Outbreak of Phoracantha semipunctata in Response to Severe Drought in a Mediterranean Eucalyptus Forest
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
2.2. Study Site and Plot Selection
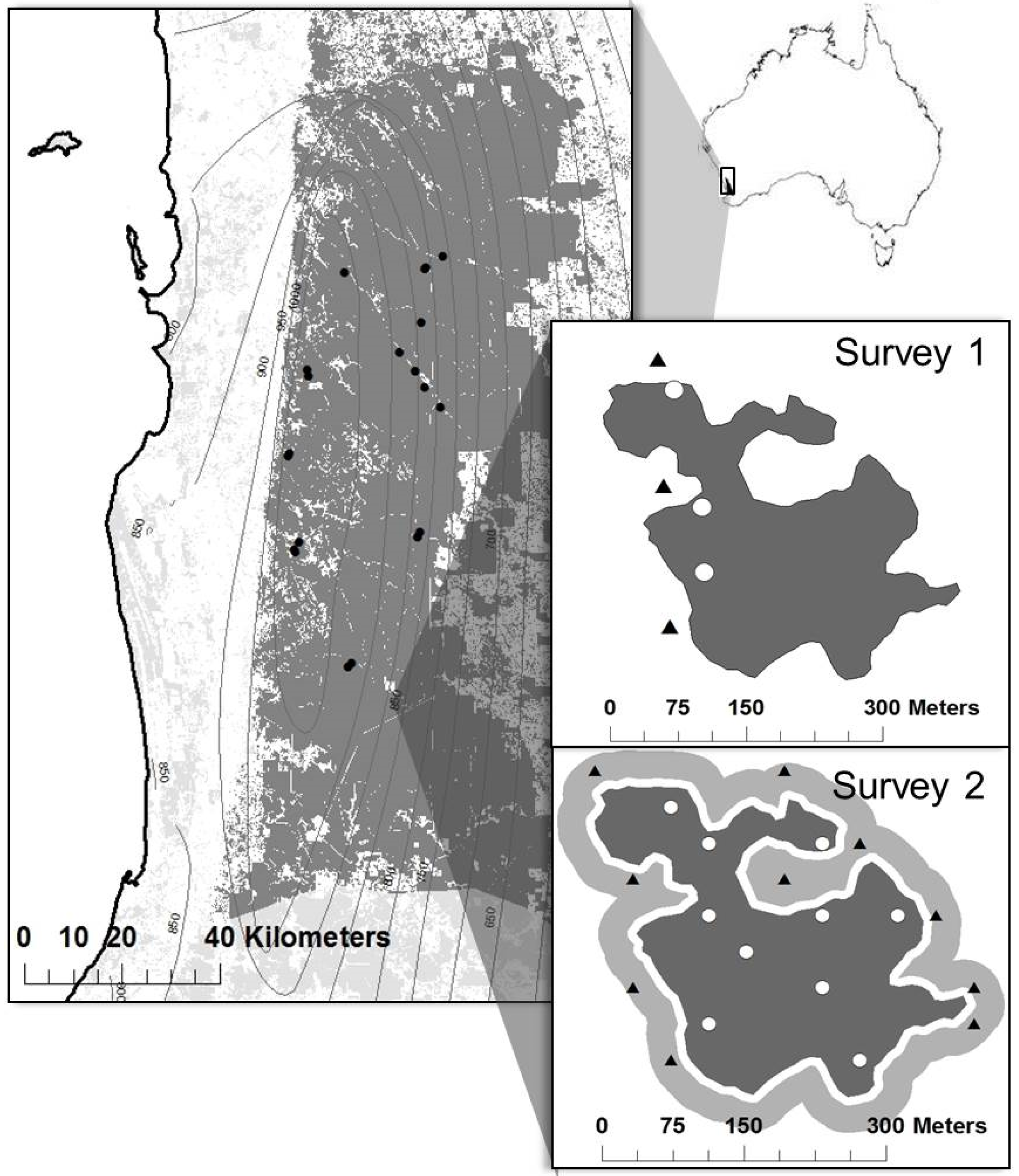
2.3. Damage and Tissue Moisture
2.4. Infestation Levels
2.5. Data Analysis
2.5.1. Damage and Tissue Moisture
2.5.2. Infestation Levels
3. Results
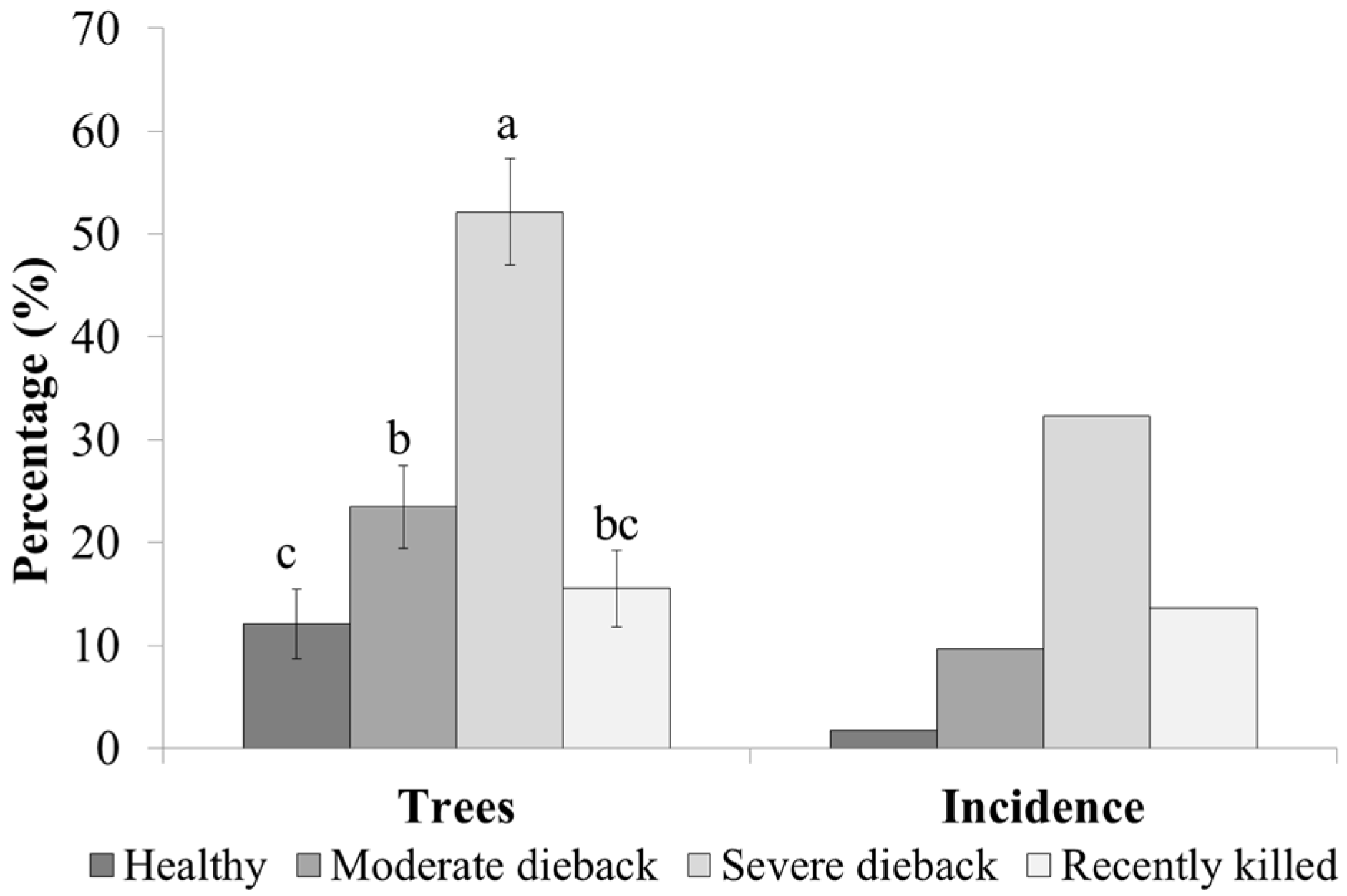
3.1. Damage and Tissue Moisture
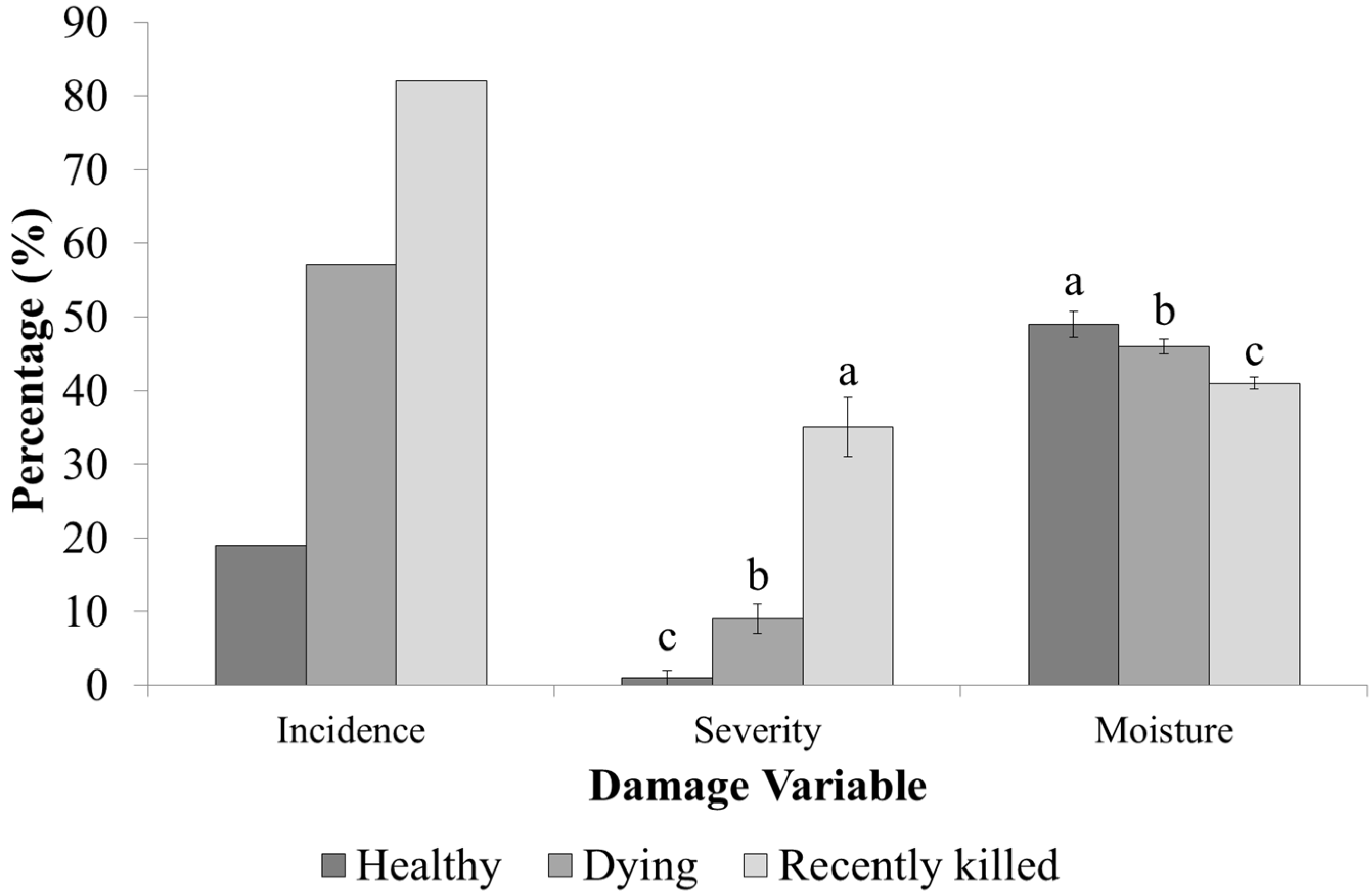
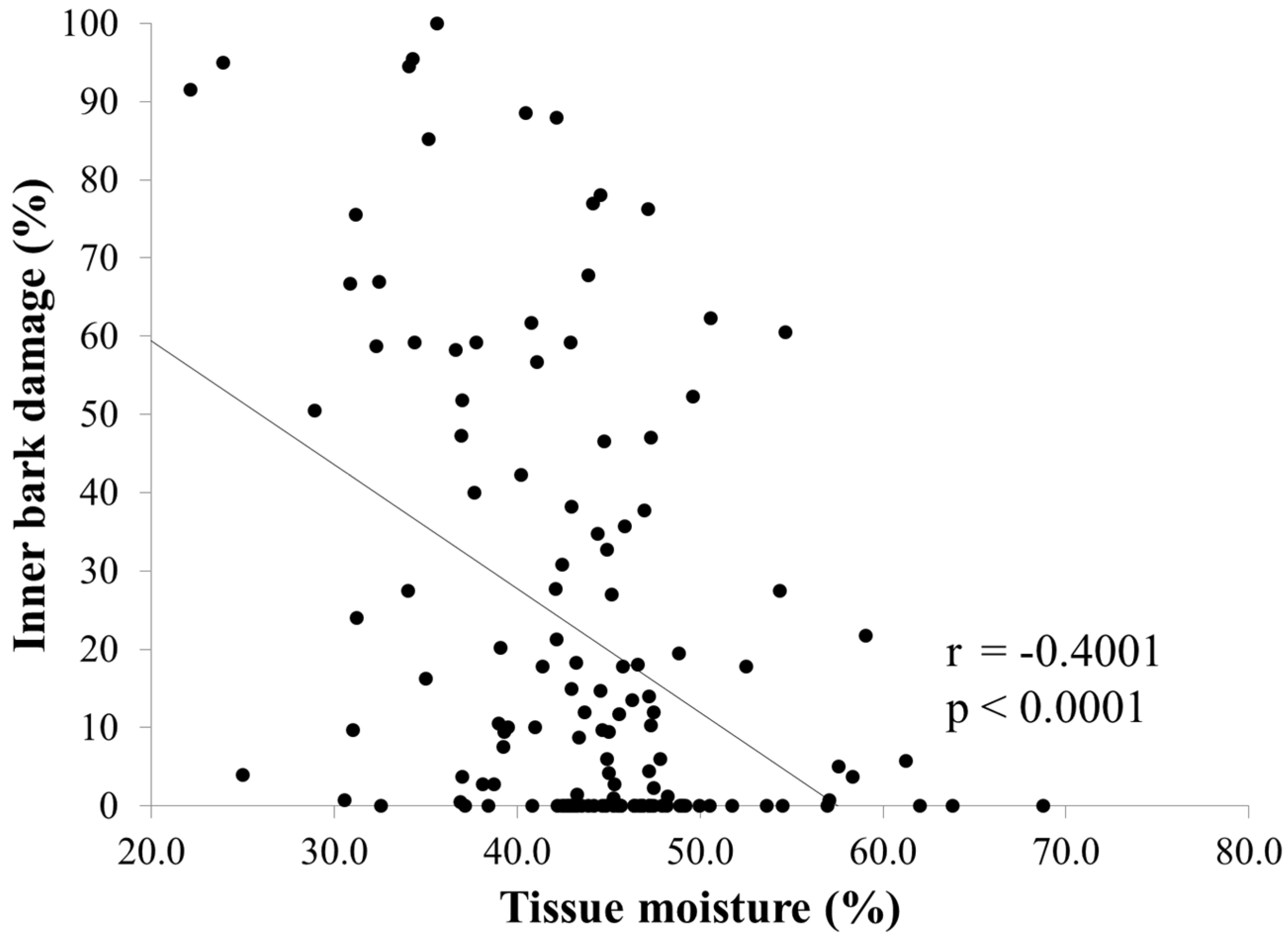
3.2. Infestation Levels
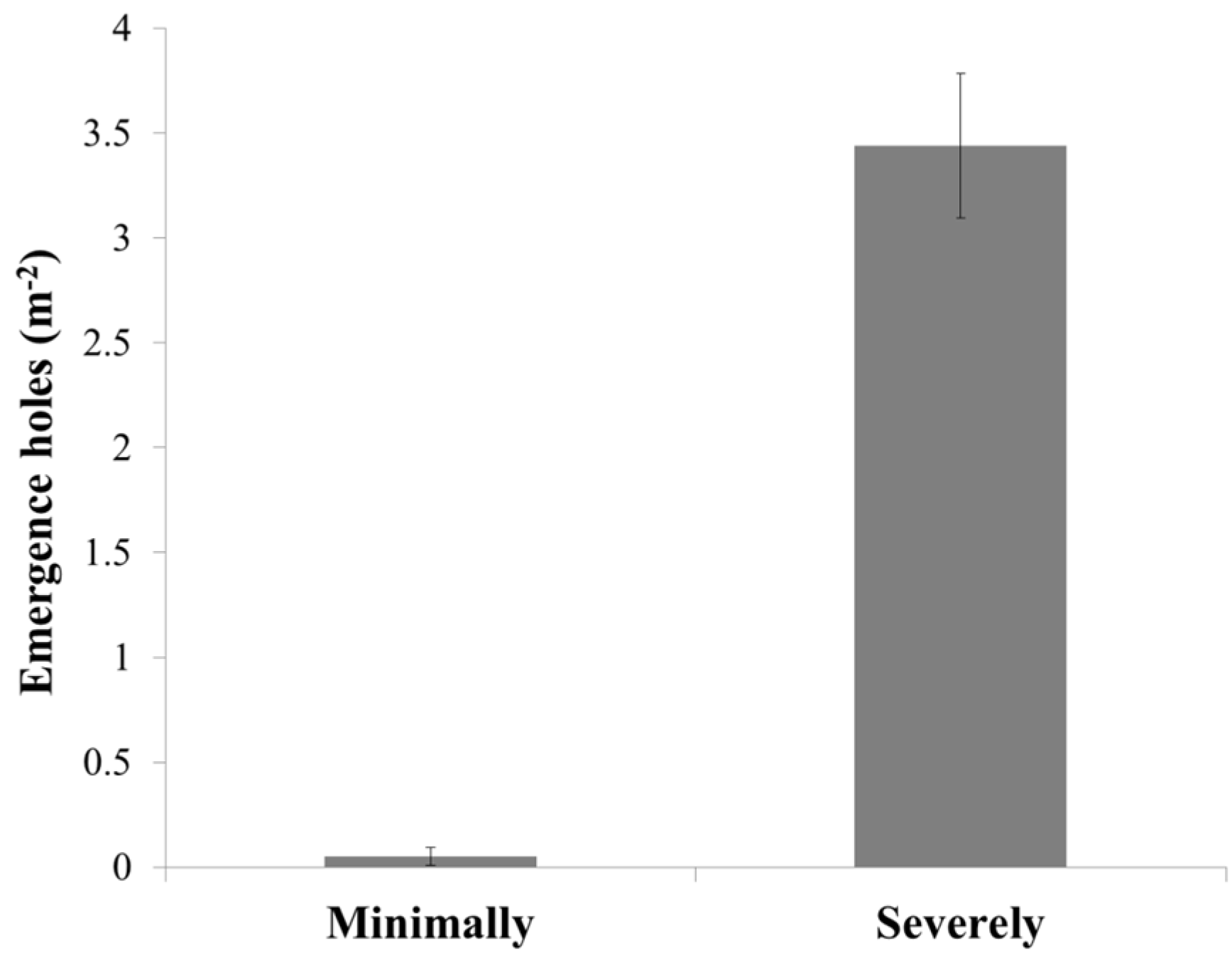

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Negrón, J.F.; McMillin, J.D.; Anhold, J.A.; Coulson, D. Bark beetle-caused mortality in a drought-affected ponderosa pine landscape in Arizona, USA. For. Ecol. Manag. 2009, 257, 1353–1362. [Google Scholar] [CrossRef]
- Hart, C.J. Drought induces spruce beetle outbreaks across northwestern Colorado. Integr. Pest Manag. Rev. 2014, 6, 247–252. [Google Scholar]
- Knight, S.K.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014. [Google Scholar]
- Bentz, B.J.; Régnière, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negrón, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. BioScience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, L11706. [Google Scholar] [CrossRef]
- Bates, B.C.; Hope, P.; Ryan, B.; Smith, I.; Charles, S. Key findings from the indian ocean climate initiative and their impact on policy development in Australia. Clim. Chang. 2008, 89, 339–354. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–219. [Google Scholar]
- Coulson, R.N.; Stephen, F.M. Impacts of insects in forest landscapes-implications for forest health management. In Invasive Forest Insects, Introduced Forest Trees, and Altered Ecosystems; Paine, T.D., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 101–125. [Google Scholar]
- Hebertson, E.G.; Jenkins, M.J. Climate factors associated with historic spruce beetle (coleoptera: Curculionidae) outbreaks in Utah and Colorado. Environ. Entomol. 2008, 37, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Boucher, T.V.; Mead, B.R. Vegetation change and forest regeneration on the Kenai Peninsula, Alaska following a spruce beetle outbreak, 1987–2000. For. Ecol. Manag. 2006, 227, 233–246. [Google Scholar] [CrossRef]
- Flower, C.E.; Gonzalez-Meler, M.A. Responses of temperate forest productivity to insect and pathogen disturbances. Annu. Rev. Plant Biol. 2015, 66, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Stephen, F.M.; Salisbury, V.B.; Oliveira, F.L. Red oak borer, enaphalodes rufulus (coleoptera: Cerambycidae), in the Ozark mountains of Arkansas, USA: An unexpected and remarkable forest disturbance. Integr. Pest Manag. Rev. 2001, 6, 247–252. [Google Scholar] [CrossRef]
- Hanks, L.M.; Paine, T.D.; Millar, J.G.; Campbell, C.D.; Schuch, U.K. Water relations of host trees and resistance to the phloem-boring beetle Phoracantha semipunctata F. (Coleoptera: Cerambycidae). Oecologia 1999, 119, 400–407. [Google Scholar] [CrossRef]
- Duffy, E.A.J. A Monograph of the Immature Stages of Australasian Timber Beetles (Cerambycidae); British Museum (Natural History), Ed.; Trustees of the British Museum: London, UK, 1963; p. 235. [Google Scholar]
- Hanks, L.M.; McElfresh, J.S.; Millar, J.G.; Paine, T.D. Phoracantha semipunctata (coleoptera: Cerambycidae), a serious pest of eucalytpus in California: Biology and laboratory-rearing procedures. Ann. Entomol. Soc. Am. 1993, 86, 95–102. [Google Scholar] [CrossRef]
- Scriven, G.T.; Reeves, E.L.; Luck, R.F. Beetle from Australia threatens eucalyptus. Calif. Agric. 1986, 40, 4–6. [Google Scholar]
- Wang, Q. A taxonomic revision of the Australian genus phoracantha newman (coleoptera: Cerambycidae). Invertebr. Taxon. 1995, 9, 865–958. [Google Scholar] [CrossRef]
- Curry, S.J. The association of insects with eucalypt dieback in south western Australia. In Eucalypt Dieback in Forests and Woodlands; Old, K.M., Kile, G.A., Eds.; CSIRO: Melbourne, Australia, 1981; pp. 130–133. [Google Scholar]
- Clarke, J. The marri borer (tryphocaria hamata). J. Agric. West. Aust. 1925, 2, 513–517. [Google Scholar]
- Hooper, R.J.; Sivasithamparam, K. Characterization of damage and biotic factors associated with the decline of eucalyptus wandoo in southwest western Australia. Can. J. For. Res. 2005, 35, 2589–2602. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.; Hardy, G. Drought and heat triggers sudden and severe dieback in a dominant Mediterranean-type woodland species. Open J. For. 2012, 2, 183–168. [Google Scholar] [CrossRef]
- Hooper, R.J.; Wills, A.; Shearer, B.L.; Sivasithamparam, K. A redescription and notes on biology of Cisseis fascigera obenberger (Coleoptera: Buprestidae) on declining eucalyptus wandoo in south-western Australia. Aust. J. Entomol. 2010, 49, 234–244. [Google Scholar] [CrossRef]
- Matusick, G.; Ruthrof, K.X.; Brouwers, N.C.; Dell, B.; Hardy, G.S.J. Sudden forest canopy collapse corresponding with extreme drought and heat in a Mediterranean-type eucalypt forest in southwestern Australia. Eur. J. For. Res. 2013, 132, 497–510. [Google Scholar] [CrossRef]
- Hanks, L.M.; Paine, T.D.; Millar, J.G. Influence of the larval environment on performance and adult body size of the wood-boring beetle Phoracantha semipunctata. Entomol. Exp. Appl. 2005, 114, 25–34. [Google Scholar] [CrossRef]
- Gentilli, J. Climate of the jarrah forest. In The Jarrah Forest: A Complex Mediterranean Ecosystem; Dell, B., Havel, J., Eds.; Kluwer Academic Publ: Dordrecht, The Netherlands, 1989; pp. 23–40. [Google Scholar]
- Marchant, N.G. Species diversity in the southwestern flora. J. R. Soc. West. Aust. 1973, 56, 23. [Google Scholar]
- McArthur, W.M.; Churchwood, H.M.; Hick, P.T. Landform and Soils of the Murray River Catchment Area of Western Australia, 3rd ed.; Management, D.O.L.R., Ed.; Commonwealth Scientific and Industrial Research Organization, Division of Land Resources Management: Melbourne, Australia, 1977; pp. 1–23. [Google Scholar]
- Brouwers, N.; Matusick, G.; Ruthrof, K.; Lyons, T.; Hardy, G. Landscape-scale assessment of tree crown dieback following extreme drought and heat in a Mediterranean eucalypt forest ecosystem. Land. Ecol. 2013, 28, 69–80. [Google Scholar] [CrossRef]
- Paine, T.D.; Dreistadt, S.H.; Millar, J.G. Eucalyptus Longhorned Borers; UC Statewide Integrated Pest Management Program; University of California: Davis, CA, USA, 2009. [Google Scholar]
- Mitchell, K. Quantitative Analysis by the Point-Centered Quarter Method; Department of Mathematics and Computer Science: Geneva, Switzerland, 2007; pp. 1–37. [Google Scholar]
- Flower, C.E.; Knight, K.S.; Rebbeck, J.; Gonzalez-Meler, M.A. The relationship between emerald ash borer (agrilus planipennis fairmaire) and ash (fraxinus spp.) tree decline: Using visual ash condition assessments and leaf isotope measurements to assess pest damage. For. Ecol. Manag. 2013, 303, 143–147. [Google Scholar] [CrossRef]
- Worrall, J.J.; Egeland, L.; Eager, T.; Mask, R.A.; Johnson, E.W.; Kemp, P.A.; Shepperd, W.D. Rapid mortality of populus tremuloides in southwestern Colorado, USA. For. Ecol. Manag. 2008, 255, 686–696. [Google Scholar] [CrossRef]
- Hanks, L.M.; Paine, T.D.; Millar, J.G. Mechanisms of resistance in eucalyptus against larvae of the eucalyptus longhorned borer (coleoptera: Cerambycidae). Environ. Entomol. 1991, 20, 1583–1588. [Google Scholar] [CrossRef]
- Fierke, M.K.; Kelley, M.B.; Stephen, F.M. Site and stand variables influencing red oak borer, enaphalodes rufulus (coleoptera: Cerambycidae), population densities and tree mortality. For. Ecol. Manag. 2007, 247, 227–236. [Google Scholar] [CrossRef]
- Timms, L.L.; Smith, S.M.; de Groot, P. Patterns in the within-tree distribution of the emerald ash borer (agrilus planipennis fairmaire) in young, green-ash plantations of south-western Ontario, Canada. Agric. For. Entomol. 2008, 8, 313–321. [Google Scholar] [CrossRef]
- Abott, I.; Loneragan, O. Ecology of Jarrah (Eucalyptus Marginata) in the Northern Jarrah Forest of Western Australia; Department of Conservation and Land Management: Perth, Australia, 1986. [Google Scholar]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Salle, A.; Nageleisen, L.-M.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Koricheva, J.; Larsson, S.; Haukioja, E. Insect performance on experimentally stressed woody plants: A meta-analysis. Ann. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynmaics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Fan, Z.; Kabrick, J.M.; Spetich, M.A.; Shifley, S.R.; Jensen, R.G. Oak mortality associated with crown dieback and oak borer attack in the ozark highlands. For. Ecol. Manag. 2008, 255, 2297–2305. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seaton, S.; Matusick, G.; Ruthrof, K.X.; Hardy, G.E.S.J. Outbreak of Phoracantha semipunctata in Response to Severe Drought in a Mediterranean Eucalyptus Forest. Forests 2015, 6, 3868-3881. https://doi.org/10.3390/f6113868
Seaton S, Matusick G, Ruthrof KX, Hardy GESJ. Outbreak of Phoracantha semipunctata in Response to Severe Drought in a Mediterranean Eucalyptus Forest. Forests. 2015; 6(11):3868-3881. https://doi.org/10.3390/f6113868
Chicago/Turabian StyleSeaton, Stephen, George Matusick, Katinka X. Ruthrof, and Giles E. St. J. Hardy. 2015. "Outbreak of Phoracantha semipunctata in Response to Severe Drought in a Mediterranean Eucalyptus Forest" Forests 6, no. 11: 3868-3881. https://doi.org/10.3390/f6113868





