Soil Organic Carbon in Particle Size and Density Fractionations under Four Forest Vegetation-Land Use Types in Subtropical China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Forest Types
- (1)
- An evergreen broad-leaved forest, which is a climax community in the study area. The dominant species were Cyclobalanopsis glauca, Castanopsis sclerophylla, Schima superba and Castanopsis eyrei. The stand was approximately 25 years old and had 70% canopy closure, with trees having an average diameter at breast height (DBH) of 14.3 cm. The dominant understory species were Litsea cubeba, Lindera glauca, Camellia fraternal and Cleyera japonica; they provided about 70% ground cover.
- (2)
- A pine forest that had an average canopy cover of 55%–75%, with trees older than 20 years and a DBH of 10–13 cm. The dominant understory species were Camellia fraterna, Vaccinium bracteatum and Symplocos stellaris.
- (3)
- A managed chestnut forest, which was converted from an evergreen broad-leaved forest or coniferous forest through clearcutting and planting. The chestnut forest was 14 years old, and the trees had a mean DBH of 14.4 cm. The stands were planted at a density of 415 trees per hectare. The stands were managed with 2–3 times of weeding/cultivation and 3 fertilizer applications per year. The rate of fertilizer application was 1 kg per tree per year with an NPK compound fertilizer (N:P2O5:K2O = 15:15:15).
- (4)
- An intensively managed bamboo forest, which was converted from a young evergreen broad-leaved forest in 1997 by clearcutting and planting. At the time of this study, the forest was 14 years old and had 1500 bamboo plants per hectare. The bamboo plants in the stands had a mean DBH of 3.5 cm. Before 2002, the bamboo forest was managed by annual tillage and fertilization (application of urea at 450 kg·ha−1·y−1 and an NPK compound fertilizer (N:P2O5:K2O = 15:15:15) at 600 kg·ha−1·y−1). After 2002, intensive management practices were used to enhance bamboo growth and bamboo shoot production. In the intensive management regime, organic materials (straw and rice chaff) were applied from November 20 to December 10 every year to cover the soil surface to increase soil temperature and maintain soil moisture content in the winter. This (mulching) involves placing 10–15 cm of rice straw at the soil surface, followed by adding 10–15 cm of rice husk. The annual rate of application was equivalent to 40 t·ha−1 of rice straw and 55 t·ha−1 of rice husk, with an approximately total annual input of 35 t·C·ha−1 [33]. The undecomposed organic residues were removed in April or May of the following year. Fertilization occurred in May, September and just before the application of the mulch material in early winter. The annual fertilizer application rates were 2.25 t·ha−1 compound fertilizer (N:P:K = 15:15:15) and 1.125 t·ha−1 urea (46% N). The land was tilled after the fertilizer application to mix the fertilizer into the soil.
2.2. Soil Sampling and Analyses
2.2.1. Soil Particle Size Fractionation
2.2.2. Soil Density Fractionation
2.2.3. Methods for pH, Organic C and N Determination
2.3. Statistical Analyses
3. Results
3.1. Soil Chemical Properties under Different Forest Vegetation-Land Use Types
| Soil Depth (cm) | Forest Type | pH | SOC (g·kg−1) | Total N (g·kg−1) | Particle Size Fractions (Mass %) | Silt-Clay | Density Fractions (Mass %) | ||
|---|---|---|---|---|---|---|---|---|---|
| Coarse Sand | Fine Sand | Light Fraction | Heavy Fraction | ||||||
| 0–20 | Broad-leaf forest | 4.32 (0.05) b | 19.89 (2.76) b | 1.31 (0.03) b | 65.1 (1.9) ab | 18.8 (2.9) a | 15.5 (4.7) c | 3.5 (0.3) b | 93.6 (0.9) a |
| Pine forest | 4.47 (0.05) a | 13.62 (0.55) b | 0.88 (0.01) d | 67.2 (3.3) a | 18.5 (0.8) a | 14.0 (2.8) c | 2.5 (0.2) c | 95.6 (0.7) a | |
| Chestnut forest | 4.10 (0.05) c | 12.17 (1.90) b | 1.05 (0.29) c | 48.1 (0.6) c | 21.9 (1.3) a | 29.5 (1.8) a | 1.5 (0.1) d | 94.3 (0.4) a | |
| Bamboo forest | 4.32 (0.05) b | 34.78 (8.36) a | 2.21 (0.04) a | 62.2 (1.9) b | 13.8 (2.9) b | 23.2 (1.5) b | 6.5 (0.5) a | 86.1 (2.9) b | |
| 20–40 | Broad-leaf forest | 4.54 (0.07) b | 10.20 (0.99) b | 0.64 (0.03) b | 46.2 (2.3) b | 25.8 (1.0) a | 26.9 (1.3) b | 1.2 (0.1) b | 95.1 (0.4) a |
| Pine forest | 4.62 (0.07) a | 8.51 (1.45) b | 0.35 (0.02) c | 59.6 (1.7) a | 18.2 (0.4) b | 21.0 (2.1) c | 0.9 (0.1) bc | 96.6 (0.6) a | |
| Chestnut forest | 4.36 (0.05) c | 6.25 (0.08) b | 0.63 (0.02) b | 33.5 (1.2) c | 20.9 (2.2) b | 44.3 (1.6) a | 0.5 (0.01) c | 94.7 (0.5) a | |
| Bamboo forest | 4.12 (0.05) d | 31.56 (10.09) a | 1.81 (0.01) a | 41.3 (2.9) b | 27.7 (1.4) a | 28.9 (4.4) b | 5.0 (0.7) a | 88.7 (1.7) b | |
3.2. Distribution of Soil C in Particle Size Fractions
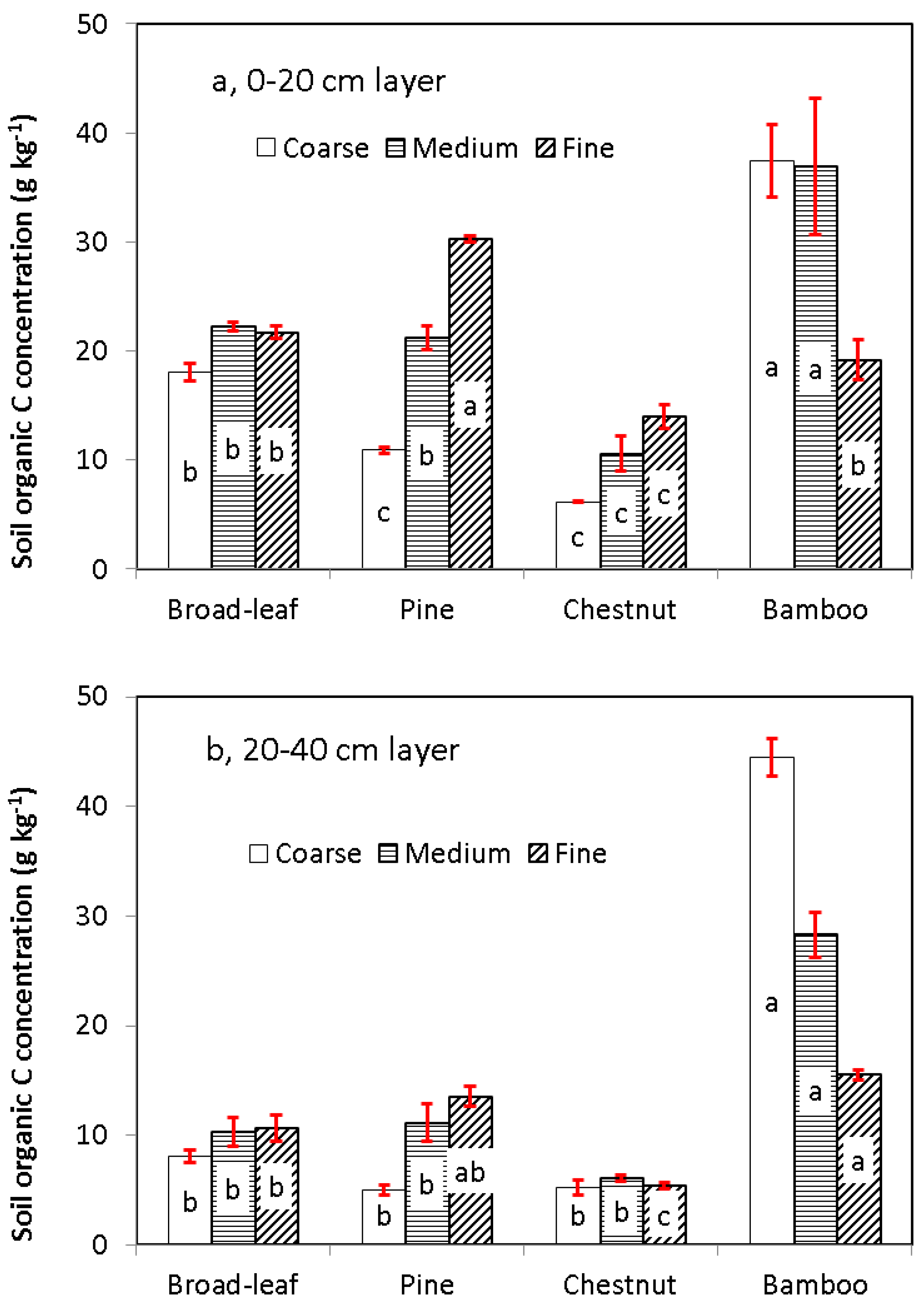

3.3. Soil Organic C in the Light and Heavy Fractions

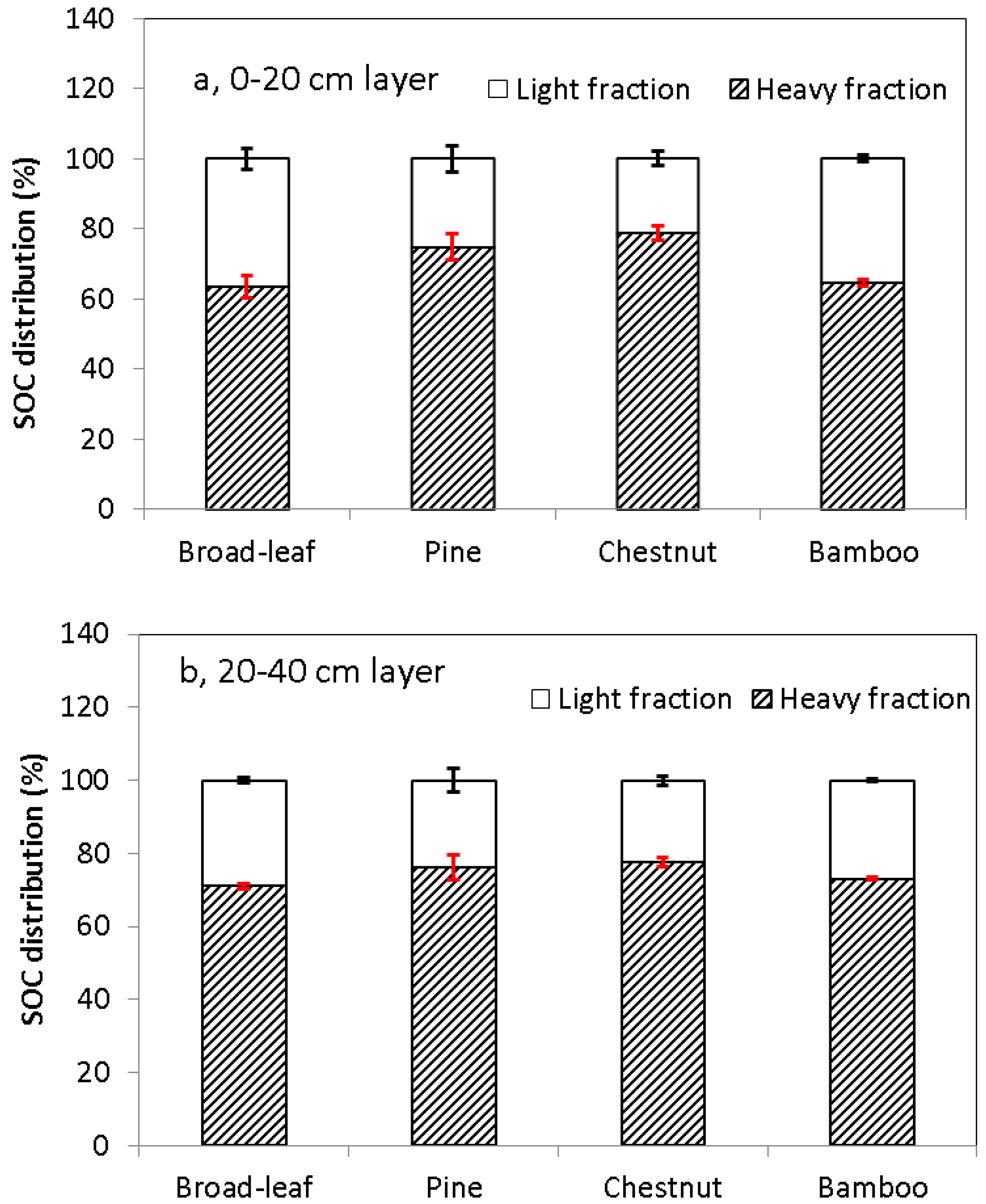
4. Discussion
4.1. Forest Vegetation-Land Use Type Affected Soil C in Particle Size Fractions
4.2. Forest Vegetation-Land Use Type Affected Soil C in Soil Density Fractions
4.3. Land Management Affected SOC Distribution
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Post, W.M.; Emanuel, M.R.; Zinke, P.J.; Stangenberger, A. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Eswaran, H.; van den Berg, E.; Reich, P. Organic carbon in soils of the world. Soil Sci. Soc. Am. J. 1993, 57, 192–194. [Google Scholar] [CrossRef]
- Batjes, N.H. The total C and N in soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Karlen, D.L.; Rosek, M.J.; Gardner, J.C.; Allan, D.L.; Alms, M.J.; Bezdicek, D.F.; Flock, M.; Huggins, D.R.; Miller, B.S.; Staben, M.L. Conservation reserve program effects on soil quality indicators. J. Soil Water Conserv. 1999, 54, 439–444. [Google Scholar]
- Smith, O.H.; Petersen, G.W.; Needelman, B.A. Environmental indicators of agroecosystems. Adv. Agron. 2000, 69, 75–97. [Google Scholar]
- Yonekura, Y.; Ohta, S.; Kiyono, Y.; Aksa, D.; Morisada, K.; Tanaka, N.; Tayasu, I. Soil organic matter dynamics in density and particle-size fractions following destruction of tropical rainforest and the subsequent establishment of Imperata grassland in Indonesian Borneo using stable carbon isotopes. Plant Soil 2013, 372, 683–699. [Google Scholar] [CrossRef]
- Teklay, T.; Chang, S.X. Temporal changes in soil carbon and nitrogen storage in a hybrid poplar chronosequence in northern Alberta. Geoderma 2008, 144, 613–619. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Soil structure and carbon cycling. Aust. J. Soil Res. 1994, 32, 1043–1068. [Google Scholar] [CrossRef]
- Six, J.; Guggenberger, G.; Paustian, K.; Haumaier, L.; Elliott, E.T.; Zech, W. Sources and composition of soil organic matter fractions between and within soil aggregates. Eur. J. Soil Sci. 2001, 52, 607–618. [Google Scholar] [CrossRef]
- Olk, D.C.; Gregorich, E.G. Overview of the symposium proceedings, “Meaningful pools in determining soil carbon and nitrogen dynamics”. Soil Soc. Am. J. 2006, 70, 967–974. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Methods for physical separation and characterization of soil organic fractions. Geoderma 1993, 56, 449–457. [Google Scholar] [CrossRef]
- Guggenberger, G.; Christensen, B.T.; Zech, W. Land use effects on the composition of organic matter in particle-size separates of soil (I): Lignin and carbohydrate signature. Eur. J. Soil Sci. 1994, 45, 449–458. [Google Scholar] [CrossRef]
- Christensen, B.T. Physical fractionation of soil and structural and functional complexity in organic matter turnover. Eur. J. Soil Sci. 2001, 52, 345–353. [Google Scholar] [CrossRef]
- Chefetz, B.; Tarchitzky, J.; Deshmukh, A.P.; Hatcher, P.G.; Chen, Y. Structural characterization of soil organic matter and humic acids in particle-size fractions of an agricultural soil. Soil Sci. Soc. Am. J. 2002, 66, 129–141. [Google Scholar] [CrossRef]
- Arevalo, C.B.M.; Chang, S.X.; Bhatti, J.S.; Sidders, D. Mineralization potential and temperature sensitivity of soil organic carbon under different land uses in the Parkland region of Alberta, Canada. Soil Sci. Soc. Am. J. 2012, 76, 241–251. [Google Scholar] [CrossRef]
- Dalal, R.C.; Mayer, R.J. Long-term trends in fertility of soil under continuous cultivation and cereal crop-ping in southern Queensland, IV. Loss of organic carbon from different density fractions. Aust. J. Soil Res. 1986, 24, 301–309. [Google Scholar] [CrossRef]
- Li, J.J.; Zhao, X.; Pan, T.H. Influence of land use on soil labile organic carbon. J. Soil Water Conserv. 2011, 25, 147–151. [Google Scholar]
- Mills, R.T.E.; Tipping, E.; Bryant, C.L.; Emmett, B.A. Long-term organic carbon turnover rates in natural and semi-natural topsoils. Biogeochemistry 2014, 118, 257–272. [Google Scholar] [CrossRef]
- Baisden, W.T.; Parfitt, R.L.; Ross, C.; Schipper, L.A.; Canessa, S. Evaluating 50 years of time-series soil radiocarbon data: Towards routine calculation of robust C residence times. Biogeochemistry 2013, 112, 129–137. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Chan, K.Y. Soil particulate organic carbon under different land use and management. Soil Use Manag. 2001, 17, 217–221. [Google Scholar] [CrossRef]
- Fang, H.J.; Yang, X.M.; Hang, X.P. Spatial distribution of particulate organic carbon and aggregate associated carbon in topsoil of a sloping farmland in the Black Soil region, Northeast China. Acta Ecol. Sin. 2006, 26, 2847–2854. [Google Scholar]
- Hassink, J.; Whitmore, A.P.; Jaromir, K. Size and density fractionation of soil organic matter and the physical capacity of soils to protect organic matter. Eur. J. Agron. 1997, 7, 189–199. [Google Scholar] [CrossRef]
- Jiang, Y.; Dou, S. Effect of organic materials application on the properties of humic substances in organo-mineral complexes of soils I. Effect of organic materials on the organo-mineral complexation and forms of humus. Acta Pedol. Sin. 1987, 24, 97–104. [Google Scholar]
- Yang, X.M.; Zhang, X.P.; Fang, H.J. Importance of agricultural soil sequestering carbon to offsetting global warming. Sci. Geogr. Sin. 2003, 23, 101–107. [Google Scholar]
- Mujuru, L.; Mureva, A.; Velthorst, E.J.; Hoosbeek, M.R. Land use and management effects on soil organic matter fractions in Rhodic Ferralsols and Haplic Arenosols in Bindura and Shamva districts of Zimbabwe. Geoderma 2013, 209, 262–272. [Google Scholar]
- Meyer, S.; Leifeld, J.; Bahn, M.; Fuhrer, J. Land-use change in subalpine grassland soils: Effect on particulate organic carbon fractions and aggregation. J. Plant Nutr. Soil Sci. 2012, 175, 401–409. [Google Scholar]
- Yu, Y.F.; Lu, L.X.; Du, H.; Peng, W.X.; Zeng, F.P.; Song, T.Q. Distribution of organic carbon and aggregation of calcareous soil in different forest types. Acta Bot. Boreali-Occident. Sin. 2013, 33, 1011–1019. [Google Scholar]
- Li, Y.F.; Zhang, J.J.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Shen, Z.M.; Wu, J.S.; Lin, L.; Wang, Z.S.; Shen, M.C. Converting native shrub forests to Chinese chestnut plantations and subsequent intensive management affected soil C and N pools. For. Ecol. Manag. 2014, 312, 161–169. [Google Scholar] [CrossRef]
- Li, Y.F.; Jiang, P.K.; Chang, S.X.; Wu, J.S.; Lin, L. Organic mulch and fertilization affect soil carbon pools and forms under intensively managed bamboo (Phyllostachys praecox) forests in southeast China. J. Soils Sediments 2010, 10, 739–747. [Google Scholar] [CrossRef]
- State Soil Survey Service of China. In Chinese Soils; China Agricultural Press: Beijing, China, 1998. (In Chinese)
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Zhou, G.M.; Jin, A.W.; He, J.C.; Wang, A.G. The influence of cultivation techniques used in covered protected plots on the high-yield property of Lei bamboo plantation for edible shoots. J. South-Cent. For.Univ. 1999, 19, 52–54. (In Chinese) [Google Scholar]
- Tan, Z.; Lal, R.; Owens, L.; Izaurralde, R.C. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007, 92, 53–59. [Google Scholar] [CrossRef]
- Griepentrog, M.; Schmidt, M.W.I. Discrepancies in utilization of density fractionation along with ultrasonic dispersion to obtain distinct pools of soil organic matter. J. Plant Nutr. Soil Sci. 2013, 176, 500–504. [Google Scholar] [CrossRef]
- Cerli, C.; Celi, L.; Kalbitz, K.; Guggenberger, G.; Kaiser, K. Separation of light and heavy organic matter fractions in soil-Testing for proper density cut-off and dispersion level. Geoderma 2012, 170, 403–416. [Google Scholar] [CrossRef]
- Crow, S.E.; Swanston, C.W.; Lajtha, K.; Brooks, J.R.; Keirstead, H. Density fractionation of forest soils: Methodological questions and interpretation of incubation results and turnover time in an ecosystem context. Biogeochemistry 2007, 85, 69–90. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis, 2nd ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Lu, R.K. Analytical Methods for Soil Agrochemistry; Chinese Agricultural Science and Technology Publishing House: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Johnsen, K.H.; Samuelson, L.J.; Sanchez, F.G.; Eaton, R.J. Soil carbon and nitrogen content and stabilization in mid-rotation, intensively managed sweetgum and loblolly pine stands. For. Ecol. Manag. 2013, 302, 144–153. [Google Scholar] [CrossRef]
- Carrington, E.M.; Hernes, P.J.; Dyda, R.Y.; Plante, A.F.; Six, J. Biochemical changes across a carbon saturation gradient: Lignin, cutin, and suberin decomposition and stabilization in fractionated carbon pools. Soil Biol. Biochem. 2012, 47, 179–190. [Google Scholar] [CrossRef]
- Jenkenson, D.S.; Hart, P.B.S.; Payner, J.H.; Pary, L.C. Modeling the turnover of organic matter in long-term experiments at Rothamsted. Intecol. Bull. 1988, 15, 1–8. [Google Scholar]
- Van Veen, J.A.; Kuikman, P.J. Soil structure aspects of decomposition of organic matter by microorganisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Carter, M.R.; Angers, D.A.; Gregorich, E.G.; Bolinder, M.A. Characterizing organic matter retention for surface soils in eastern Canada using density and particle size fractions. Can. J. Soil Sci. 2003, 83, 11–23. [Google Scholar] [CrossRef]
- Zhang, J.J.; Li, Y.F.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Liu, J.; Wu, J.S.; Shen, Z.M. Understory vegetation control affected greenhouse gas emissions and labile organic carbon pools in an intensively managed Chinese chestnut plantation. Plant Soil 2014, 376, 363–375. [Google Scholar] [CrossRef]
- Hu, Y.L.; Zeng, D.H.; Chang, S.X.; Mao, R. Dynamics of soil and root C stocks following afforestation of croplands with poplars in a semi-arid region in northeast China. Plant Soil 2013, 368, 619–627. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Y.F.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Liu, J.; Lin, L. Converting paddy fields to Lei bamboo (Phyllostachys praecox) stands affected soil nutrient concentrations, labile organic carbon pools, and organic carbon chemical compositions. Plant Soil 2013, 367, 249–261. [Google Scholar] [CrossRef]
- Barrios, E.; Buresh, R.J.; Sprent, J.I. Organic matter in soil particle size and density fraction from maize and legume cropping systems. Soil Biol. Biochem. 1996, 28, 185–193. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.P.; Liang, A.Z.; Fang, H.J.; Yang, X.M. Spatial distribution of density fractions of soil organic carbon at a sloping farmland of a Black soil. Chin. J. Soil Sci. 2008, 39, 1023–1029. [Google Scholar]
- Xu, S.Q.; Cui, S.Y.; Chen, F.; Xiao, X.P.; Zhang, H.L. Effect of tillage on content of density fractions of paddy soil organic carbon and its spatial distribution. J. Agro-Environ. Sci. 2011, 30, 127–132. [Google Scholar]
- Boone, R.D. Light fraction soil organic matter: Origin and contribution to net nitrogen mineralization. Soil Biol. Biochem. 1994, 26, 1459–1468. [Google Scholar] [CrossRef]
- Xie, J.S.; Yang, Y.S.; Xie, M.S.; Chen, G.S.; Yang, Z.J.; Huang, S.D. Effect of vegetation restoration on soil organic matter of light fraction in eroded degraded Red soil in subtropics China. Acta Pedol. Sin. 2008, 45, 170–177. [Google Scholar]
- Strickland, T.C.; Sollins, P. Improved method for separating light and heavy fraction organic material from soil. Soil Sci. Soc. Am. J. 1987, 51, 1390–1393. [Google Scholar] [CrossRef]
- Wu, J.G.; Zhang, X.Q.; Wang, Y.H.; Xu, D.Y. The effect of land use changes on the distribution of soil organic carbon in physical fractionation of soil. Sci. Silvae Sin. 2002, 38, 19–29. [Google Scholar]
- Liu, L.X.; Wang, J.C.; Liu, J.X.; Wang, X. Analytical study of biochemical properties and ability to protect organic-carbon on Chinese pine forest Gray Cinnamon forest soil in Ziwulin Mountain, Northwest China. J. Soil Water Conserv. 2010, 24, 94–99. [Google Scholar]
- Ladd, J.N.; Amato, M. Studies of nitrogen immobilization and mineralization in calcareous soils—IV. Changes in the organic nitrogen of light and heavy subfractions of silt-and fine clay-size particles during nitrogen turnover. Soil Biol. Biochem. 1980, 12, 185–189. [Google Scholar]
- Williams, A.; Xing, B.S.; Veneman, P. Effect of cultivation on soil organic matter and aggregate stability. Pedosphere 2005, 15, 255–262. [Google Scholar]
- Yin, Y.; Cai, Z.C.; Qin, S.W. Dynamics of fluvo-aquic soil organic matter fractions under long-term fertilization. Chin. J. Appl. Ecol. 2005, 16, 875–878. [Google Scholar]
- Shi, J.P.; Zhang, F.D.; Lin, J. Effect of long-term fertilization on organo-mineral complex status in soils. Plant Nutr. Fertil. Sci. 2002, 8, 131–136. [Google Scholar]
- Swanston, C.W.; Caldwell, B.A.; Homann, P.S.; Ganio, L.; Sollins, P. Carbon dynamics during a long-term incubation of separate and recombined density fractions from seven forest soils. Soil Biol.Biochem. 2002, 34, 1121–1130. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhang, J.J.; Chang, S.X.; Jiang, P.K.; Zhou, G.M.; Fu, S.L.; Yan, E.R.; Wu, J.S.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Baritz, R.; Seufert, G.; Montanarella, L.; van Ranst, E. Carbon concentrations and stocks in forest soils of Europe. For. Ecol. Manag. 2010, 260, 262–277. [Google Scholar] [CrossRef]
- Yang, Y.S.; Xie, J.S.; Sheng, H.; Chen, G.S.; Li, X. The impact of land use/cover change on soil organic carbon stocks and quality in mid-subtropical mountainous area of southern China. Acta Geogr. Sin. 2007, 62, 1123–1131. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shang, S.; Jiang, P.; Chang, S.X.; Song, Z.; Liu, J.; Sun, L. Soil Organic Carbon in Particle Size and Density Fractionations under Four Forest Vegetation-Land Use Types in Subtropical China. Forests 2014, 5, 1391-1408. https://doi.org/10.3390/f5061391
Shang S, Jiang P, Chang SX, Song Z, Liu J, Sun L. Soil Organic Carbon in Particle Size and Density Fractionations under Four Forest Vegetation-Land Use Types in Subtropical China. Forests. 2014; 5(6):1391-1408. https://doi.org/10.3390/f5061391
Chicago/Turabian StyleShang, Suyun, Peikun Jiang, Scott X. Chang, Zhaoliang Song, Juan Liu, and Lei Sun. 2014. "Soil Organic Carbon in Particle Size and Density Fractionations under Four Forest Vegetation-Land Use Types in Subtropical China" Forests 5, no. 6: 1391-1408. https://doi.org/10.3390/f5061391




