Effects of Visual Grading on Northern Red Oak (Quercus rubra L.) Seedlings Planted in Two Shelterwood Stands on the Cumberland Plateau of Tennessee, USA
Abstract
:1. Introduction
- Visual grading would result in two distinct size classes that differed in height, root-collar diameter (RCD), and number of roots, when compared to the overall nursery population;
- The larger seedling grade will exhibit the highest survival, greatest growth, and have the best competitive ability seven-years after planting in a regeneration harvest;
- Seedling grade will have minimal effects on growth or survival in the mid-story removal stand;
- Planting shock, identified by stem dieback and mortality, will be more pronounced for the larger seedling grade and in the first year after out-planting.
2. Methods
2.1. Study Areas
2.1.1. Midstory-Removal Stand
2.1.2. Shelterwood Harvest
2.2. Experimental Material and Treatment Design
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Grading Effects on Seedling Quality
| Height (cm) | RCD (mm) | No. of FOLR | ||||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | |
| Premium | 121 (2) a | 42–176 | 11.5 (0.1) a | 5.8–16.7 | 16 (0.3) a | 4–30 |
| Standard | 101 (2) b | 25–167 | 9.6 (0.1) b | 5.8–17.6 | 15 (0.3) b | 4–29 |
| Sample | 79 (2) c | 15–162 | 8.5 (0.1) c | 3.2–13.7 | 12 (0.5) c | 1–30 |
| Premium | Standard | Sample | |
|---|---|---|---|
| Height-RCD | 0.28 (<0.0001) | 0.55 (<0.0001) | 0.83 (<0.0001) |
| RCD-FOLR | 0.32 (<0.0001) | 0.59 (<0.0001) | 0.77 (<0.0001) |
| Height-FOLR | 0.05 (0.4223) | 0.39 (<0.0001) | 0.62 (<0.0001) |
3.2. Seedling Grade Effects on Survival, Growth, and Competitive Ability
3.2.1. Midstory-Removal Stand
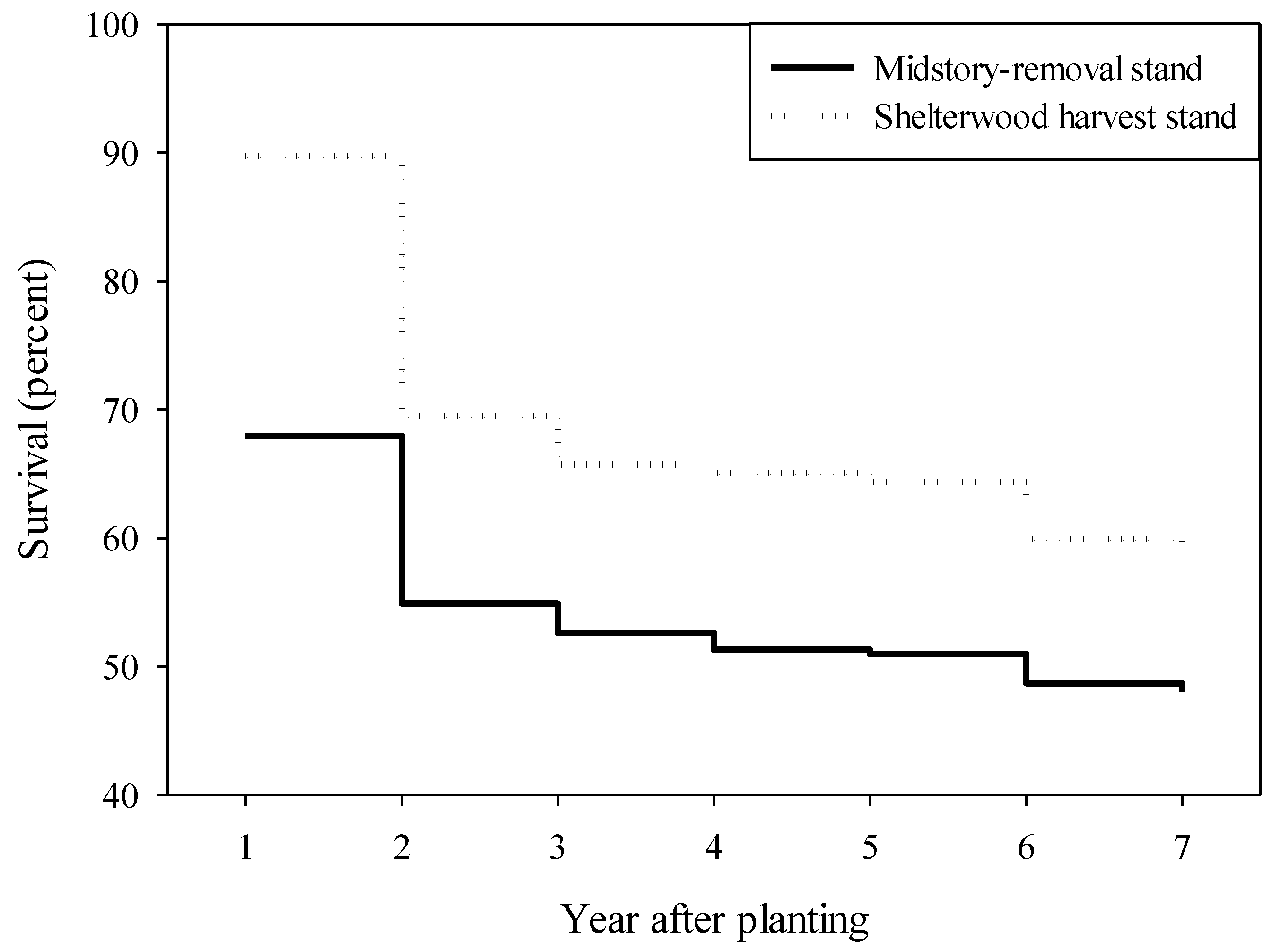
| Seedling Grade | Year Since Planting | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DDF | F | p | DDF | F | P | DDF | F | p | ||
| Midstory removal | ||||||||||
| Height a | 317 | 5.0 | 0.0267 | 1085 | 6.9 | <0.0001 | 1085 | 1.8 | 0.0937 | |
| BD a | 359 | 22.6 | <0.0001 | 931 | 28.5 | <0.0001 | 931 | 0.3 | 0.9638 | |
| Stem dieback | 212 | 3.2 | 0.0738 | 920 | 20.6 | <0.0001 | 920 | 0.8 | 0.6079 | |
| Shelterwood Harvest | ||||||||||
| Height a | 264 | 18.5 | <0.0001 | 917 | 74.3 | <0.0001 | 917 | 1.3 | 0.2403 | |
| BD a | 329 | 12.6 | 0.0004 | 852 | 197.7 | <0.0001 | 852 | 0.7 | 0.6671 | |
| Stem dieback | 235 | 0.1 | 0.7794 | 1078 | 10.4 | <0.0001 | 1078 | 1.1 | 0.3808 | |
| Understory dominance | 230 | 2.7 | 0.1043 | 348 | 13.5 | <0.0001 | 348 | 0.7 | 0.6225 | |
| FTG in understory | 235 | 0.0 | 0.9998 | 716 | 9.1 | <0.0001 | 716 | 1.0 | 0.4283 | |
| FTG in midstory, year 3 | 183 | 0.6 | 0.4554 | -- | -- | -- | -- | -- | -- | |
| FTG in midstory, year 5 | 182 | 0.0 | 0.8743 | -- | -- | -- | -- | -- | -- | |
| FTG in midstory, year 7 | 172 | 0.1 | 0.2493 | -- | -- | -- | -- | -- | -- | |
| Premium | Standard | |||||||
|---|---|---|---|---|---|---|---|---|
| Year Since Planting | Mean | Std. error | Range | Mean | Std. error | Range | ||
| Midstory removal | ||||||||
| Height (cm) | ||||||||
| 0 | 118 | 2.7 | a | 57–182 | 102 | 2.2 | e | 40–152 |
| 1 | 113 | 2.9 | b | 11–166 | 101 | 2.4 | e | 10–142 |
| 2 | 112 | 3.2 | bc | 43–157 | 103 | 2.6 | de | 30–144 |
| 3 | 111 | 3.4 | abd | 35–166 | 103 | 2.7 | de | 35–155 |
| 4 | 100 | 3.5 | ef | 13–162 | 95 | 2.9 | f | 23–165 |
| 5 | 105 | 3.6 | ce | 19–168 | 102 | 2.9 | e | 29–172 |
| 6 | 104 | 3.7 | cdef | 18–181 | 100 | 3.0 | ef | 28–187 |
| 7 | 102 | 3.7 | ef | 21–173 | 102 | 3.1 | de | 33–186 |
| BD (mm) | ||||||||
| 0 | 11.0 | 0.20 | e | 7.1–18.2 | 9.9 | 0.20 | fg | 6.0–18.0 |
| 1 | 10.8 | 0.21 | de | 1.9–19.4 | 9.8 | 0.21 | fg | 1.9–16.9 |
| 2 | 9.7 | 0.14 | g | 7.7–12.3 | 8.6 | 0.14 | h | 5.9–12.2 |
| 3 | 10.4 | 0.25 | ef | 7.5–17.5 | 9.4 | 0.25 | g | 6.1–21.0 |
| 4 | 10.8 | 0.26 | d | 2.1–17.7 | 9.7 | 0.26 | fg | 3.6–16.0 |
| 5 | 11.0 | 0.26 | d | 3.0–17.5 | 9.9 | 0.26 | fg | 3.6–14.6 |
| 6 | 12.9 | 0.27 | b | 2.8–20.4 | 11.8 | 0.27 | c | 4.3–18.0 |
| 7 | 13.4 | 0.27 | a | 2.9–23.2 | 12.1 | 0.27 | c | 7.3–19.1 |
| Shelterwood Harvest | ||||||||
| Height (cm) | ||||||||
| 0 | 123 | 3.1 | f | 45–168 | 100 | 3.1 | gh | 46–180 |
| 1 | 119 | 3.3 | f | 5–174 | 100 | 3.3 | h | 4–174 |
| 2 | 124 | 3.5 | f | 20–172 | 104 | 3.4 | gh | 12–179 |
| 3 | 126 | 3.7 | f | 14–233 | 108 | 3.6 | g | 21–237 |
| 4 | 147 | 3.8 | e | 17–298 | 126 | 3.7 | f | 20–281 |
| 5 | 187 | 10.2 | cd | 7–397 | 159 | 9.8 | e | 7–419 |
| 6 | 211 | 10.3 | b | 11–451 | 181 | 9.9 | d | 18–473 |
| 7 | 241 | 10.3 | a | 17–526 | 201 | 9.9 | bc | 16–495 |
| BD (mm) | ||||||||
| 0 | 11.1 | 0.32 | hi | 6.8–19.3 | 9.8 | 0.32 | jk | 5.5–16.2 |
| 1 | 11.6 | 0.34 | h | 1.9–19.1 | 10.3 | 0.34 | i | 2.5–17.8 |
| 2 | 10.7 | 0.36 | ij | 2.3–17.4 | 9.5 | 0.35 | k | 2.0–15.2 |
| 3 | 15.0 | 0.37 | f | 2.5–27.6 | 13.6 | 0.37 | g | 4.2–25.4 |
| 4 | 17.7 | 0.38 | e | 4.0–37.1 | 16.0 | 0.38 | f | 4.2–32.2 |
| 5 | 22.4 | 0.99 | cd | 5.1–47.4 | 19.7 | 0.95 | d | 3.9–45.8 |
| 6 | 25.7 | 1.00 | b | 5.4–54.0 | 22.2 | 0.96 | c | 3.6–48.3 |
| 7 | 29.5 | 1.00 | a | 7.6–63.7 | 25.9 | 0.96 | b | 7.8–54.2 |
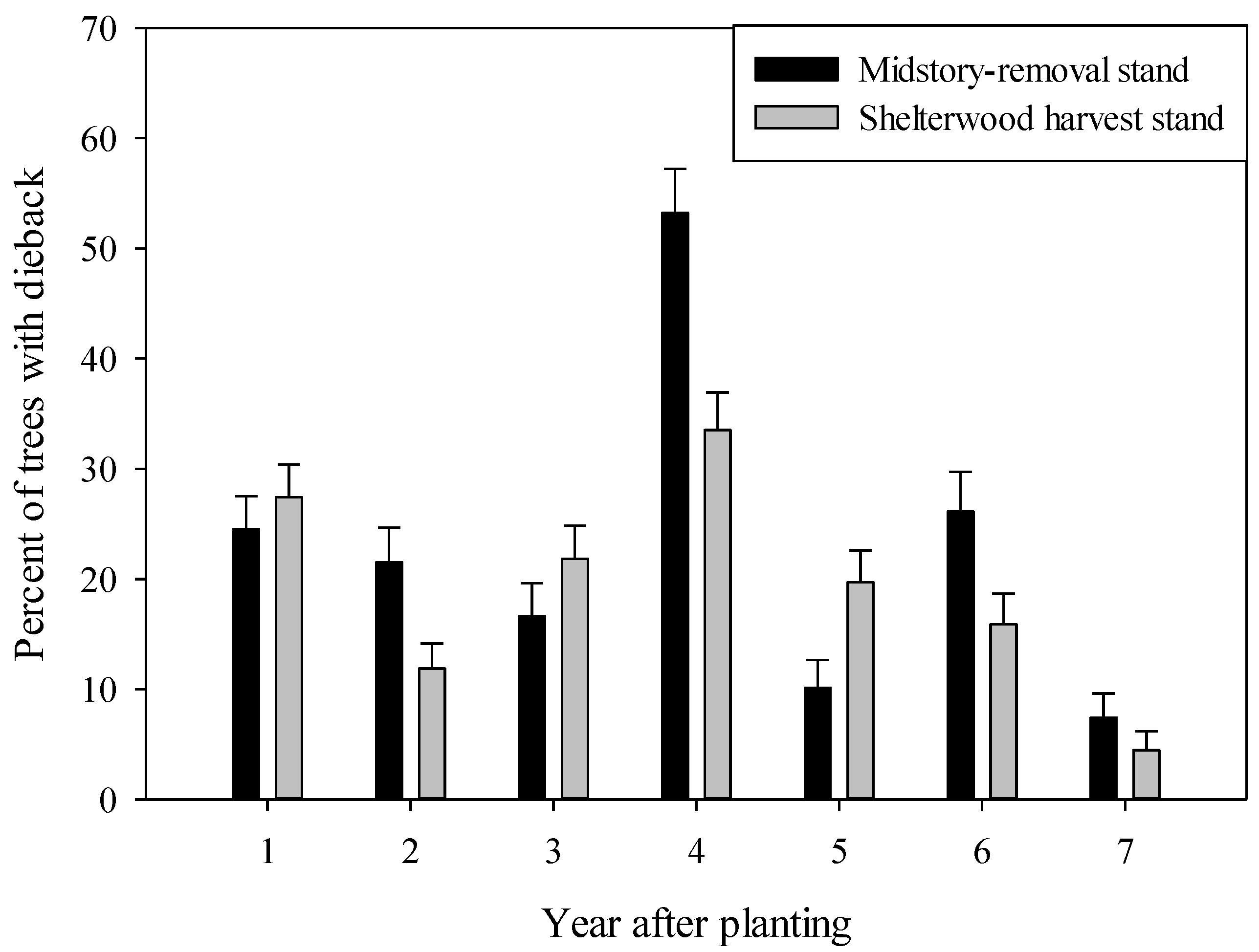
3.2.2. Shelterwood Harvest
| Year | Understory Dominance | Free-to-Grow in Understory | ||||
|---|---|---|---|---|---|---|
| 1 | 53 | 4.2 | bc | 78 | 2.8 | ab |
| 2 | 46 | 5.0 | c | 69 | 3.2 | bc |
| 3 | 38 | 5.1 | c | 65 | 3.5 | c |
| 5 | 78 | 4.2 | a | 83 | 2.7 | a |
| 7 | 67 | 3.6 | ab | 72 | 3.4 | bc |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abrams, M.D. Fire and the development of oak forests. BioSci. 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Dyer, J.M. Revisiting the deciduous forests of eastern North America. BioSci. 2006, 56, 341–352. [Google Scholar] [CrossRef]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Loftis, D.L. Predicting post-harvest performance of advance red oak reproduction in the southern Appalachians. For. Sci. 1990, 36, 908–916. [Google Scholar]
- Iverson, L.R.; Hutchinson, T.F.; Prasad, A.M.; Peters, M.P. Thinning, fire, and oak regeneration across a heterogeneous landscape in eastern US: 7-year results. For. Ecol. Manag. 2008, 255, 3035–3050. [Google Scholar] [CrossRef]
- McShea, W.J.; Healy, W.M. Oaks and acorns as a Foundation for ecosystem management. In Oak Forest Ecosystems: Ecology and Management for Wildlife; The Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 1–12. [Google Scholar]
- Oswalt, C.M.; Turner, J.A. Status of Hardwood Forest Resources in the Appalachian Region Including Estimates of Growth and Removals; Southern Research Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 2009; Resource Bulletin 142; p. 16.
- Oswalt, C.M.; Oswalt, S.N.; Johnson, T.G.; Brandeis, C.; Randolph, K.C.; King, C.R. Tennessee’s Forests, 2009; Southern Research Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 2012; Resource Bulletin 189; p. 136.
- Major, K.C.; Nosko, P.; Kuehne, C.; Campbell, D.; Bauhus, J. Regeneration dynamics of non-native northern red oak (Quercus rubra L.) populations as influenced by environmental factors: A case study in managed hardwood forests of southwestern Germany. For. Ecol. Manag. 2013, 291, 144–153. [Google Scholar] [CrossRef]
- Loftis, D.L. Preharvest herbicide control of undesirable vegetation in Southern Appalachian hardwoods. South. J. Appl. For. 1978, 2, 51–54. [Google Scholar]
- Arthur, M.A.; Alexander, H.D.; Dey, D.C.; Schweitzer, C.J.; Loftis, D.L. Refining the oak-fire hypothesis for management of oak-dominated forests of the eastern United States. J. For. 2012, 110, 257–266. [Google Scholar] [CrossRef]
- Brose, P.H.; Dey, D.C.; Phillips, R.J.; Waldrop, T.A. A Meta-Analysis of the Fire-Oak Hypothesis: Does Prescribed Burning Promote Oak Reproduction in Eastern North America? For. Sci. 2013, 59, 322–334. [Google Scholar] [CrossRef]
- Loftis, D.L. A shelterwood method for regenerating red oak in the Southern Appalachians. For. Sci. 1990, 36, 917–929. [Google Scholar]
- Parrott, D.L.; Lhotka, J.M.; Stringer, J.W.; Dillaway, D.N. Seven-Year Effects of Midstory Removal on Natural and Underplanted Oak Reproduction. North J. Appl. For. 2012, 29, 182–189. [Google Scholar] [CrossRef]
- Clark, S.L.; Schlarbaum, S.E.; Kormanik, P.P. Visual grading and quality of 1–0 northern red oak seedlings. South. J. Appl. For. 2000, 24, 93–97. [Google Scholar]
- Kormanik, P.P.; Sung, S.S.; Kormanik, T.L. Toward a single nursery protocol for oak seedlings. In Proceedings of the 22nd Southern Forest Tree Improvement Conference, Atlanta, GA, USA, 14–17 June 1993; Lantz, C.W., Moorhead, D., Eds.; Southern Forest Tree Improvement Committee: Springfield, VA, USA, 1994; pp. 89–98. [Google Scholar]
- Pope, P.E. A historical perspective of planting and seeding oaks: Progress, problems, and status. In Proceedings of the Oak Regeneration: Serious Problems, Practical Recommendations; Knoxville, TN, USA, 8–10 September 1992, Loftis, D.L., McGee, C.E., Eds.; Southeastern Forest Experiment Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 1993; pp. 224–239. [Google Scholar]
- Dey, D.C.; Gardiner, E.S.; Schweitzer, C.J.; Kabrick, J.M.; Jacobs, D.F. Underplanting to sustain future stocking of oak (Quercus) in temperate deciduous forests. New For. 2012, 43, 955–978. [Google Scholar] [CrossRef]
- Spetich, M.A.; Dey, D.C.; Johnson, P.S.; Graney, D.L. Competitive capacity of Quercus rubra L. planted in Arkansas’ Boston Mountains. For. Sci. 2002, 48, 504–517. [Google Scholar]
- Schuler, J.L.; Robison, D.J. Performance of Northern Red Oak enrichment plantings in naturally regenerating Southern Appalachian hardwood stands. New For. 2010, 40, 119–130. [Google Scholar] [CrossRef]
- Morrissey, R.C.; Jacobs, D.F.; Davis, A.S.; Rathfon, R.A. Survival and competitiveness of Quercus rubra regeneration associated with planting stocktype and harvest opening intensity. New For. 2010, 40, 273–287. [Google Scholar] [CrossRef]
- Buckley, D.S.; Sharik, T.L.; Isebrands, J.G. Regeneration of northern red oak: Positive and negative effects of competitor removal. Ecology 1998, 79, 65–78. [Google Scholar] [CrossRef]
- Dey, D.C.; Jacobs, D.F.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial regeneration of major oak (Quercus) species in the eastern United States—A review of the literature. For. Sci. 2008, 54, 77–106. [Google Scholar]
- Struve, D.K. Root regeneration in transplanted deciduous nursery stock. HortSci. 1990, 25, 266–270. [Google Scholar]
- Thompson, J.R.; Schultz, R.C. Root system morphology of Quercus rubra L. planting stock and 3-year field performance in Iowa. New For. 1995, 9, 225–236. [Google Scholar] [CrossRef]
- Struve, D.K.; Burchfield, L.; Maupin, C. Survival and growth of transplanted large- and small-caliper red oaks. J. Arbor. 2000, 26, 162–169. [Google Scholar]
- Wilson, E.R.; Vitols, K.C.; Park, A. Root characteristics and growth potential of container and bare-root seedlings of red oak (Quercus rubra L.) in Ontario, Canada. New For. 2007, 34, 163–176. [Google Scholar] [CrossRef]
- Waston, W.T. Influence of tree size on transplant establishment and growth. Horttech. 2005, 15, 118–122. [Google Scholar]
- Johnson, P.S.; Dale, C.D.; Davidson, K.R. Planting northern red oak in the Missouri Ozarks: A prescription. North. J. Appl. For. 1986, 3, 66–68. [Google Scholar]
- Weigel, D.R.; Johnson, P.S. Planting Northern Red Oak in the Ozark Highlands: A Shelterwood Prescription; North Central Forest Experiment Station, Forest Service, US Department of Agriculture: St. Paul, MN, USA, 1998.
- Schlarbaum, S.E.; Kormanik, P.P.; Tibbs, T.; Barber, L.R. Oak seedlings: Quality improved available now, genetically improved available soon. In Proceedings of the 25th Annual Hardwood Symposium, Cashiers, NC, USA, 7–10 May 1997; Myers, D.A., Ed.; National Hardwood Lumber Association: Memphis, TN, USA, 1997; pp. 123–128. [Google Scholar]
- Kormanik, P.P.; Sung, S.S.; Zarnoch, S.J.; Tibbs, T.G. Artificial regeneration of northern red oak and white oak on high-quality sites: Effects of root morphology and relevant biological characteristics. In Beyond 2001: A Silvicultural Odyssey to Sustaining Terrestrial and Aquatic Ecosystems, Proceedings of the 2001 National Silvicultural Workshop; Pacific Northwest Research Station, Forest Service, US Department of Agriculture: Portland, OR, USA, 2002; pp. 83–91. [Google Scholar]
- Kormanik, P.P.; Sung, S.S.; Kass, D.; Zarnoch, S.J. Effect of Seedling size and First-Order Lateral Roots on Early Development of Northern Red Oak on a Mesic Site: Eleventh Year Results; Southern Research Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 2002; pp. 332–337.
- Oswalt, C.M.; Clatterbuck, W.K.; Houston, A.E. Impacts of deer herbivory and visual grading on the early performance of high-quality oak planting stock in Tennessee, USA. For. Ecol. Manag. 2006, 229, 128–135. [Google Scholar] [CrossRef]
- Clark, S.L.; Schlarbaum, S.E.; Keyser, T.L.; Schweitzer, C.J.; Spetich, M.A.; Simon, D.; Warburton, G.S. Response of planted northern red oak seedlings to regeneration harvesting, Midstory removal, and prescribed burning. In Proceedings of the 18th Biennial Southern Silvicultural Research Conference; Knoxville, TN, USA, 3–5 March 2015, Southern Research Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 2015. (in press) [Google Scholar]
- Smalley, G.W. Classification and Evaluation of Forest Sites in the Cumberland Mountains; Southern Forest Experiment Station, Forest Service, US Department of Agriculture: New Orleans, LA, USA, 1984; p. 84.
- Braun, E.L. Deciduous Forests of Eastern North America; The Blakiston Co.: Philadelphia, PA, USA, 1950; p. 596. [Google Scholar]
- Druckenbrod, D.L.; Dale, V.H.; Olsen, L.M. Comparing current and desired ecological conditions at a landscape scale in the Cumberland Plateau and Mountains, USA. J. Land Use Sci. 2006, 1, 169–189. [Google Scholar] [CrossRef]
- Clatterbuck, W.K.; Smalley, G.W.; Turner, J.A.; Travis, A. Natural History and Land Use History of Cumberland Plateau Forests in Tennessee; National Council for Air and Stream Improvement Inc.: Cary, NC, USA, 2006; Special Report No. 06–01; pp. 1–37. [Google Scholar]
- Gribko, L.S.; Jones, W.E. Test of the float method of assessing northern red oak acorn condition. Tree Planters’ Notes 1995, 46, 143–147. [Google Scholar]
- Sander, I.L.; Johnson, P.S.; Rogers, R. Evaluating Oak Advance Reproduction in the Missouri Ozarks; North Central Forest Experiment Station, Forest Service, US Department of Agriculture: St. Paul, MN, USA, 1984; p. 12.
- Johnson, P.S.; Rogers, R. A method for estimating the contribution of planted hardwoods to future stocking. For. Sci. 1985, 31, 883–891. [Google Scholar]
- SAS Institute. SAS/STAT 12.3 User’s Guide; SAS Institute: Cary, NC, USA, 2012. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.S. Generalized linear mixed models: A practical quide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Stroup, W.W. Rethinking the Analysis of Non-Normal Data in Plant and Soil Science. Agron. J. 2014, 106, 1–17. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [PubMed]
- DAWG. Design and Analysis Web Guide. Available online: http://dawg.utk.edu/ (accessed on 23 June 2013).
- Dey, D.C.; Parker, W.C. Morphological indicators of stock quality and field performance of red oak (Quercus rubra L.) seedlings underplanted in a central Ontario shelterwood. New For. 1997, 14, 145–156. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Salifu, K.F.; Seifert, J.R. Relative contribution of initial root and shoot morphology in predicting field performance of hardwood seedlings. New For. 2005, 30, 235–251. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Zaczek, J.J.; Steiner, K.C.; Bowersox, T.W. Performance of northern red oak planting stock. North. J. Appl. For. 1993, 10, 105–111. [Google Scholar]
- Ward, J.S. Influence of Initial Seedling Size and Browse Protection on Height Growth: 5 Year Results; Pacific Northwest Research Station, Forest Service, US Department of Agriculture: Portland, OR, USA, 1996; pp. 127–134.
- Kormanik, P.P.; Sung, S.J.S.; Kormanik, T.L.; Schlarbaum, S.E.; Zarnoch, S.J. Effect of acorn size on development of northern red oak 1–0 seedlings. Can. J. For. Res. 1998, 28, 1805–1813. [Google Scholar] [CrossRef]
- Gordon, A.M.; Simpson, J.A.; Williams, P.A. Six-year response of red oak seedlings planted under a shelterwood in central Ontario. Can. J. For. Res. 1995, 25, 603–613. [Google Scholar] [CrossRef]
- United States Drought Monitor. Tabular Data Archive: Campbell County, TN, USA. Available online: http://droughtmonitor.unl.edu/MapsAndData/DataTables.aspx (accessed on 13 July 2015).
- Jacobs, D.F.; Salifu, K.F.; Davis, A.S. Drought susceptibility and recovery of transplanted Quercus rubra seedlings in relation to root system morphology. Ann. For. Sci. 2009, 66, 504. [Google Scholar] [CrossRef]
- Kolb, T.E.; Steiner, K.C.; McCormick, L.H.; Bowersox, T.W. Growth response of northern red-oak and yellow-poplar seedlings to light, soil moisture and nutrients in relation to ecological strategy. For. Ecol. Manag. 1990, 38, 65–78. [Google Scholar] [CrossRef]
- Schweitzer, C.J.; Gardiner, E.S.; Loftis, D.L. Response of Sun-Grown and Shade-Grown Northern Red oak Seedlings to Outplanting in Clearcuts and Shelterwoods in North Alabama; Southern Research Station, Forest Service, US Department of Agriculture: Asheville, NC, USA, 2006; Volume 92, pp. 269–274.
- McGee, C.E. Elevation of seed sources and planting sites affects phenology and development of Red Oak seedlings. For. Sci. 1974, 20, 160–164. [Google Scholar]
- Pinto, J.R.; Dumroese, R.K.; Davis, A.S.; Landis, T.D. Conducting seedling stocktype trials: A new approach to an old question. J. For. 2011, 109, 293–299. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, S.L.; Schlarbaum, S.E.; Schweitzer, C.J. Effects of Visual Grading on Northern Red Oak (Quercus rubra L.) Seedlings Planted in Two Shelterwood Stands on the Cumberland Plateau of Tennessee, USA. Forests 2015, 6, 3779-3798. https://doi.org/10.3390/f6103779
Clark SL, Schlarbaum SE, Schweitzer CJ. Effects of Visual Grading on Northern Red Oak (Quercus rubra L.) Seedlings Planted in Two Shelterwood Stands on the Cumberland Plateau of Tennessee, USA. Forests. 2015; 6(10):3779-3798. https://doi.org/10.3390/f6103779
Chicago/Turabian StyleClark, Stacy L., Scott E. Schlarbaum, and Callie J. Schweitzer. 2015. "Effects of Visual Grading on Northern Red Oak (Quercus rubra L.) Seedlings Planted in Two Shelterwood Stands on the Cumberland Plateau of Tennessee, USA" Forests 6, no. 10: 3779-3798. https://doi.org/10.3390/f6103779





