The Impact of Moss Species and Biomass on the Growth of Pinus sylvestris Tree Seedlings at Different Precipitation Frequencies
Abstract
:1. Introduction
2. Experimental Section
2.1. Moss Communities
2.2. Experimental Design
2.3. N2-Fixation and Available Nutrients
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Moss Species
| Tree Seedling | ||||||
|---|---|---|---|---|---|---|
| Height | Shoot biomass | Root biomass | Total biomass | Shoot:root ratio | ||
| Moss species (S) | 5.381 (<0.001) | 4.975 (<0.001) | 11.796 (<0.001) | 8.631 (<0.001) | 2.462 (0.048) | 104.114 (<0.001) |
| Moss biomass class (MBC) 1 | 14.756 (<0.001) | 3.881 (0.010) | 14.682 (<0.001) | 8.821 (<0.001) | 6.886 (<0.001) | 55.788 (<0.001) |
| Precipitation frequency (W) | 1.319 (0.253) | 0.102 (0.750) | 1.038 (0.099) | 0.491 (0.485) | 0.122 (0.728) | 23.620 (<0.001) |
| S × MBC | 1.807 (0.071) | 1.934 (0.051) | 2.229 (0.023) | 2.237 (0.022) | 0.477 (0.888) | 3.228 (0.001) |
| S × W | 0.930 (0.448) | 1.895 (0.114) | 2.520 (0.043) | 2.342 (0.057) | 0.516 (0.724) | 2.523 (0.060) |
| MBC × W | 0.940 (0.423) | 2.131 (0.099) | 1.016 (0.387) | 1.658 (0.178) | 0.810 (0.490) | 1.808 (0.148) |
| S × MBC × W | 1.017 (0.429) | 0.765 (0.649) | 0.783 (0.632) | 0.798 (0.619) | 0.660 (0.744) | 0.541 (0.843) |
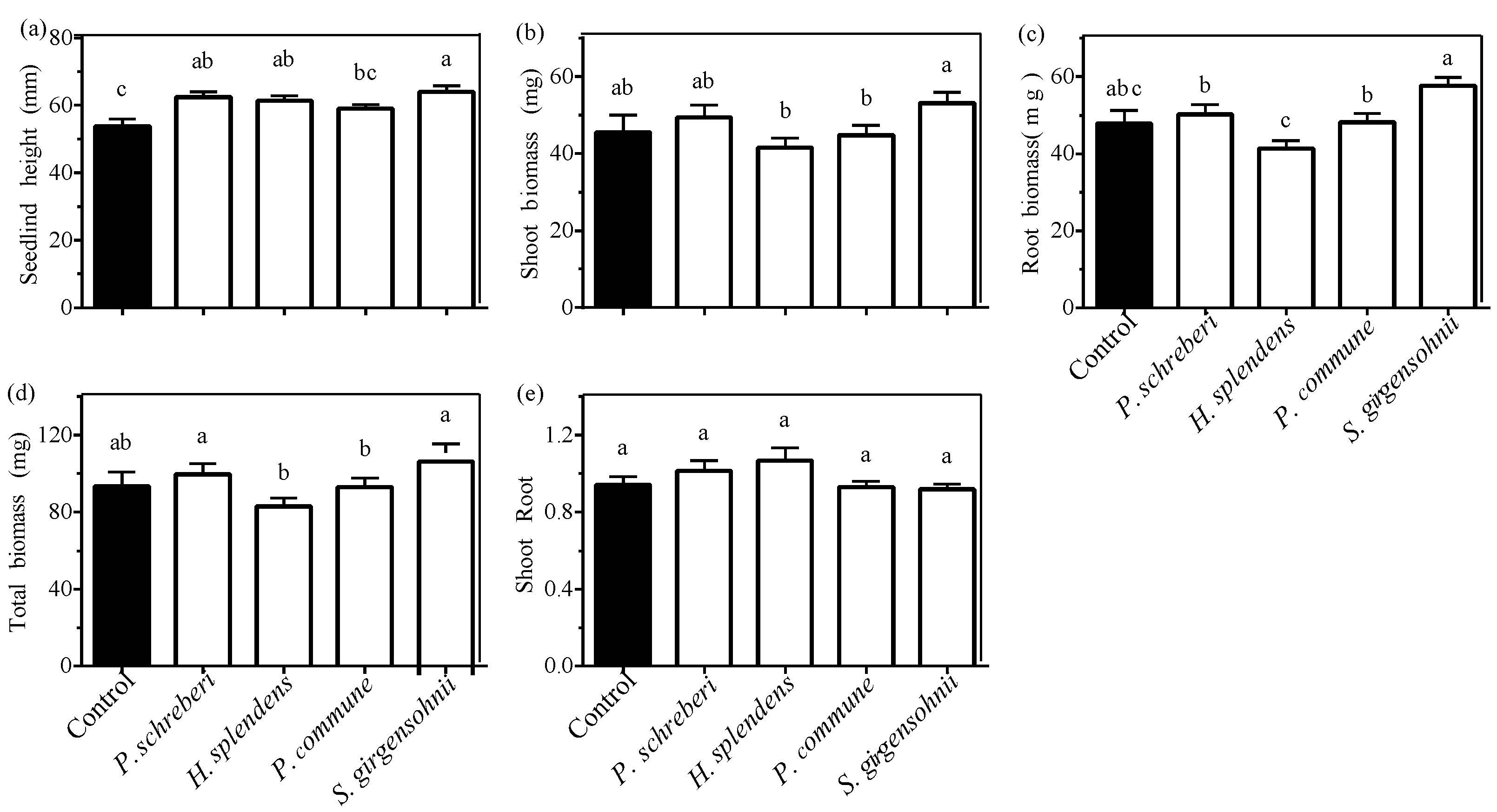
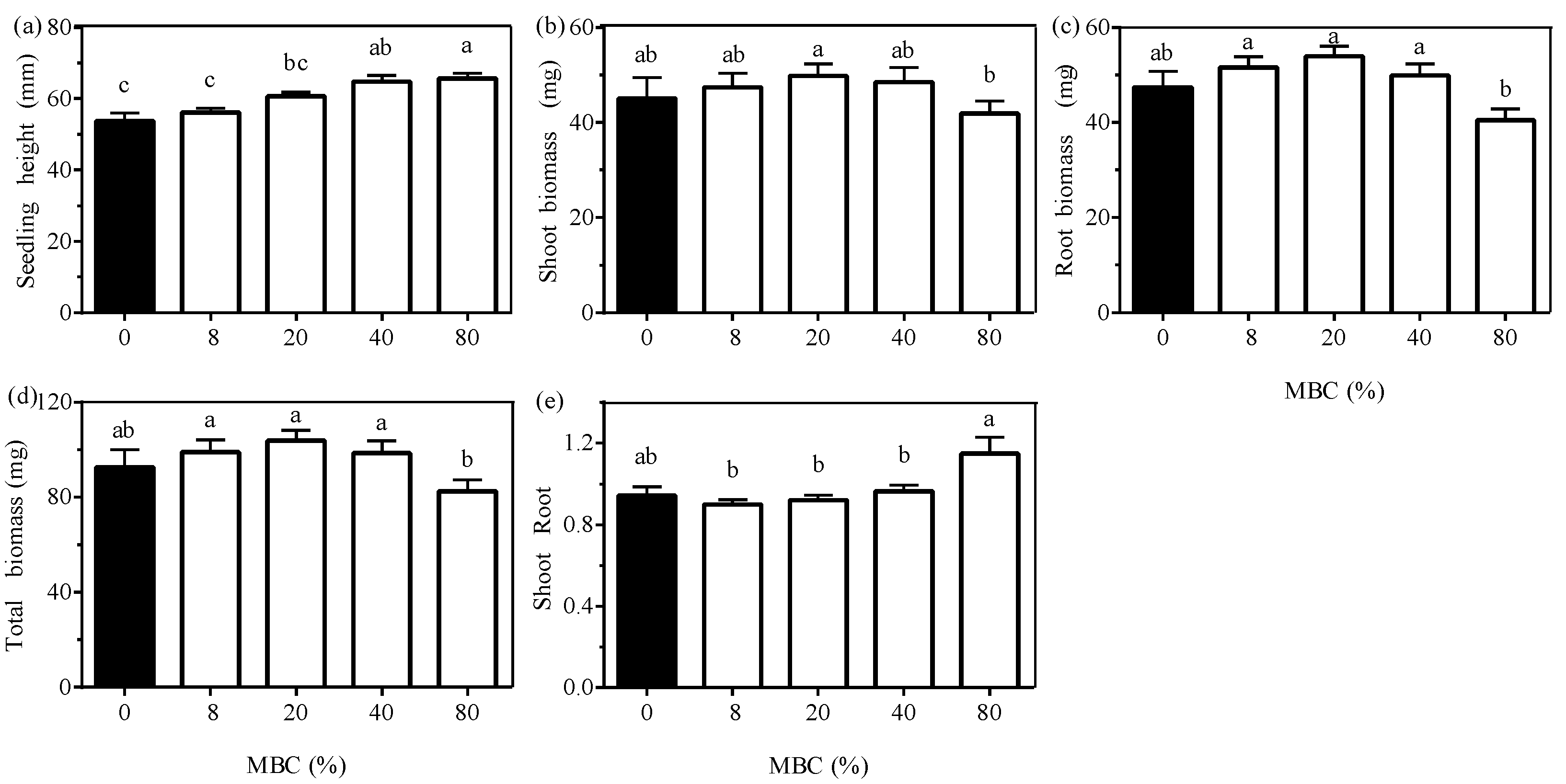
3.2. Effect of Moss Biomass
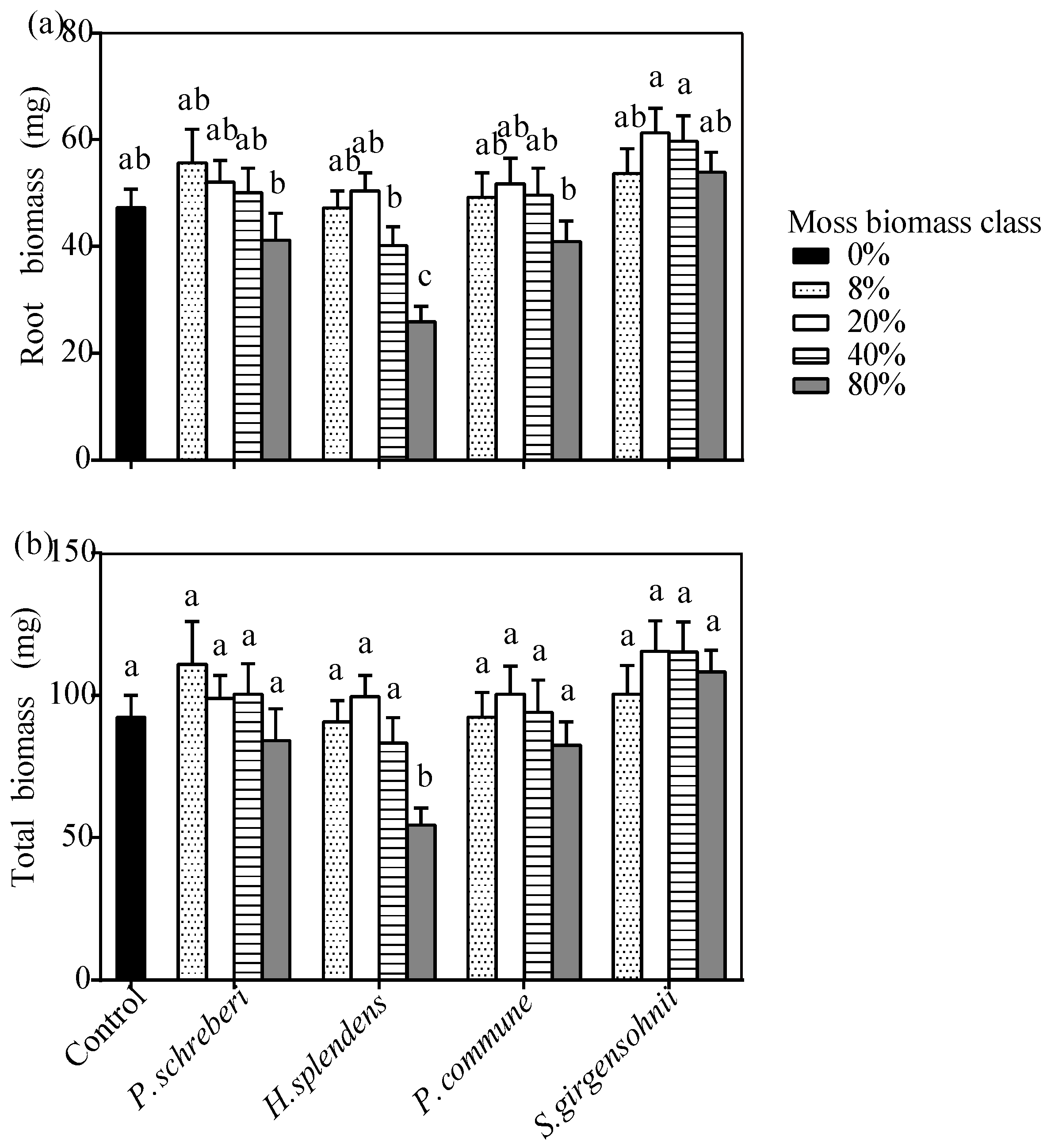
3.3. Effects of the Interaction between Moss Species and Moss Biomass
| Tree Seedling | |||||||
|---|---|---|---|---|---|---|---|
| Height | Shoot biomass | Root biomass | Total biomass | Shoot:root ratio | |||
| P. schreberi | MBC 1 | 5.595 (0.001) | 1.005 (0.417) | 2.439 (0.063) | 1.646 (0.182) | 1.518 (0.215) | 20.621 (<0.001) |
| W | 0.459 (0.502) | 0.072 (0.789) | 0.081 (0.777) | 0.099 (0.754) | 0.089 (0.767) | 6.081 (0.020) | |
| Moss species | Height | Shoot biomass | Root biomass | Total biomass | Shoot:root ratio | Moss growth | |
| H. splendens | MBC 1 | 7.587 (<0.001) | 6.729 (<0.001) | 14.863 (<0.001) | 11.290 (<0.001) | 2.392 (0.067) | 12.073 (<0.001) |
| W | 1.071 (0.307) | 0.188 (0.667) | 0.082 (0.776) | 0.166 (0.686) | 0.404 (0.528) | 14.329 (0.001) | |
| P. commune | MBC 1 | 2.977 (0.032) | 0.605 (0.662) | 2.147 (0.094) | 1.149 (0.349) | 1.439 (0.240) | 3.016 (0.045) |
| W | 0.877 (0.355) | 4.913 (0.033) | 7.783 (0.008) | 6.712 (0.014) | 0.120 (0.731) | 0.202 (0.656) | |
| S. girgensohnii | MBC 1 | 18.315 (<0.001) | 2.129 (0.095) | 3.085 (0.026) | 2.754 (0.041) | 2.252 (0.080) | 35.191 (<0.001) |
| W | 1.479 (0.231) | 2.711 (0.108) | 3.164 (0.083) | 3.503 (0.069) | 0.006 (0.938) | 10.050 (0.003) | |
3.4. Precipitation Effect
3.5. Moss Growth
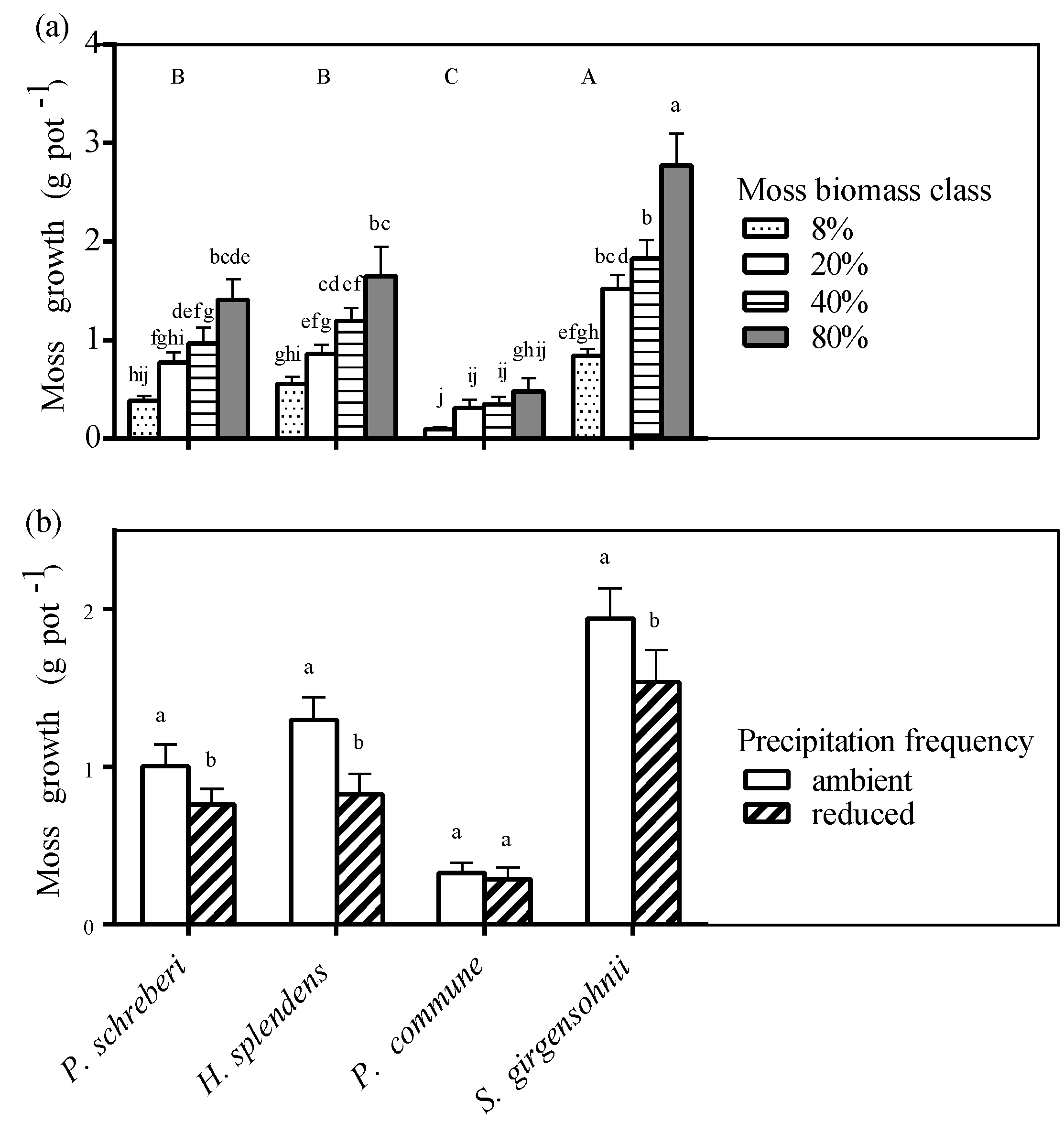
3.6. Effect of Moss Species on N2-Fixation and Nutrient Availability
| Response Variable | N2 Fixation | NH4+-N | NO3−-N | PO43−-P |
|---|---|---|---|---|
| Moss species (S) | 16.780 (<0.001) | 1.097 (0.371) | 6.340 (<0.001) | 5.368 (0.001) |
| Precipitation frequency (W) | 0.772 (0.387) | 0.368 (0.548) | 0.068 (0.795) | 0.138 (0.711) |
| S × W | 1.903 (0.151) | 0.312 (0.868) | 0.348 (0.844) | 0.213 (0.930) |

3.7. Discussion
4. Conclusions
Supplementary Information
| Species | Natural Monoculture Moss Cushion Biomass (DW g·m−2) | Cushion Dry/Fresh Weight Ratio |
|---|---|---|
| Pleurozium schreberi 1 | 651 ± 172 | 0.078 ± 0.004 |
| Hylocomium splendens 2 | 569 ± 144 | 0.098 ± 0.010 |
| Polytrichum commune 2 | 661 ± 146 | 0.176 ± 0.013 |
| Sphagnum girgensohnii 1 | 592 ± 44 | 0.055 ± 0.001 |
| Mean | 618 | |
| 80% of mean weight | 495 |

Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Gundale, M.J.; Gustafsson, H.; Nilsson, M.-C. The sensitivity of nitrogen fixation by a feathermoss-cyanobacteria association to litter and moisture variability in young and old boreal forests. Can. J. For. Res. 2009, 39, 2542–2549. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Bond-Lamberty, B.; Euskirchen, E.; Talbot, J.; Frolking, S.; McGuire, A.D.; Tuittila, E.S. The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol. 2012, 196, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Hyppönen, M.; Hallikainen, V.; Niemelä, J.; Rautio, P. The contradictory role of understory vegetation on the success of Scots pine regeneration. Silva Fenn. 2013, 47. doi:org/10.14214/sf.903. [Google Scholar]
- Steijlen, I.; Nilsson, M.-C.; Zackrisson, O. Seed regeneration of Scots pine in boreal forest stands dominated by lichen and feather moss. Can. J. For. Res. 1995, 25, 713–723. [Google Scholar] [CrossRef]
- Hörnberg, G.; Ohlson, M.; Zackrisson, O. Influence of bryophytes and microrelief conditions on Picea abies seed regeneration patterns in boreal old-growth swamp forests. Can. J. For. Res. 1997, 27, 1015–1023. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Hermanutz, L.; Marino, P.M. Feathermoss seedbeds facilitate black spruce seedling recruitment in the forest–tundra ecotone (Labrador, Canada). Oikos 2011, 120, 1263–1271. [Google Scholar] [CrossRef]
- Zackrisson, O.; Dahlberg, A.; Norberg, G.; Nilsson, M.-C.; Jäderlund, A. Experiments on the effects of water availability and exclusion of fungal hyphae on nutrient uptake and establishment of Pinus sylvestris seedlings in carpets of the moss Pleurozium schreberi. Ecoscience 1998, 5, 77–85. [Google Scholar]
- Weber, M.G.; van Cleve, K. Nitrogen transformations in feather moss and forest floor layers of interior Alaska black spruce ecosystems. Can. J. For. Res. 1984, 14, 278–290. [Google Scholar] [CrossRef]
- Nilsson, M.-C.; Steijlen, I.; Zackrisson, O. Time-restricted seed regeneration of Scots pine in sites dominated by feather moss after clear-cutting. Can. J. For. Res. 1996, 26, 945–953. [Google Scholar] [CrossRef]
- Simard, M.-J.; Bergeron, Y.; Sirois, L. Conifer seedling recruitment in a southeastern Canadian boreal forest: The importance of substrate. J. Veg. Sci. 1998, 9, 575–582. [Google Scholar] [CrossRef]
- Blok, D.; Heijmans, M.M.P.D.; Schaepman-Strub, G.; van Ruijven, J.; Parmentier, F.J.W.; Maximov, T.C.; Berendse, F. The cooling capacity of mosses: Controls on water and energy fluxes in a Siberian tundra site. Ecosystems 2011, 14, 1055–1065. [Google Scholar] [CrossRef] [Green Version]
- Oleskog, G.; Sahlen, K. Effects of seedbed substrate on moisture conditions and germination of Scots pine (Pinus sylvestris) seeds in a mixed conifer stand. New For. 2000, 20, 119–133. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Bodegom, P.M.; Cornelissen, J.H. Dominant bryophyte control over high-latitude soil temperature fluctuations predicted by heat transfer traits, field moisture regime and laws of thermal insulation. Funct. Ecol. 2013, 27, 1442–1454. [Google Scholar] [CrossRef]
- Jackson, B.G.; Martin, P.; Nilsson, M.-C.; Wardle, D.A. Response of feather moss associated N2 fixation and litter decomposition to variations in simulated rainfall intensity and frequency. Oikos 2011, 120, 570–581. [Google Scholar] [CrossRef]
- Bay, G.; Nahar, N.; Oubre, M.; Whitehouse, M.J.; Wardle, D.A.; Zackrisson, O.; Nilsson, M.C.; Rasmussen, U. Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytol. 2013, 200, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Danielsson, Å; Svensson, B.H. Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant Soil 2013, 362, 271–278. [Google Scholar]
- Schmalholz, M.; Hylander, K. Succession of bryophyte assemblages following clear-cut logging in boreal spruce-dominated forests in south-central Sweden—Does retrogressive succession occur? Can. J. For. Res. 2009, 39, 1871–1880. [Google Scholar]
- Palviainen, M.; Finer, L.; Mannerkoski, H.; Piirainen, S.; Starr, M. Responses of ground vegetation species to clear-cutting in a boreal forest: Aboveground biomass and nutrient contents during the first 7 years. Ecol. Res. 2005, 20, 652–660. [Google Scholar] [CrossRef]
- Donath, T.W.; Eckstein, R.L. Effects of bryophytes and grass litter on seedling emergence vary by vertical seed position and seed size. Plant Ecol. 2010, 207, 257–268. [Google Scholar] [CrossRef]
- Soudzilovskaia, N.A.; Graae, B.J.; Douma, J.C.; Grau, O.; Milbau, A.; Shevtsova, A.; Wolters, L.; Cornelissen, J.H.C. How do bryophytes govern generative recruitment of vascular plants? New Phytol. 2011, 190, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H.C. The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Michel, P.; Lee, W.G.; During, H.J.; Cornelissen, J.H.C. Species traits and their non-additive interactions control the water economy of bryophyte cushions. J. Ecol. 2012, 100, 222–231. [Google Scholar] [CrossRef]
- Hájek, T.; Vicherová, E. Desiccation tolerance of Sphagnum revisited: A puzzle resolved. Plant Biol. 2014, 16, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.C.; Oliver, M.J.; Wood, A.J.; Alpert, P.; Stark, L.R.; Cleavitt, N.L.; Mishler, B.D. Desiccation-tolerance in bryophytes: A review. Bryologist 2007, 110, 595–621. [Google Scholar] [CrossRef]
- Jentsch, A.; Beierkuhnlein, C. Research frontiers in climate change: Effects of extreme meteorological events on ecosystems. Comptes Rendus Geosci. 2008, 340, 621–628. [Google Scholar] [CrossRef]
- Lindo, Z.; Nilsson, M.C.; Gundale, M.J. Bryophyte-cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Glob. Chang. Biol. 2013, 19, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Chapin III, F.; Oechel, W.; van Cleve, K.; Lawrence, W. The role of mosses in the phosphorus cycling of an Alaskan black spruce forest. Oecologia 1987, 74, 310–315. [Google Scholar] [CrossRef]
- Lindo, Z.; Gonzalez, A. The bryosphere: An integral and influential component of the earth’s biosphere. Ecosystems 2010, 13, 612–627. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin III, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Parker, W.C.; Watson, S.R.; Cairns, D.W. The role of hair-cap mosses (Polytrichum spp.) in natural regeneration of white spruce (Picea glauca (moench) voss). For. Ecol. Manag. 1997, 92, 19–28. [Google Scholar]
- Zamfir, M. Effects of bryophytes and lichens on seedling emergence of alvar plants: Evidence from greenhouse experiments. Oikos 2000, 88, 603–611. [Google Scholar] [CrossRef]
- Wardle, D.A.; Lagerström, A.; Nilsson, M.C. Context dependent effects of plant species and functional group loss on vegetation invasibility across an island area gradient. J. Ecol. 2008, 96, 1174–1186. [Google Scholar] [CrossRef]
- Heijmans, M.M.P.D.; Arp, W.J.; Chapin, F.S. Controls on moss evaporation in a boreal black spruce forest. Glob. Biogeochem. Cycles 2004, 18, 1–8. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment; Cambridge University Press: Cambridge, UK, 2007; p. 996. [Google Scholar]
- May, W. Potential future changes in the characteristics of daily precipitation in Europe simulated by the HIRHAM regional climate model. Clim. Dyn. 2008, 30, 581–603. [Google Scholar] [CrossRef]
- Schlyter, P.; Stjernquist, I.; Bärring, L.; Jönsson, A.M.; Nilsson, C. Assessment of the impacts of climate change and weather extremes on boreal forests in northern Europe, focusing on Norway spruce. Clim. Res. 2006, 31, 75–84. [Google Scholar] [CrossRef]
- Rixen, C.; Mulder, C.P. Improved water retention links high species richness with increased productivity in arctic tundra moss communities. Oecologia 2005, 146, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Gundale, M.J.; Nilsson, M.; Bansal, S.; Jaderlund, A. The interactive effects of temperature and light on biological nitrogen fixation in boreal forests. New Phytol. 2012, 194, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.V.; Collins, N.J.; Callaghan, C.H. Photosynthesis, growth and reproduction of Hylocomium splendens and Polytrichum commune in Swedish lapland. Strategies of growth and population dynamics of tundra plants 4. Oikos 1978, 31, 73–88. [Google Scholar]
- Gustafsson, L.; Hallingbäck, T. Bryophyte flora and vegetation of managed and virgin coniferous forests in south-west Sweden. Biol. Conserv. 1988, 44, 283–300. [Google Scholar] [CrossRef]
- Fenton, N.J.; Bergeron, Y. Facilitative succession in a boreal bryophyte community driven by changes in available moisture and light. J. Veg. Sci. 2006, 17, 65–76. [Google Scholar] [CrossRef]
- Wern, L. Luftfuktighet, Variationer i Sverige; SMHI: Norrköping, Sweden, 2013; p. 68. [Google Scholar]
- DeLuca, T.H.; Zackrisson, O.; Nilsson, M.C.; Sellstedt, A. Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 2002, 419, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Gundale, M.J.; From, F.; Bach, L.H.; Nordin, A. Anthropogenic nitrogen deposition in boreal forests has a minor impact on the global carbon cycle. Glob. Chang. Biol. 2014, 20, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Dehlin, H.; Nilsson, M.-C.; Wardle, D.A.; Shevtsova, A. Effects of shading and humus fertility on growth, competition, and ectomycorrhizal colonization of boreal forest tree seedlings. Can. J. For. Res. 2004, 34, 2573–2586. [Google Scholar] [CrossRef]
- Jeschke, M.; Kiehl, K. Effects of a dense moss layer on germination and establishment of vascular plants in newly created calcareous grasslands. Flora 2008, 203, 557–566. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Romanovsky, V.E.; Harden, J.W.; McGuire, A.D. The effect of moisture content on the thermal conductivity of moss and organic soil horizons from black spruce ecosystems in interior Alaska. Soil Sci. 2009, 174, 646–651. [Google Scholar] [CrossRef]
- Økland, R.H.; Økland, T. Population biology of the clonal moss Hylocomium splendens in Norwegian boreal spruce forests. II. Effects of density. J. Ecol. 1996, 84, 63–69. [Google Scholar]
- Castro, J. Seed mass versus seedling performance in Scots pine: A maternally dependent trait. New Phytol. 1999, 144, 153–161. [Google Scholar] [CrossRef]
- Zackrisson, O.; Nilsson, M.-C.; Dahlberg, A.; Jäderlund, A. Interference mechanisms in conifer-ericaceae-feathermoss communities. Oikos 1997, 78, 209–220. [Google Scholar] [CrossRef]
- Kellomäki, S. Book 2: Forest Resources and Sustainable Management; Fapet Oy: Helsinki, Finland, 1998. [Google Scholar]
- Ohlson, M.; Zackrisson, O. Tree establishment and microhabitat relationships in north Swedish peatlands. Can. J. For. Res. 1992, 22, 1869–1877. [Google Scholar] [CrossRef]
- Kuuluvainen, T.; Juntunen, P. Seedling establishment in relation to microhabitat variation in a windthrow gap in a boreal Pinus sylvestris forest. J. Veg. Sci. 1998, 9, 551–562. [Google Scholar] [CrossRef]
- Gornall, J.L.; Woodin, S.J.; Jonsdottir, I.S.; van der Wal, R. Balancing positive and negative plant interactions: How mosses structure vascular plant communities. Oecologia 2011, 166, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.; Hanslin, H.M.; Bakken, S. Testing for positive density-dependent performance in four bryophyte species. Ecology 2001, 82, 70–88. [Google Scholar] [CrossRef]
- Lavoie, M.; Paré, D.; Bergeron, Y. Relationships between microsite type and the growth and nutrition of young black spruce on post-disturbed lowland black spruce sites in eastern Canada. Can. J. For. Res. 2007, 37, 62–73. [Google Scholar] [CrossRef]
- Jackson, B.G.; Nilsson, M.-C.; Wardle, D.A. The effects of the moss layer on the decomposition of intercepted vascular plant litter across a post-fire boreal forest chronosequence. Plant Soil 2013, 367, 199–214. [Google Scholar] [CrossRef]
- Gundale, M.J.; Deluca, T.H.; Nordin, A. Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Glob. Chang. Biol. 2011, 17, 2743–2753. [Google Scholar] [CrossRef]
- Fenton, N.J.; Bergeron, Y.; Pare, D. Decomposition rates of bryophytes in managed boreal forests: Influence of bryophyte species and forest harvesting. Plant Soil 2010, 336, 499–508. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stuiver, B.M.; Wardle, D.A.; Gundale, M.J.; Nilsson, M.-C. The Impact of Moss Species and Biomass on the Growth of Pinus sylvestris Tree Seedlings at Different Precipitation Frequencies. Forests 2014, 5, 1931-1951. https://doi.org/10.3390/f5081931
Stuiver BM, Wardle DA, Gundale MJ, Nilsson M-C. The Impact of Moss Species and Biomass on the Growth of Pinus sylvestris Tree Seedlings at Different Precipitation Frequencies. Forests. 2014; 5(8):1931-1951. https://doi.org/10.3390/f5081931
Chicago/Turabian StyleStuiver, Babs M., David A. Wardle, Michael J. Gundale, and Marie-Charlotte Nilsson. 2014. "The Impact of Moss Species and Biomass on the Growth of Pinus sylvestris Tree Seedlings at Different Precipitation Frequencies" Forests 5, no. 8: 1931-1951. https://doi.org/10.3390/f5081931




