Nitrogen Transfer to Forage Crops from a Caragana Shelterbelt
Abstract
:1. Introduction
2. Experimental Section
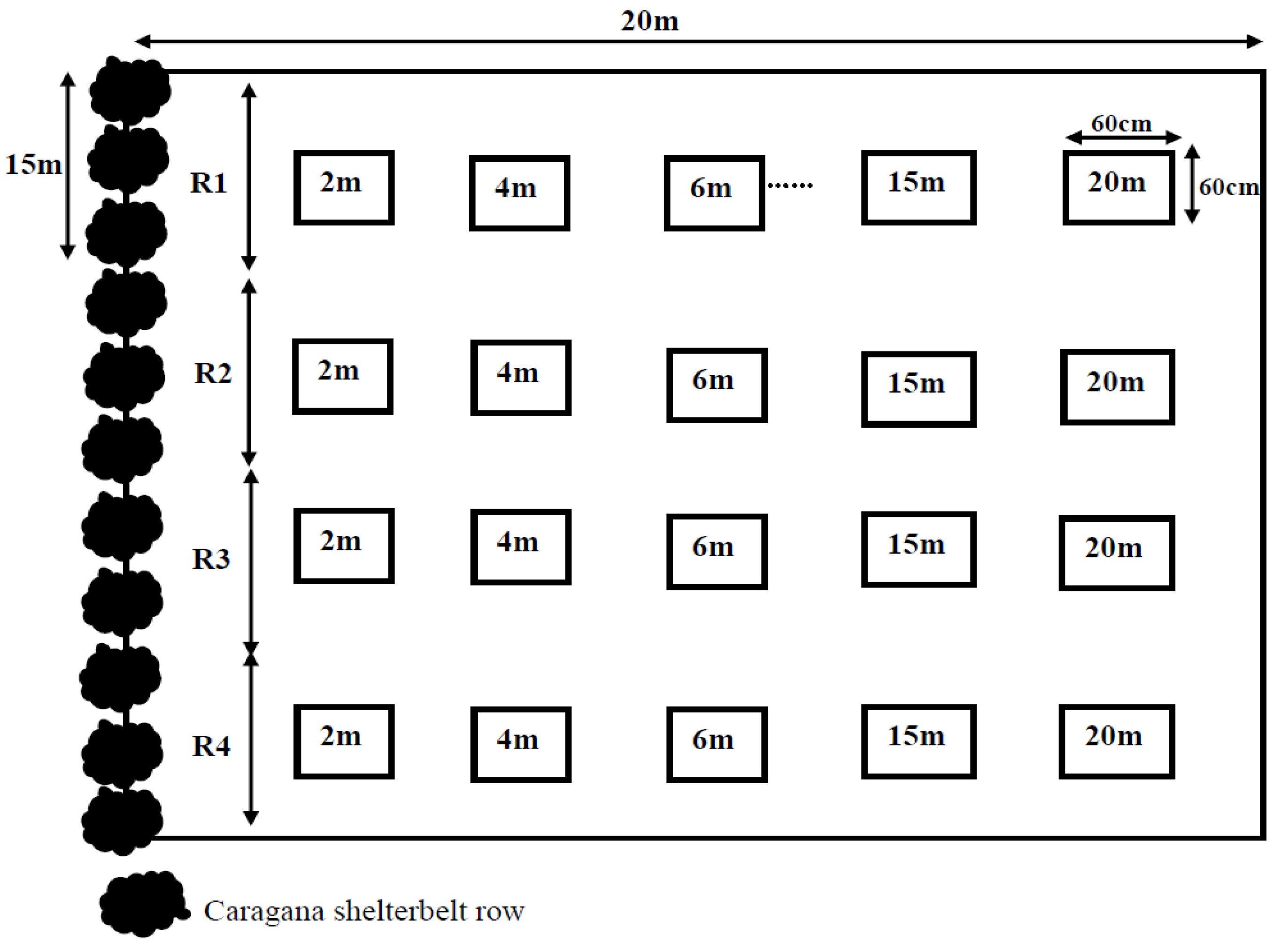
2.1. Experimental Setup
| Distance (m) | pH | EC (mS cm–1) | P | NO3− | NH4+ | S | OC | K+ | Mg2+ | Ca2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | cmol (+) kg−1 | ||||||||
| 0–15 cm | ||||||||||
| 2 | 8.04 † | 0.26 | 22.04 | 8.27 | 3.72 | 5.66 | 7.55 | 0.70 | 1.86 | 9.83 |
| 4 | 8.05 | 0.17 | 26.21 | 2.40 | 3.09 | 5.15 | 7.05 | 0.62 | 1.66 | 9.29 |
| 6 | 8.06 | 0.19 | 21.34 | 1.52 | 2.85 | 4.47 | 7.00 | 0.52 | 1.98 | 9.50 |
| 15 | 8.01 | 0.22 | 27.23 | 2.23 | 3.36 | 7.65 | 7.20 | 0.63 | 2.21 | 7.75 |
| 20 | 7.93 | 0.26 | 31.94 | 2.99 | 3.77 | 9.00 | 7.45 | 0.65 | 2.44 | 7.27 |
| 15–30 cm | ||||||||||
| 2 | 8.21 | 0.20 | 6.40 | 1.50 | 4.27 | 4.18 | 4.50 | 0.36 | 2.28 | 9.07 |
| 4 | 8.31 | 0.16 | 6.31 | 1.02 | 3.74 | 4.25 | 5.10 | 034 | 2.06 | 9.32 |
| 6 | 8.37 | 0.15 | 11.48 | 1.25 | 3.85 | 4.29 | 4.95 | 0.33 | 2.11 | 8.86 |
| 15 | 8.17 | 0.20 | 9.50 | 1.17 | 3.32 | 6.55 | 5.90 | 0.34 | 2.35 | 7.79 |
| 20 | 8.26 | 0.23 | 8.51 | 0.87 | 3.07 | 11.16 | 5.15 | 0.29 | 2.91 | 8.21 |
| 30–45 cm | ||||||||||
| 2 | 6.40 | 0.13 | 5.36 | 0.22 | 3.33 | 3.37 | 2.45 | 0.23 | 2.58 | 6.13 |
| 4 | 8.49 | 0.18 | 9.77 | 0.29 | 3.94 | 4.18 | 3.00 | 0.31 | 3.10 | 8.34 |
| 6 | 8.45 | 0.20 | 6.09 | 0.67 | 3.66 | 4.11 | 3.55 | 0.24 | 2.70 | 8.27 |
| 15 | 8.44 | 0.21 | 5.64 | 0.49 | 3.30 | 7.80 | 3.45 | 0.23 | 2.75 | 7.90 |
| 20 | 8.56 | 0.29 | 5.16 | 0.46 | 4.25 | nd ‡ | 3.80 | 0.25 | 3.34 | 7.79 |
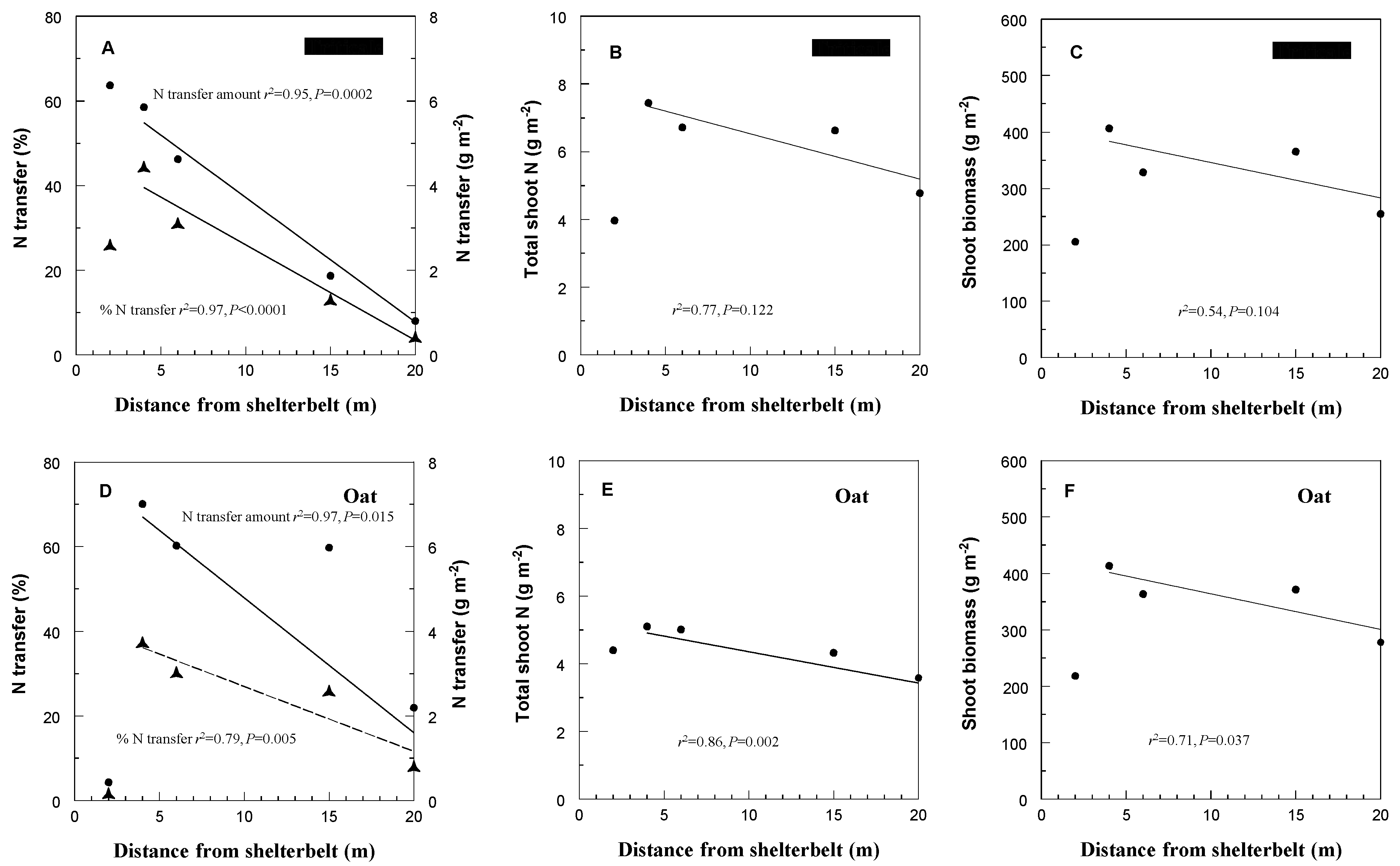
3. Results and Discussion
| Caragana | Triticale | Oats | ||||
|---|---|---|---|---|---|---|
| Distance (m) | δ15N (per mil) | Std Dev | δ15N (per mil) | Std Dev | δ15N (per mil) | Std Dev |
| 0 | 4.55 | 0.44 | - | - | - | - |
| 2 | - | - | 7.03 | 0.65 | 8.175 | 1.20 |
| 4 | - | - | 7.38 | 0.44 | 5.845 | 0.75 |
| 6 | - | - | 8.22 | 0.78 | 6.268 | 0.18 |
| 15 | - | - | 8.99 | 1.91 | 6.285 | 0.19 |
| 20 | - | - | 10.83 | 1.19 | 7.935 | 1.18 |
| P-value | 0.0028 | 0.0047 | ||||
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- One Hundred Years of Prairie Forestry. Available online: http://www.ourspace.uregina.ca/handle/10294/129 (accessed on 25 March 2015).
- Arha, K.; Josling, T.; Sumner, D.A.; Thompson, B.H. National Forum on U.S. Agricultural Policy and the 2007 Farm Bill: Conserving the Ecological Integrity and Economic Resilience of American Working Lands. Woods Institute for the Environment, Stanford University: California, CA, USA, 2007; pp. 1–257. Available online: http://media.law.stanford.edu/publications/archive/pdf/farmbill_book. pdf (accessed on 23 March 2015).
- Public Fundraising Regulatory Association. A Study of Tree Planting on the Canadian Prairies; Government of Canada-PFRA: Regina, SK, Canada, 1976; pp. 1–184.
- Sutton, V. An Evaluation of Landowner’s Attitudes toward Field Shelterbelts in Manitoba: A Case Study in the Lyleton Area. Master’s Thesis, University of Manitoba, Winnipeg, Manitoba, Canada, 1983. [Google Scholar]
- Scholten, H. Snow distribution on crop fields. Agric. Ecosyst. Environ. 1988, 22–23, 363–380. [Google Scholar] [CrossRef]
- Kort, J. Benefits of windbreaks to field and forage crops. Agric. Ecosyst. Environ. 1988, 22–23, 165–190. [Google Scholar] [CrossRef]
- Jose, S.; Gillespie, A.R.; Seifert, J.R.; Biehle, D.J. Defining competition vectors in a temperate alley cropping system in the Midwestern USA: 2. Competition for water. Agroforest. Syst. 2000, 48, 41–59. [Google Scholar] [CrossRef]
- Gillespie, A.R.; Jose, S.; Mengel, D.B.; Hoover, W.L.; Pope, P.E.; Seifert, J.R. Defining competition vectors in a temperate alley cropping system in the Midwestern USA: 1. Production physiology. Agroforest. Syst. 2000, 48, 25–40. [Google Scholar] [CrossRef]
- Zolotukhin, A.I. Allelopathic effect of shrubs used in steppe afforestation. Ekologiya 1980, 11, 13–17. [Google Scholar]
- George, E.J. Effect of Tree Windbreaks and Slat Barriers on Wind Velocity and Crop Yields; USDA Agricultural Research Service: Washington, DC, USA, 1971; p. 23.
- Stoeckeler, J.H. The design of shelterbelts in relation to crop yield improvement. World Crop. 1965, 17, 3–8. [Google Scholar]
- George, E.J. Tree and shrub species for the northern Great Plains. Cir. USDA. 1953, 912, 46. [Google Scholar]
- Brandle, J.R.; Johnson, B.B.; Dearmont, D.D. Windbreak economics: The case of winter wheat production in Eastern Nebraska. J. Soil Water Conserv. 1984, 39, 339–343. [Google Scholar]
- Dietz, D.R.; Slabaugh, P.E.; Bonner, F.T. Caragana arborescens Lam.: Siberian peashrub. In The Woody Plant Seed Manual; Franklin, T., Karrfalt, R.P., Eds.; Department of Agriculture, Forest Service: Washington, DC, USA, 2008; Volume 727, pp. 321–323. [Google Scholar]
- Moukoumi, J.; Hynes, R.K.; Dumonceaux, T.J.; Town, J.; Bélanger, N. Characterization and genus identification of rhizobial symbionts from Caragana arborescens in western Canada. Can. J. Micro. 2013, 59, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Z.; Zhao, H.L. Soil properties and plant species in an age sequence of Caragana microphylla plantations in the Horqin Sandy Land, north China. Ecol. Eng. 2003, 20, 223–235. [Google Scholar] [CrossRef]
- Moukoumi, J.; Farrell, R.E.; van Rees, K.J.C.; Hynes, R.K.; Bélanger, N. Growth and nitrogen dynamics of juvenile short rotation intensive cultures of pure and mixed Salix miyabeana and Caragana arborescens. Bioenergy Res. 2012, 5, 719–732. [Google Scholar] [CrossRef]
- Issah, G.; Kimaro, A.A.; Kort, J.; Knight, J.D. Quantifying biological nitrogen fixation of agroforestry shrub species using 15N dilution techniques under greenhouse conditions. Agroforest. Syst. 2014, 8, 607–617. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. The soils of Indian Head Rural Municipality. In Saskatchewan Soil Survey, 156; Saskatchewan Institute of Pedology, University of Saskatchewan: Saskatoon, SK, Canada, 1986. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil sampling and methods of analysis. In Canadian Society of Soil Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Malhi, S.S.; Johnston, A.M.; Schoenau, J.J.; Wang, Z.H.; Vera, C.L. Seasonal biomass accumulation and nutrient uptake of wheat, barley and oat on a Black Chernozem soil in Saskatchewan. Can. J. Plant Sci. 2006, 86, 1005–1014. [Google Scholar] [CrossRef]
- SAS Institute. version 9.2; SAS/STAT user’s guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Read, R.A. Tree windbreaks for the central Great Plains. In Agriculture Handbook 250; USDA forest Service: Washington, DC, USA, 1964; p. 68. [Google Scholar]
- Lyles, L.; Tatarko, J.; Dickerson, J.D. Windbreak effects on soil water and wheat yield. Trans. ASAE 1984, 20, 69–72. [Google Scholar] [CrossRef]
- Henderson, D.C.; Chapman, R. Caragana arborescens invasion in Elk Island National Park, Canada. Nat. Areas J. 1996, 26, 261–266. [Google Scholar] [CrossRef]
- Rao, A.V.; Giller, K.E. Nitrogen fixation and its transfer from Leucaena to grass using 15N. For. Ecol. Manag. 1993, 61, 221–227. [Google Scholar] [CrossRef]
- Arnebrandt, K.; Ek, H.; Finlay, R.D.; Soderstrom, B. Nitrogen translocation between Alnus lutinosa (L.) Gaertn. seedlings inoculated with Frankia sp. and Pinus contorta Doug, ex Loud seedlings connected by a common ectomycorrhizal mycelium. New Phytol. 1993, 124, 231–242. [Google Scholar] [CrossRef]
- Ekbland, A.; Huss-Danell, K. Nitrogen fixation by Alnus incana and nitrogen transfer from A. incana to Pinus sylvestris influenced by macronutrients and ectomycorrhiza. New Phytol. 1995, 131, 453–459. [Google Scholar] [CrossRef]
- Roggy, J.C.; Moiroud, A.; Lensi, R.; Domenach, A.M. Estimating N transfer between N2-fixing ctinorhizal species and the non-N2-fixing Prunus avium under partially controlled conditions. Biol. Fert. Soils 2004, 39, 312–319. [Google Scholar]
- Binkley, D.; Sollins, P.; McGill, W.B. Natural abundance of nitrogen-15 as a tool for tracing alder-fixed nitrogen. Soil Sci. Soc. Am. J. 1985, 49, 444–447. [Google Scholar] [CrossRef]
- Snoeck, D.; Zapata, F.; Domenach, A.M. Isotopic evidence of the transfer of nitrogen fixed by legumes to coffee trees. Biotech. Agron. Soc. Environ. 2000, 4, 95–100. [Google Scholar]
- Sierra, J.; Nygren, P. Transfer of N fixed by a legume tree to the associated grass in a tropical silvopastoral system. Soil. Biol. Biochem. 2006, 3, 1893–1903. [Google Scholar] [CrossRef]
- Kremer, R.J.; Li, J. Developing weed-suppressive soils through improved soil quality management. Soil Tillage Res. 2003, 72, 193–202. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Kremer, R.J.; Adamson, B.W.; Anderson, S.H. Variations in soil aggregate stability and enzyme activities in a temperate agroforestry practice. Appl. Soil Ecol. 2008, 39, 153–160. [Google Scholar] [CrossRef]
- Paudel, B.R.; Udawatta, R.P.; Kremer, R.J.; Anderson, S.H. Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl. Soil Ecol. 2011, 48, 125–132. [Google Scholar] [CrossRef]
- Hogberg, P. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Daudin, D.; Sierra, J. Spatial and temporal variation of below-ground N transfer from a leguminous tree to an associated grass in an agroforestry system. Agri. Ecosyst. Environ. 2008, 126, 275–280. [Google Scholar] [CrossRef]
- Isaac, M.E.; Harmand, J.M.; Lesueur, D.; Lelon, J. Tree age and soils phosphorus conditions influence N2-fixation rates and soil N dynamics in natural populations of Acacia senegal. For. Ecol. Manag. 2013, 261, 582–588. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issah, G.; Kimaro, A.A.; Kort, J.; Knight, J.D. Nitrogen Transfer to Forage Crops from a Caragana Shelterbelt. Forests 2015, 6, 1922-1932. https://doi.org/10.3390/f6061922
Issah G, Kimaro AA, Kort J, Knight JD. Nitrogen Transfer to Forage Crops from a Caragana Shelterbelt. Forests. 2015; 6(6):1922-1932. https://doi.org/10.3390/f6061922
Chicago/Turabian StyleIssah, Gazali, Anthony A. Kimaro, John Kort, and J. Diane Knight. 2015. "Nitrogen Transfer to Forage Crops from a Caragana Shelterbelt" Forests 6, no. 6: 1922-1932. https://doi.org/10.3390/f6061922





