0 | 144 | 24 | Cwa | 20.1 | 1210 | LVd | very clayey | 1.4 |
| CB3 (6) | E. grandis × E. urophylla | 3.0 × 2.0 | 144 | 24 | Cwa | 20.1 | 1210 | CXBd | loam | 1.6 |
| PA (7) | E. grandis (Parb.) | 3.0 × 2.5 | 180 | 24 | Cwa | 20.8 | 1249 | LVAd | clayey | 11.0 |
| VOT (8) | E.grandis × E. urophylla | 3.0 × 2.0 | 96 | 16 | Cwa | 21.3 | 1287 | PVAd | very clayey | 1.8 |
| Site * | Clay | C | P 1 | pH | K | Ca | Mg | CEC | Al sat. | BS |
|---|---|---|---|---|---|---|---|---|---|---|
| g·kg−1 | g·dm−3 | mg·kg−1 | CaCl2 | ____________mmolc·dm−3__________ | % | % | ||||
| AG | 167 | 12.2 | 4.0 | 3.9 | 4.8 | 4.8 | 2.4 | 70.7 | 52.2 | 17 |
| ANG | 100 | 10.4 | 8.5 | 4.0 | 0.6 | 5.0 | 2.5 | 47.0 | 44.5 | 17 |
| BOT | 100 | 8.7 | 8.3 | 4.0 | 0.4 | 4.0 | 5.0 | 49.5 | 36.9 | 25 |
| ITA | 193 | 11.0 | 2.4 | 2.6 | 0.9 | 2.1 | 2.4 | 65.6 | 67.8 | 8 |
| ALT | 67 | 11.6 | 6.2 | 4.3 | 0.3 | 11.0 | 4.0 | 58.0 | 20.7 | 28 |
| CB1 | 478 | 16.2 | 1.4 | 3.9 | 1.2 | 1.7 | 2.3 | 94.9 | 78.2 | 5 |
| CB2 | 653 | 20.3 | 1.4 | 4.4 | 2.7 | 15.2 | 9.3 | 107.3 | 31.8 | 25 |
| CB3 | 272 | 12.8 | 3.5 | 4.1 | 1.4 | 9.9 | 9.5 | 102.5 | 43.9 | 21 |
| PA | 365 | 12.2 | 2.1 | 4.1 | 1.9 | 6.8 | 4.2 | 58.0 | 33.8 | 22 |
| VOT | 670 | 29.0 | 2.2 | 4.0 | 5.0 | 5.0 | 2.1 | 125.6 | 58.4 | 9 |
2.4. Statistical Analysis
3. Results
3.1. Distribution of Organic and Inorganic P Fractions
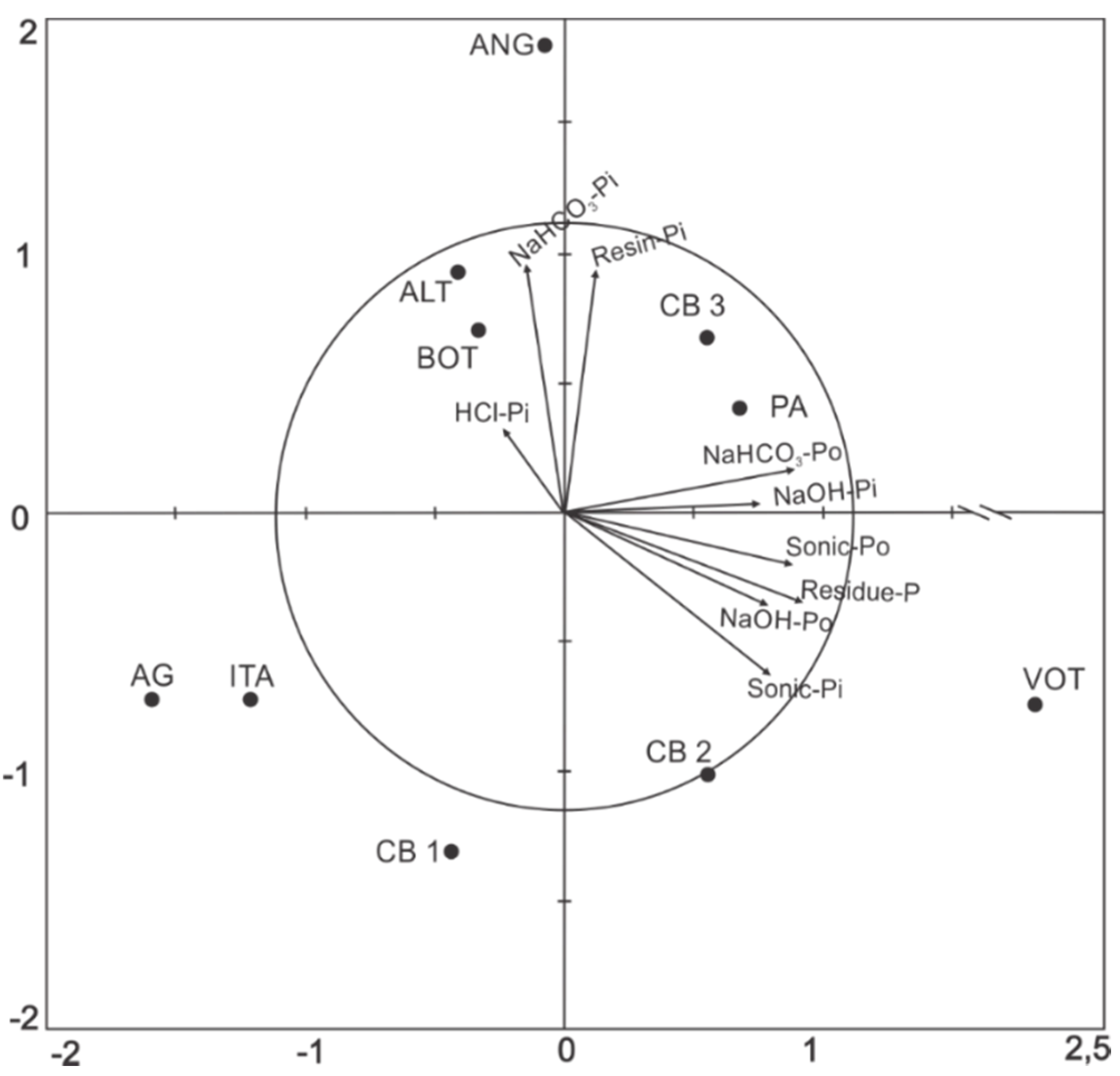
| Site * | Resin Pi | NaHCO3 Pi | NaHCO3 Po | NaOH Pi | NaOH Po | Sonic Pi | Sonic Po | HCl Pi | Residue P |
|---|---|---|---|---|---|---|---|---|---|
| ________________________________________________________mg·kg−1________________________________________________________ | |||||||||
| AG | 3.4 C | 4.4 C | 19.1 B | 45.0 B | 33.8 E | 7.7 B | 5.6 D | 3.4 C | 81.9 F |
| ANG | 8.5 A | 12.9 A | 23.6 B | 53.5 B | 59.1 D | 7.3 B | 16.0 C | 3.1 C | 98.3 F |
| BOT | 6.9 A | 7.0 B | 26.7 B | 52.2 B | 59.2 D | 7.9 B | 17.6 C | 3.6 C | 108.7 F |
| ITA | 2.5 C | 6.4 B | 17.9 B | 47,2 B | 62.0 D | 12.3 B | 13.3 C | 6.2 B | 142.3 E |
| ALT | 5.2 B | 8.2 B | 22.6 B | 83.1 A | 19.9 F | 10.9 B | 8.5 D | 10.5 A | 150.9 E |
| CB1 | 2.8 C | 2.8 C | 21.0 B | 73.2 A | 78.5 C | 43.3 A | 18.0 C | 3.7 C | 169.9 D |
| CB2 | 4.3 B | 2.1 C | 25.8 B | 76.1 A | 98.0 B | 52.0 A | 46.5 B | 3.2 C | 214.6 B |
| CB3 | 5.0 B | 7.2 B | 35.0 A | 79.3 A | 79.3 C | 18.1 B | 17.0 C | 6.6 B | 165.9 D |
| PA | 5.2 B | 7.4 B | 25.8 B | 84.5 A | 38.3 E | 26.3 B | 58.4 A | 4.0 C | 188.6 C |
| VOT | 3.5 C | 4.4 C | 38.2 A | 73.9 A | 166.8 A | 49.6 A | 67.7 A | 2.6 C | 332.3 A |
| Mean | 4.7 | 6.3 | 25.6 | 66.8 | 69.5 | 23.5 | 26.9 | 4.7 | 165.3 |
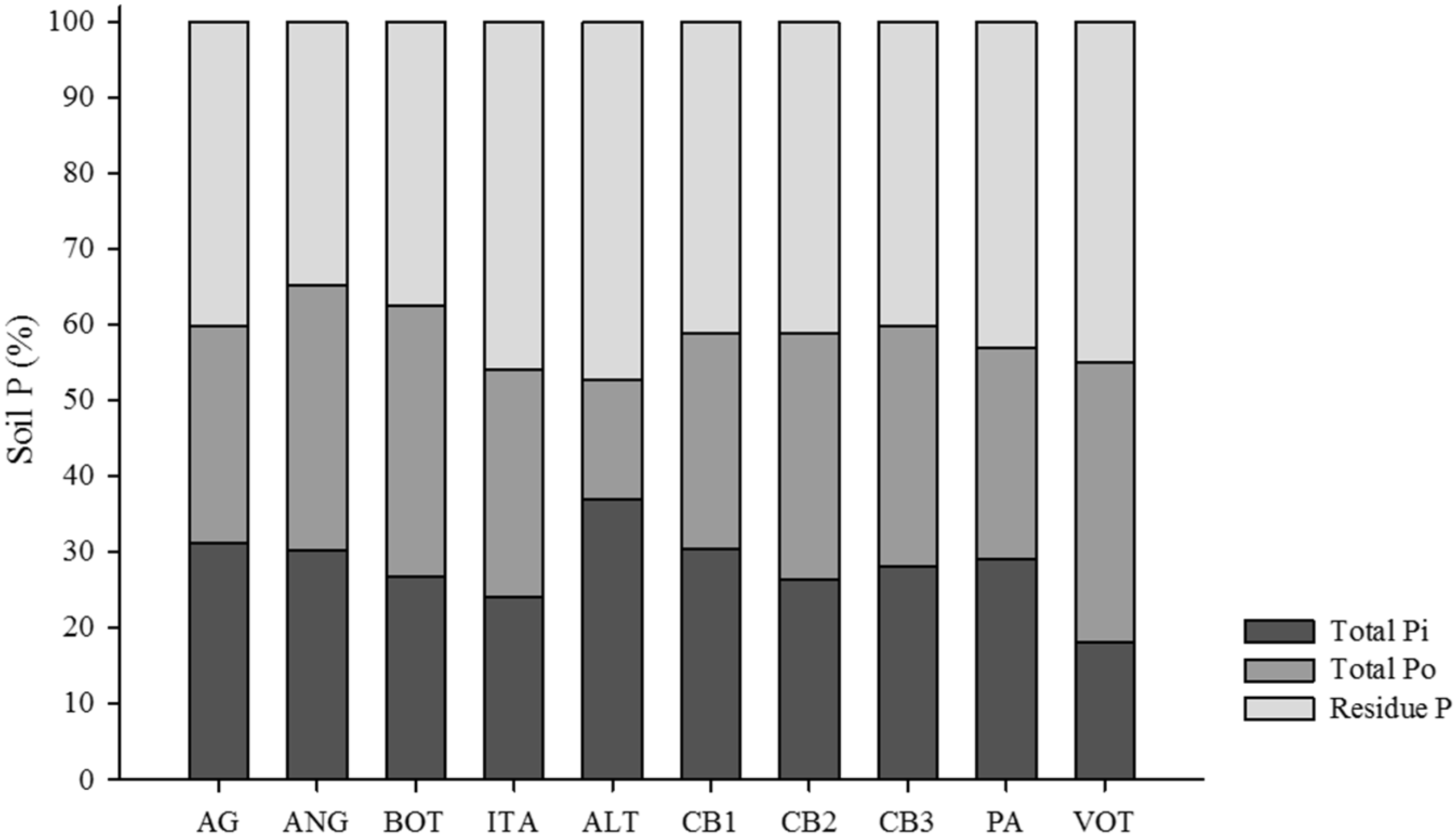
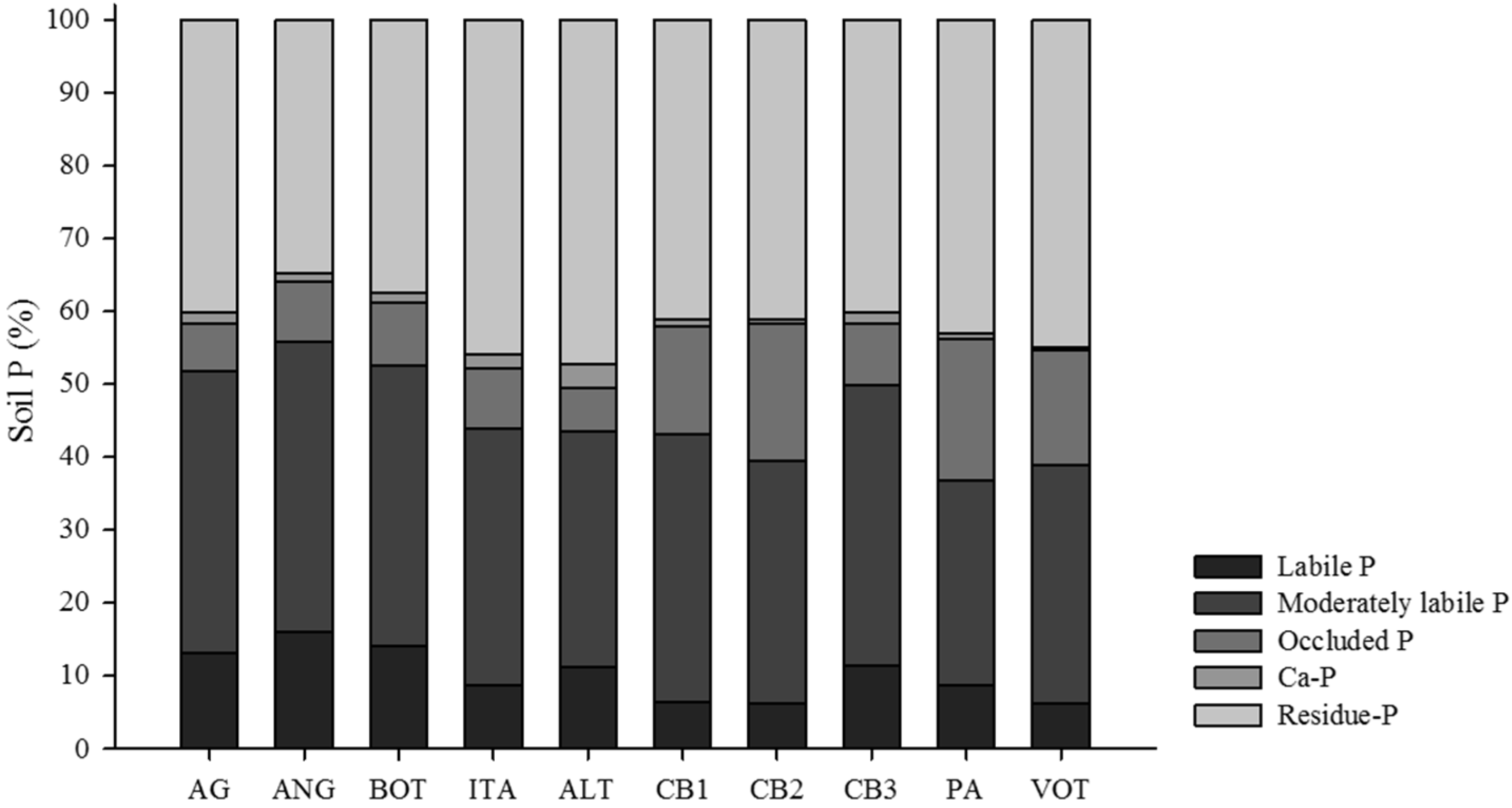
3.2. Relations between P Fractions and Soil Attributes
| Fractions | Resin Pi | NaHCO3 Pi | NaHCO3 Po | NaOH Pi | NaOH Po | Sonic Pi | Sonic Po | HCl Pi | Residue P |
|---|---|---|---|---|---|---|---|---|---|
| Resin Pi | 1 | 0.78 ** | 0.13 | −0.05 | −0.27 | −0.45 | −0.11 | −0.05 | −0.37 |
| NaHCO3 Pi | - | 1 | −0.04 | −0.16 | −0.40 | −0.69 * | −0.28 | 0.23 | −0.43 |
| NaHCO3 Po | - | - | 1 | 0.48 | 0.71 * | 0.41 | 0.60 | −0.17 | 0.70 * |
| NaOH Pi | - | - | - | 1 | 0.17 | 0.54 | 0.50 | 0.31 | 0.58 |
| NaOH Po | - | - | - | - | 1 | 0.74 * | 0.65 * | −0.49 | 0.83 ** |
| Sonic Pi | - | - | - | - | - | 1 | 0.73 * | −0.39 | 0.82 ** |
| Sonic Po | - | - | - | - | - | - | 1 | −0.45 | 0.85 ** |
| HCl Pi | - | - | - | - | - | - | - | 1 | −0.18 |
| Residue P | - | - | - | - | - | - | - | - | 1 |
| Fractions | Clay | C | pH | SB | Al sat. | CEC |
|---|---|---|---|---|---|---|
| Resin Pi | −0.47 | −0.43 | 0.42 | 0.04 | −0.58 | −0.56 |
| NaHCO3 Pi | −0.69 * | −0.55 | −0.03 | −0.27 | −0.34 | −0.68 * |
| NaHCO3 Po | 0.47 | 0.59 | 0.41 | 0.41 | −0.15 | 0.61 |
| NaOH Pi | 0.46 | 0.37 | 0.59 | 0.52 | −0.37 | 0.41 |
| NaOH Po | 0.79 ** | 0.88 *** | 0.04 | 0.15 | 0.37 | 0.82 ** |
| Sonic Pi | 0.98 *** | 0.86 ** | 0.28 | 0.35 | 0.23 | 0.82 ** |
| Sonic Po | 0.78** | 0.73 * | 0.27 | 0.29 | −0.08 | 0.51 |
| HCl Pi | −0.48 | −0.37 | −0.06 | 0.12 | −0.37 | −0.24 |
| Residue P | 0.84 ** | 0.91 *** | 0.18 | 0.29 | 0.10 | 0.78 ** |
3.3. Soil P Transformations
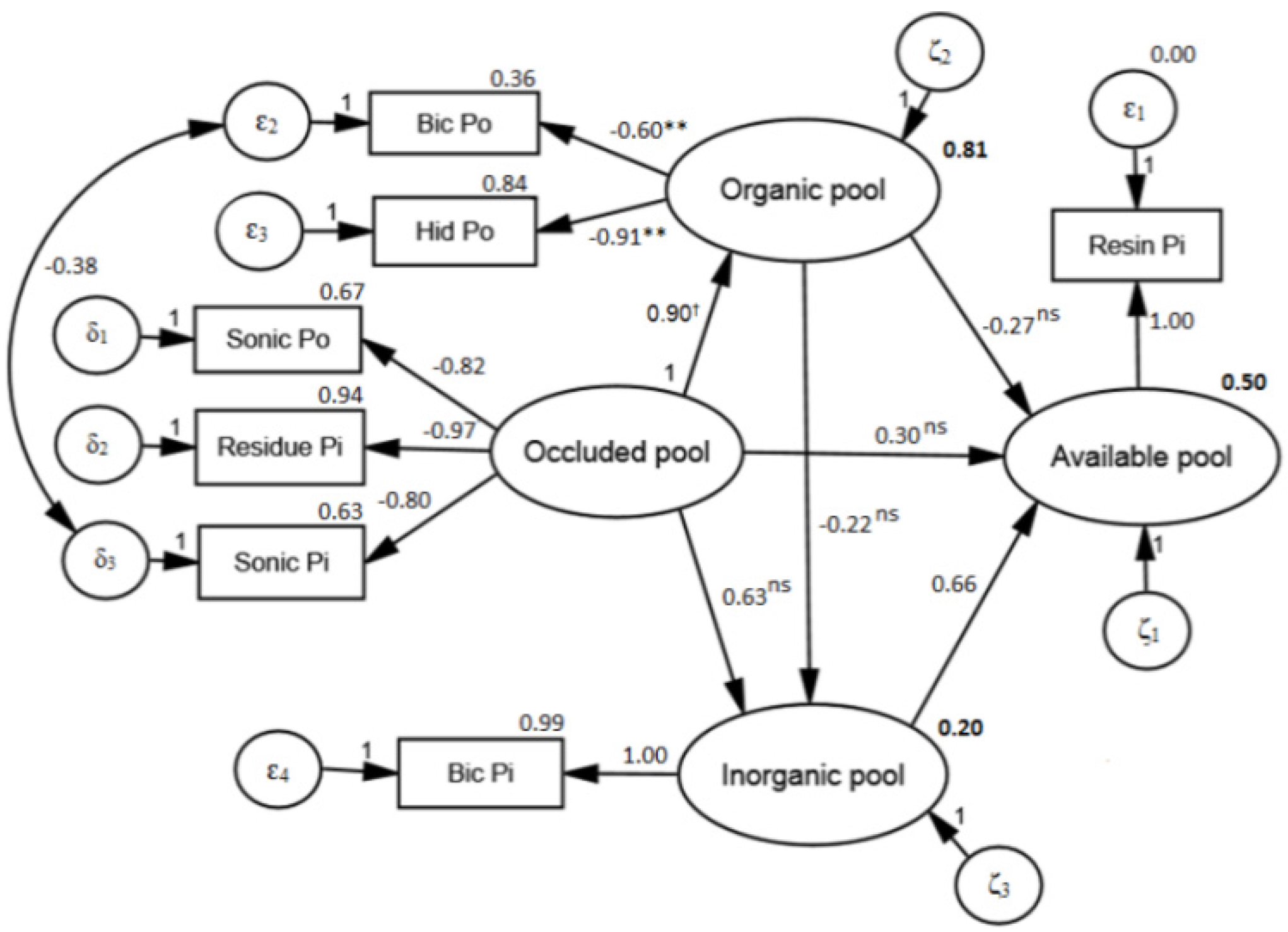
4. Discussion
4.1. Distribution of Organic and Inorganic P Fractions
4.2. Structural Model
5. Conclusions
- There is great dissimilarity across forestry sites regarding P fractions in soil, caused by differences in the physical and chemical attributes of soil and in forest management practices.
- The fractionation procedure of P establishes the potential for labile fractions to supply Eucalyptus trees, which is not possible in routine methods for analysis of soil fertility.
- The structural model is adequate to identify functional pools of P in soil and to identify transformation processes of P in soil, based on the source–sink relationships.
- The available P pool is highly dependent on inorganic and occluded pool and the organic pool acts predominantly as a sink of P on available and inorganic pools. This demonstrates the high level of recalcitrance of the organic pool and the fact that Eucalyptus plants must access pools of limited-availability P in order to meet their nutritional demands.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Booth, T.H. Eucalypt plantations and climate change. For. Ecol. Manag. 2013, 301, 28–34. [Google Scholar] [CrossRef]
- Laclau, J.P.; Goncalves, J.L.D.; Stape, J.L. Perspectives for the management of eucalypt plantations under biotic and abiotic stresses. For. Ecol. Manag. 2013, 301, 1–5. [Google Scholar] [CrossRef]
- Bazani, J.H.; Gonçalves, J.L.M.; Rocha, J.H.T.; Melo, E.S.A.C.; Prieto, M. Nutrição fosfatada em plantações de eucalipto. Info. Agron. 2014, 148, 1–11. [Google Scholar]
- Gonçalves, J.L.M.; Alvares, C.A.; Higa, A.R.; Silva, L.D.; Alfenas, A.C.; Stahl, J.; Ferraz, S.F.B.; Lima, W.P.; Brancalion, P.H.S.; Hubner, A.; et al. Integrating genetic and silvicultural strategies to minimize abiotic and biotic constraints in Brazilian eucalypt plantations. For. Ecol. Manag. 2013, 301, 6–27. [Google Scholar] [CrossRef]
- Gonçalves, J.L.M. Fertilização de Plantação de Eucalipto. In Encontro brasileiro de Silvicultura, 2011; Gonçalves, J.L.M., Pulito, A.P., Arthur Junior, J.C., Silva, L.D., Eds.; Anais Piracicaba: Piracicaba, Brazil, 2011; pp. 85–114. [Google Scholar]
- Turner, B.L.; Engelbrecht, B.M.J. Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry. 2011, 103, 297–315. [Google Scholar] [CrossRef]
- Yang, X.; Post, W.M. Phosphorus transformation as a function of pedogenesis: A synthesis of soil phosphorus data using Hedley fractionation method. Biogeosciences 2011, 8, 2907–2916. [Google Scholar] [CrossRef]
- Condron, L.M.; Turner, B.L.; Cade-Menun, B.J. Chemistry and dynamics of soil organic phosphorus. In Phosphorus: Agriculture and the Environment, Agronomy Monograph 46; Sims, J.T., Sharpley, A.N., Eds.; SSSA: Madison, WI, USA, 2005; pp. 87–121. [Google Scholar]
- Cunha, G.M.; Gama-Rodrigues, A.C.; Costa, G.S.; Velloso, A.C.X. Fósforo orgânico em solos sob florestas montanas, pastagens e eucalipto no Norte Fluminense. Rev. Bras. Ciênc. Solo 2007, 31, 667–672. [Google Scholar] [CrossRef]
- Turner, B.L. Resource partitioning for soil phosphorus: A hypothesis. J. Ecol. 2008, 96, 698–702. [Google Scholar] [CrossRef]
- Zaia, F.C.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F. Formas de fósforo no solo sob leguminosas florestais, floresta secundária e pastagem no Norte Fluminense. Rev. Bras. Ciênc. Solo 2008, 32, 1191–1197. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil-phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Condron, L.M.; Newman, S. Revisiting the fundamentals of phosphorus fractionation of sediments and soils. J. Soils Sediment. 2011, 11, 830–840. [Google Scholar] [CrossRef]
- Cross, A.F.; Schlesinger, W.H. A literature review and evaluation of the Hedley fractionation: Applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 1995, 64, 197–214. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. J. Plant Nutr. Soil Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Tiessen, H.; Stewart, J.W.B.; Cole, C.V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. [Google Scholar] [CrossRef]
- Guo, F.; Yost, R.S. Partitioning soil phosphorus into three discrete pools of differing availability. Soil Sci. 1998, 163, 822–832. [Google Scholar] [CrossRef]
- Cardoso, I.; Janssen, B.; Oenema, O.; Kuyper, T. Phosphorus pools in Oxisols under shaded and unshaded coffee systems on farmers’ fields in Brazil. Agrofor. Syst. 2003, 58, 55–64. [Google Scholar] [CrossRef]
- Frizano, J.; Vann, D.R.; Johnson, A.H.; Johnson, C.M.; Vieira, I.C.G.; Zarin, D.J. Labile phosphorus in soils of forest fallows and primary forest in the Bragantina region, Brazil. Biotropica 2003, 35, 2–11. [Google Scholar] [CrossRef]
- Gatiboni, L.C.; Brunetto, G.; Rheinheimer, D.S.; Kaminsk, J. Fracionamento químico das formas de fósforo do solo: Uso e limitações. Tóp. Ciênc. Solo 2013, 8, 141–187. [Google Scholar]
- Gama-Rodrigues, A.C.; Sales, M.V.S.; Silva, P.S.D.; Comerford, N.B.; Cropper, W.P.; Gama-Rodrigues, E.F. An exploratory analysis of phosphorus transformations in tropical soils using structural equation modeling. Biogeochemistry 2014, 118, 453–469. [Google Scholar] [CrossRef]
- Barros, N.F.; Novais, R.F. Eucalipto. In Recomendações para uso de Corretivos e Fertilizantes em Minas Gerais, 5th ed.; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; UFV: Viçosa, Brazil, 1999; pp. 303–305. [Google Scholar]
- Empresa Brasileira de Pesquisa Agropecuária—Embrapa. Manual de Análises Químicas de Solos, Plantas e Fertilizantes; Empresa Brasileira de Pesquisa Agropecuária: Brasília, Brazil, 1999. [Google Scholar]
- Alvarez, V.V.H.; Ribeiro, A.C. Calagem. In Recomendação Para Uso de Corretivos e Fertilizantes em Minas Gerais, 5th ed.; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; UFV: Viçosa, Brazil, 1999; pp. 43–60. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Dick, W.A.; Tabatabai, M.A. Determination of orthophosphate in aqueous solutions containing labile organic and inorganic phosphorus compounds. J. Environ. Qual. 1977, 6, 82–85. [Google Scholar] [CrossRef]
- Zaia, F.C.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Moço, M.K.S.; Fontes, A.G.; Machado, R.C.R.; Baligar, V.C. Carbon, nitrogen, organic phosphorus, microbial biomass and N mineralization in soils under cacao agroforestry systems in Bahia, Brazil. Agrofor. Syst. 2012, 86, 197–212. [Google Scholar] [CrossRef]
- Lima, A.M.N.; Silva, I.R.; Neves, J.C.L.; Novais, R.F.; Barros, N.F.; Mendonça, E.S.; Smyth, T.J.; Moreira, M.S.; Leite, F.P. Soil organic carbon dynamics following afforestation of degraded pastures with eucalyptus in Southeastern Brazil. For. Ecol. Manag. 2006, 235, 219–231. [Google Scholar] [CrossRef]
- Barreto, P.A.B.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Barros, N.F. Nitrogen balance in soil under eucalyptus plantations. Rev. Bras. Ciênc. Solo 2012, 36, 1239–1248. [Google Scholar] [CrossRef]
- Friesen, D.K.; Rao, I.M.; Thomas, R.J.; Oberson, A.; Sanz, J.I. Phosphorus acquisition and cycling in crop and pasture systems in low fertility tropical soils. Plant Soil 1997, 196, 289–294. [Google Scholar] [CrossRef]
- Araújo, M.S.B.; Schaefer, C.E.R.; Sampaio, E.V.S.B. Soil phosphorus fractions from toposequences of semi-arid Latosols and Luvisols in northeastern Brazil. Geoderma 2004, 119, 309–321. [Google Scholar] [CrossRef]
- Beck, M.A.; Sanchez, P.A. Soil-phosphorus fraction dynamics during 18 years of cultivation on a typic paleudult. Soil Sci. Soc. Am. J. 1994, 58, 1424–1431. [Google Scholar] [CrossRef]
- Tiessen, H. Phosphorus dynamics in tropical soils. In Phosphorus: Agriculture and the Environment, Agronomy Monograph; Sims, J.T., Sharpley, A.N., Eds.; SSSA: Madison, WI, USA, 2005; pp. 253–326. [Google Scholar]
- Sales, M.V.S.; Gama-Rodrigues, A.C.; Comerford, N.B.; Cropper, W.P.; Gama-Rodrigues, E.F.; Oliveira, P.H.G. Respecification of structural equation models for the P cycle in tropical soils. Nutr. Cycl. Agroecosyst. 2015, 102, 347–358. [Google Scholar] [CrossRef]
- Dieter, D.; Elsenbeer, H.; Turner, B.L. Phosphorus fractionation in lowland tropical rainforest soils in central Panama. Catena 2010, 2, 118–125. [Google Scholar] [CrossRef]
- Xavier, F.A.S.; Almeida, E.F.; Cardoso, I.M.; Mendonca, E.S. Soil phosphorus distribution in sequentially extracted fractions in tropical coffee-agroecosystems in the Atlantic Forest biome, Southeastern Brazil. Nutr. Cycl. Agroecosyst. 2011, 89, 31–44. [Google Scholar] [CrossRef]
- Turner, B.L. Organic phosphorus in Madagascan rice soils. Geoderma 2006, 136, 279–288. [Google Scholar] [CrossRef]
- Oliveira, R.I.; Gama-Rodrigues, A.C.; Gama-Rodrigues, E.F.; Zaia, F.C.; Pereira, M.G.; Fontana, A. Organic phosphorus in diagnostic surface horizons of different Brazilian soil orders. Rev. Bras. Ciênc. Solo 2014, 38, 1411–1420. [Google Scholar] [CrossRef]
- Schmitt, D.E.; Comin, J.J.; Gatiboni, L.C.; Tiecher, T.; Lorensini, F.; Melo, G.W.B.; Girotto, E.; Guardini, R.; Heinzen, J.; Brunetto, G. Phosphorus fractions in sandy soils of vineyards in southern Brazil. Rev. Bras. Ciênc. Solo 2013, 37, 472–481. [Google Scholar] [CrossRef]
- Bellote, A.F.J.; Sarruge, J.R.; Haag, H.P.; Oliveira, G.D. Extração e exportação de nutriente pelo Eucalyptus grandis Hill ex-Maiden em função da idade: 1-Macronutrientes. IPEF Piracicaba 1980, 20, 1–23. [Google Scholar]
- Poggiani, F.; Couto, H.T.Z.; Corradini, L.; Fazzio, E.C.M. Exportação de biomassa e nutrientes através da exploração dos troncos e das copas de um povoamento de Eucalyptus saligna. IPEF Piracicaba 1983, 25, 37–39. [Google Scholar]
- Santana, R.C.; Barros, N.F.; Neves, J.C.L. Biomassa e conteúdo de nutrientes de procedências de Eucalyptus grandis e Eucalyptus saligna em alguns sítios florestais do Estado de São Paulo. Sci. For. 1999, 56, 155–169. [Google Scholar]
- Santana, R.C.; Barros, N.F.; Novais, R.F.; Leite, H.G.; Comerford, N.B. Alocação de nutrientes em plantios de eucalipto no Brasil. Rev. Bras. Ciênc. Solo 2008, 32, 2723–2733. [Google Scholar] [CrossRef]
- Laclau, J.P.; Deleporte, P.; Ranger, J.; Bouillet, J.P.; Kazotti, G. Nutrient dynamics throughout the rotation of Eucalyptus clonal stands in Congo. Ann. Bot. 2003, 91, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.; Nogueira, G.S.; Santana, R.C.; Leite, H.G.; Oliveira, M.L.R.; Almado, R.P. Produção e acúmulo de nutrientes em povoamento de eucalipto em consequência da intensidade do desbaste e da fertilização. Pesq. Agropec. Bras. 2012, 47, 1555–1562. [Google Scholar] [CrossRef]
- Gama-Rodrigues, A.C.; Barros, N.F. Ciclagem de nutrientes em floresta natural e plantios de eucalipto e de dandá no sudeste da Bahia, Brasil. Rev. Árvore 2002, 26, 193–207. [Google Scholar]
- Gatiboni, L.C.; Kaminski, J.; Rheinheimer, D.S.; Flores, J.P.C. Biodisponibilidade de formas de fósforo acumuladas em solo sob sistema plantio direto. Rev. Bras. Ciênc. Solo 2007, 31, 691–699. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Celi, L.; Barberis, E. Abiotic stabilization of organic phosphorus in the environment. In Organic Phosphorus in the Environment; Turner, B.L., Frossard, E., Baldwin, D., Eds.; CABI Publishing: Wallingford, WI, USA, 2005; pp. 113–132. [Google Scholar]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Aust. J Soil 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Brundrett, M.C.; Raven, J.A.; Hopper, S.D. Plant mineral nutrition in ancient landscapes: High plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 2010, 334, 11–31. [Google Scholar] [CrossRef]
- Radersma, S.; Grierson, P. Phosphorus mobilization in agroforestry: Organic anions, phosphatase activity and phosphorus fractions in the rhizosphere. Plant Soil 2004, 259, 209–219. [Google Scholar] [CrossRef]
- Grierson, P.F.; Adams, M.A. Plant species affect acid phosphatase, ergosterol and microbial P in a Jarrah (Eucalyptus marginata Donn ex Sm.) forest in south-western Australia. Soil Bio. Biochem. 2000, 32, 1817–1927. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Binkley, D.; Caldwell, B.A. Effects of dinitrogen-fixing trees on phosphorus biogeochemical cycling in contrasting forests. Soil Sci. Soc. Am. J. 1995, 59, 1452–1458. [Google Scholar] [CrossRef]
- Barto, E.K.; Alt, F.; Oelmann, Y.; Wilcke, W.; Rillig, M. Contributions of biotic and abiotic factors to soil aggregation across a land use gradient. Soil Biol. Biochem. 2010, 42, 2316–2324. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.G.; Gama-Rodrigues, A.C.; Gonçalves, J.L.d.M.; Gama-Rodrigues, E.F.; Sales, M.V.d.S.; Aleixo, S. Labile and Non-Labile Fractions of Phosphorus and Its Transformations in Soil under Eucalyptus Plantations, Brazil. Forests 2016, 7, 15. https://doi.org/10.3390/f7010015
Costa MG, Gama-Rodrigues AC, Gonçalves JLdM, Gama-Rodrigues EF, Sales MVdS, Aleixo S. Labile and Non-Labile Fractions of Phosphorus and Its Transformations in Soil under Eucalyptus Plantations, Brazil. Forests. 2016; 7(1):15. https://doi.org/10.3390/f7010015
Chicago/Turabian StyleCosta, Marlon Gomes, Antonio Carlos Gama-Rodrigues, José Leonardo de Moraes Gonçalves, Emanuela Forestieri Gama-Rodrigues, Marcus Vinicius da Silva Sales, and Seldon Aleixo. 2016. "Labile and Non-Labile Fractions of Phosphorus and Its Transformations in Soil under Eucalyptus Plantations, Brazil" Forests 7, no. 1: 15. https://doi.org/10.3390/f7010015






