A Comparison of Invasive Acer platanoides and Native A. saccharum First-Year Seedlings: Growth, Biomass Distribution and the Influence of Ecological Factors in a Forest Understory
Abstract
:1. Introduction
2. Methods
2.1. Field-Site Description
2.2. Experimental Setting
2.3. Descriptive Variables
| Parameter | Calculation |
|---|---|
| RMF (Root Mass Fraction) | Root mass/plant mass |
| SMF (Stem Mass Fraction) | Stem mass/plant mass |
| LMF (Leaf Mass Fraction) | Foliage mass/plant mass |
| SLA (Specific Leaf Area) | Foliage area/foliage mass (cm2/g) |
| LAR (Leaf Area Ratio) | Foliage area/total plant mass (cm2 leaf/g plant) |
| RGR (Relative Growth Rate) | (ln y2 – ln y1)/(t2 – t1), where y = diameter (RGR-D), length (RGR-H) or total biomass (RGR-B), t is in days |
| NAR (Net Assimilation Rate per foliar area) | RGR-B/LAR (g/cm2 × day) |
2.4. Ecological Factors
2.4.1. Leaf Herbivory and Pathogens
2.4.2. Light
2.4.3. Soil pH
2.4.4. Soil Moisture
2.5. Data Analysis
3. Results
3.1. Comparison of A. Platanoides and A. Saccharum Seedlings
| Parameters | Before transplantation | At harvest | ||||
| A. platanoides | A. saccharum | F- or Q-test | A. platanoides | A. saccharum | F- or Q-test | |
| n = 120 | n = 120 | n = 240 | n = 120 | n = 117 | n = 237 | |
| Diameter (mm) | 3.60 (0.41) | 3.40 (0.44) | 11.15** | 5.02 (0.78) | 4.26 (0.57) | 48.73*** |
| Height (cm) | 19.2 (5.5) | 20.0 (5.1) | 1.52 1 | 22.1 (6.4) | 21.9 (5.2) | 0.02 2 |
| Number of leaves | 13.7 (4.0) | 11.5 (2.3) | 23.91*** | 11.1 (4.6) | 9.6 (3.8) | 11.23** |
| RGR-D (×10−3 mm/mm×day) | – | – | – | 3.5 (1.6) | 2.4 (1.3) | 31.74*** |
| RGR-H (×10−3 cm/cm×day) | – | – | – | 1.4 (1.5) | 1.0 (0.8) | 2.25 1 |
| Biomass | n = 55 | n = 56 | n = 111 | n = 55 | n = 56 | n = 111 |
| Total (g) | 1.93 (0.44) | 1.56 (0.34) | 19.95*** | 3.22 (1.12) | 2.12 (0.76) | 45.16*** |
| Roots (g) | 0.44 (0.14) | 0.28 (0.08) | 40.36*** | 1.04 (0.42) | 0.74 (0.29) | 22.30*** |
| Shoot (g) | 0.44 (0.12) | 0.35 (0.10) | 13.13*** | 0.99 (0.38) | 0.66 (0.22) | 33.05*** |
| Leaves (g) | 1.06 (0.22) | 0.93 (0.17) | 10.50** | 1.19 (0.46) | 0.72 (0.33) | 62.02*** 1 |
| Foliar area (cm2) | – | – | – | 298.9 (108.1) | 172.6 (77.6) | 89.45*** 1 |
| Biomass fractions | – | – | – | – | – | – |
| RMF | 0.22 (0.04) | 0.178 (0.03) | 37.10*** | 0.32 (0.07) | 0.36 (0.05) | 7.75** |
| SMF | 0.22 (0.01) | 0.221 (0.03) | 0.09 2 | 0.31 (0.05) | 0.32 (0.07) | 0.53 |
| LMF | 0.55 (0.05) | 0.602 (0.04) | 39.23*** 2 | 0.37 (0.07) | 0.33 (0.08) | 9.27** 1 |
| SLA (cm2/g) | – | – | – | 259 (46) | 243 (33) | 14.14*** 1 |
| LAR (cm2/g) | – | – | – | 94.7 (22.2) | 78.9 (21.0) | 14.82*** |
| RGR-B (×10−3 g/g×day) | – | – | – | 5.1 (2.8) | 2.8 (3.1) | 22.22*** |
| NAR (×10−1 g/m2×day) | – | –- | – | 5.65 (3.95) | 2.81 (7.05) | 14.38*** 1 |
| Herbivory (%) | – | – | – | 11.2 (10.2) | 13.9 (14.0) | 0.851 |
3.2. The Influence of Ecological Factors on Seedling Growth Parameters
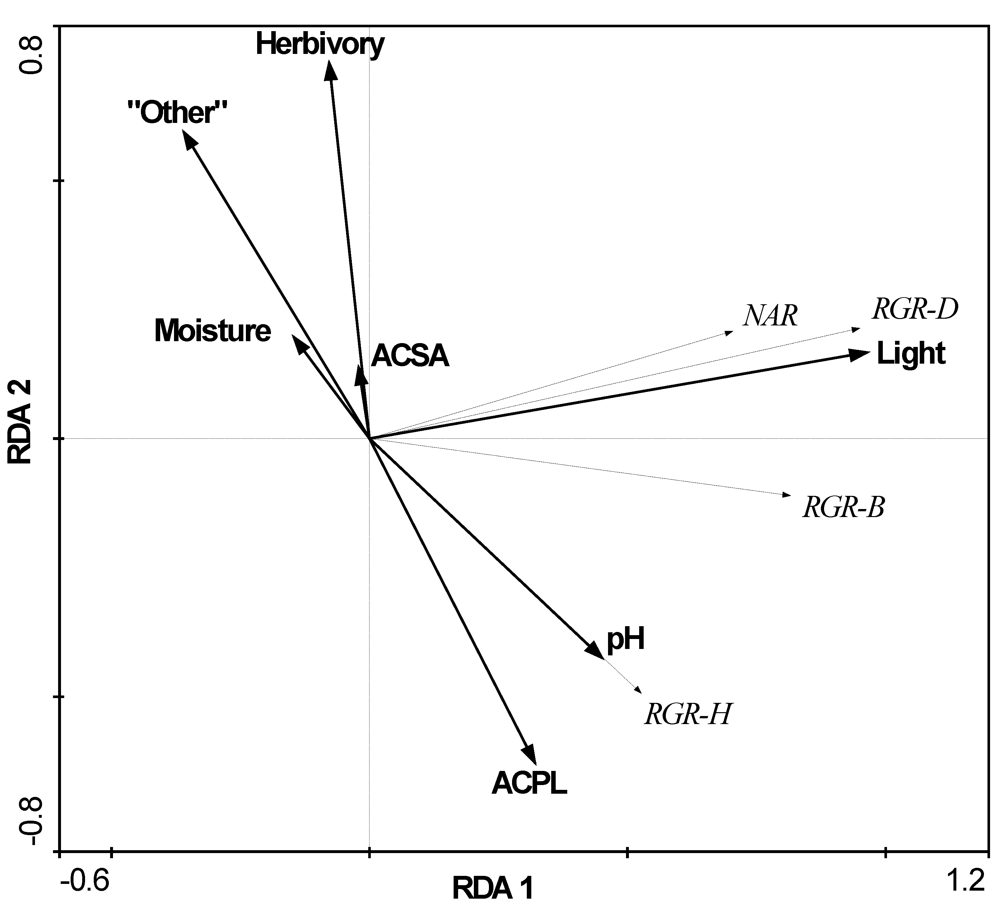
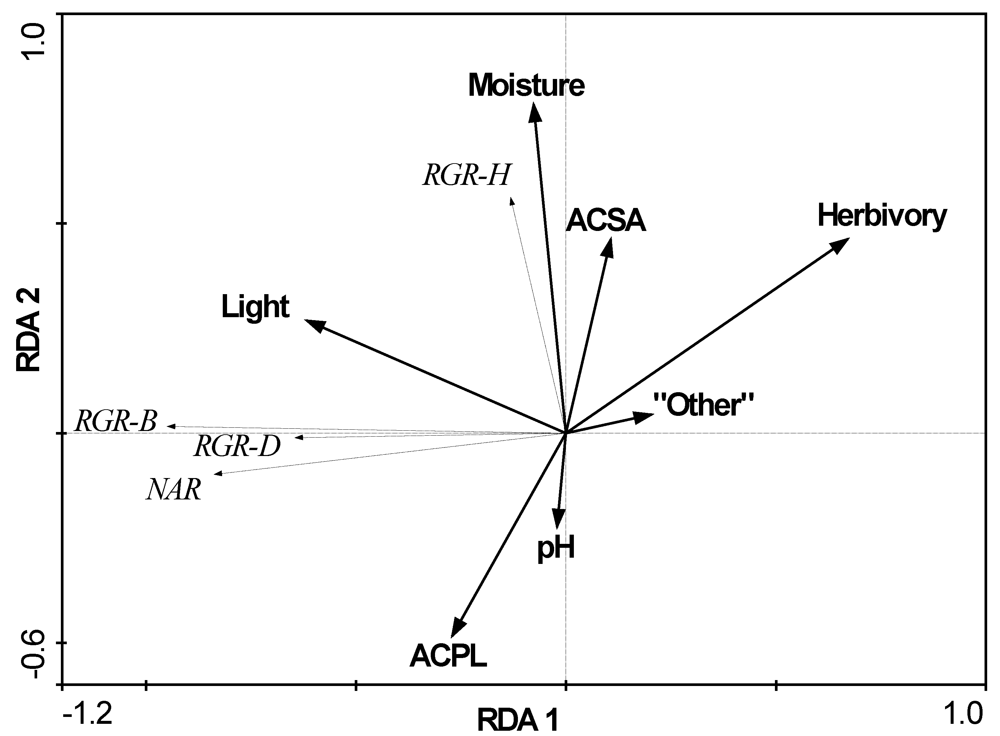
| Factors | A. platanoides canopy | A. saccharum canopy | Other native spp. canopy | ACPL vs. ACSA F- or U-test | ACPL vs. all native spp. F or U-test |
|---|---|---|---|---|---|
| n = 96 | n = 80 | n = 64 | n = 176 | n = 240 | |
| Light (%) | 2.53 (1.34) | 1.69 (0.87) | 1.43 (0.64) | 22.36*** 2 | 38.67*** 2 |
| Soil moisture (%) | 12.4 (3.2) | 13.7 (4.8) | 13.4 (2.8) | 0.69 1 | 1.41 1 |
| Soil pH | 5.98 (0.55) | 5.67 (0.40) | 5.45 (0.62) | 3.58 | 5.79* 1 |
| Herbivory (%) | 9.5 (8.8), n = 48 | 15.2 (15.7), n = 39 | 14.4 (11.1), n = 24 | 2.44 1, n = 87 | 3.711, n = 111 |
4. Discussion
4.1. Growth, Biomass Distribution and Survival of A. Platanoides and A. Saccharum Seedlings
4.2. The Influence of Ecological Factors
4.2.1. Growth and Understory Light Levels
4.2.2. The influence of Leaf Herbivory on Seedling Growth
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Eco. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Reichard, S.H.; Hamilton, C.W. Predicting invasions of woody plants introduced into North America. Conserv. Bio. 1997, 11, 193–203. [Google Scholar]
- Martin, P.H.; Canham, C.D.; Marks, P.L. Why forests appear resistant to exotic plant invasions: Intentional introductions, stand dynamics, and the role of shade tolerance. Front. Ecol. Environ. 2008, 6, 142–149. [Google Scholar]
- Canham, C.D. Suppression and release during canopy recruitment in Acer saccharum. Bull. Torrey Bot. Club 1985, 112, 134–145. [Google Scholar] [CrossRef]
- Wangen, S.R.; Webster, C.R. Potential for multiple lag phases during biotic invasions: Reconstructing an invasion of the exotic tree Acer platanoides. J. Appl. Ecol. 2006, 43, 258–268. [Google Scholar] [CrossRef]
- Webster, C.R.; Jenkins, M.A.; Jose, S. Woody invaders and the challenges they pose to forest ecosystems in the eastern United States. J. For. 2006, 104, 366–374. [Google Scholar]
- Herron, P.M.; Martine, C.T.; Latimer, A.M.; Leicht-Young, S.A. Invasive plants and their ecological strategies: Prediction and explanation of woody plant invasion in New England. Divers. Distrib. 2007, 13, 633–644. [Google Scholar]
- Santamour, F.S.; McArdle, A.J. Checklist of cultivated maples. III. Acer platanoides L. J. Arboric. 1982, 8, 241–246. [Google Scholar]
- Nowak, D.J.; Rowntree, R.A. History and range of Norway maple. J. Arboric. 1990, 16, 291–296. [Google Scholar]
- Anderson, R. Disturbance as a factor in the distribution of sugar maple and the invasion of Norway maple into a modified woodland. Rhodora 1999, 101, 264–273. [Google Scholar]
- Webb, S.L.; Pendergast, T.H.; Dwyer, M.E. Response of native and exotic maple seedling banks to removal of the exotic, invasive Norway maple (Acer platanoides). J. Torrey Bot. Soc. 2001, 128, 141–149. [Google Scholar] [CrossRef]
- Bertin, R.I.; Manner, M.E.; Larrow, B.F.; Cantwell, T.W.; Berstene, E.M. Norway maple (Acer platanoides) and other non-native trees in urban woodlands of central Massachusetts. J. Torrey Bot. Soc. 2005, 132, 225–235. [Google Scholar] [CrossRef]
- Webster, C.R.; Nelson, K.; Wangen, S.R. Stand dynamics of an insular population of an invasive tree, Acer platanoides. For. Ecol. Manag. 2005, 208, 85–99. [Google Scholar] [CrossRef]
- Wangen, S.R.; Webster, C.R.; Griggs, J.A. Spatial characteristics of the invasion of Acer platanoides on a temperate forested island. Biol. Invasions 2006, 8, 1001–1012. [Google Scholar] [CrossRef]
- Midy, J.; Brisson, J.; Boivin, P. Invasion du Parc du Mont-Royal par l'érable de Norvège: Evaluation de la Situation, Causes, Conséquences. Rapport d'Activités Final Présenté à la Direction des Sports, des Parcs et des Espaces Verts, Ville de Montréal; Institut de Recherche en Biologie Végétale: Montreal, QC, Canada, 2007. [Google Scholar]
- Webb, S.L.; Dwyer, M.; Kaunzinger, C.K.; Wyckoff, P.H. The myth of the resilient forest: Case study of the invasive Norway maple (Acer platanoides). Rhodora 2000, 102, 332–354. [Google Scholar]
- Fang, W. Spatial analysis of an invasion front of Acer platanoides: dynamic inferences from static data. Ecography 2005, 28, 283–294. [Google Scholar] [CrossRef]
- Martin, P.H.; Marks, P.L. Intact forests provide only weak resistance to a shade-tolerant invasive Norway maple (Acer platanoides L). J. Ecol. 2006, 94, 1070–1079. [Google Scholar] [CrossRef]
- Martin, P.H.; Canham, C.D. Dispersal and recruitment limitation in native versus exotic tree species: Life-history strategies and Janzen-Connell effects. Oikos 2010, 119, 807–824. [Google Scholar]
- Martin, P.H.; Canham, C.D.; Kobe, R.K. Divergence from the growth–survival trade-off and extreme high growth rates drive patterns of exotic tree invasions in closed-canopy forests. J. Ecol. 2010, 98, 778–789. [Google Scholar]
- Wyckoff, P.H.; Webb, S.L. Understory influence of the invasive Norway maple (Acer platanoides). Bull. Torrey Bot. Club 1996, 123, 197–205. [Google Scholar] [CrossRef]
- Martin, P.H. Norway maple (Acer platanoides) invasion of a natural forest stand: Understory consequence and regeneration pattern. Biol. Invasions 1999, 1, 215–222. [Google Scholar] [CrossRef]
- Galbraith-Kent, S.L.; Handel, S.N. Invasive Acer platanoides inhibits native sapling growth in forest understorey communities. J. Ecol. 2008, 96, 293–302. [Google Scholar] [CrossRef]
- Webb, S.L.; Kaunzinger, C.K. Biological invasion of the Drew University (New Jersey) forest preserve by Norway maple (Acer platanoides L.). Bull. Torrey Bot. Club 1993, 120, 343–349. [Google Scholar] [CrossRef]
- Godman, R.M.; Yawney, H.W.; Tubbs, C.H. Sugar maple (Acer saccharum Marsh). In Sylvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA Forest Service: Washington, DC, USA, 1990; Volume 2, pp. 78–91. [Google Scholar]
- Marks, P.L.; Gardescu, S. A case study of sugar maple (Acer saccharum) as a forest seedling bank species. J. Torrey Bot. Soc. 1998, 125, 287–296. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A., Jr.; Kobe, R.K.; Ribbens, E. Forest models defined by field measurements: Estimation, error analysis and dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Morrison, J.A.; Mauck, K. Experimental field comparison of native and non-native maple seedlings: Natural enemies, ecophysiology, growth and survival. J. Ecol. 2007, 95, 1036–1049. [Google Scholar] [CrossRef]
- Mack, R.N. Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar]
- Thiffault, C. État de situation sur les bois de l’arrondissement historique et naturel du mont Royal; Direction régionale de Montréal, Ministère de l’Environnement du Québec: Montreal, QC, Canada, 2003. [Google Scholar]
- Boivin, R. La végétation forestière du Mont-Royal (Montréal, Québec); Département de sciences biologiques; Université de Montréal: Montreal, QC, Canada, 1989. [Google Scholar]
- Brisson, J.; Bouchard, A. Érablière à caryer. In Manuel de Foresterie; MultiMondes: Sainte-Foy, QC, Canada, 2009; pp. 206–215. [Google Scholar]
- Adams, J.M.; Fang, W.; Callaway, R.M.; Cipollini, D.; Newell, E. Transatlantic Acer platanoides Invasion Network (TRAIN). A cross-continental test of the Enemy Release Hypothesis: Leaf herbivory on Acer platanoides (L.) is three times lower in North America than in its native Europe. Biol. Invasions 2009, 11, 1005–1016. [Google Scholar] [CrossRef]
- Lapointe, M.; Brisson, J. Tar spot disease on Norway maple in North America: Quantifying the impacts of a reunion between an invasive tree species and its adventive natural enemy. Ecoscience 2011, 18, 63–69. [Google Scholar]
- Paquette, A.; Bouchard, A.; Cogliastro, A. A less restrictive technique for the estimation of understory light under variable weather conditions. For. Ecol. Manag. 2007, 242, 800–804. [Google Scholar]
- JMP Statistical Discovery Software, version 7.0.1, SAS Institute Inc.: Cary, NC, USA, 2007.
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Meiners, S.J. Seed and seedling ecology of Acer saccharum and Acer platanoides: A contrast between native and exotic congeners. Northeast. Naturalist 2005, 12, 23–32. [Google Scholar] [CrossRef]
- Kloeppel, B.D.; Abrams, M.D. Ecophysiological attributes of the native Acer saccharum and the exotic Acer platanoides in urban oak forests in Pennsylvania, USA. Tree Physiol. 1995, 15, 739–746. [Google Scholar]
- Paquette, A.; Fontaine, B.; Berninger, F.; Dubois, K.; Lechowicz, M.J.; Messier, C.; Posada, J.M.; Valladares, F.; Brisson, J. Seedlings of the exotic norway maple have greater plasticity and late season height growth than native sugar maple in canopy gaps. 2012. submitted. [Google Scholar]
- Sanford, N.L.; Harrington, R.A.; Fownes, J.H. Survival and growth of native and alien woody seedlings in open and understory environments. For. Ecol. Manag. 2003, 183, 377–385. [Google Scholar]
- Walters, M.B.; Reich, P.B. Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 2000, 81, 1887–1901. [Google Scholar] [CrossRef]
- Walters, M.B.; Reich, P.B. Are shade tolerance, survival, and growth linked? Low light and, nitrogen effects on hardwood seedlings. Ecology 1996, 77, 841–853. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Canham, C.D.; Martin, P.H. Neighbourhood models of the effects of the invasive Acer platanoides on tree seedling dynamics: Linking impacts on communities and ecosystems. J. Ecol. 2008, 96, 78–90. [Google Scholar]
- Gómez-Aparicio, L.; Canham, C.D. Neighborhood models of the effects of invasive tree species on ecosystem processes. Ecol. Monogr. 2008, 78, 69–86. [Google Scholar]
- Reinhart, K.O.; Greene, E.; Callaway, R.M. Effects of Acer platanoides invasion on understory plant communities and tree regeneration in the northern Rocky Mountains. Ecography 2005, 28, 573–582. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Gurnee, J.; Tirado, R.; Callaway, R.M. Invasion through quantitative effects: Intense shade drives native decline and invasive success. Ecol. Appl. 2006, 16, 1821–1831. [Google Scholar]
- Lapointe, M. Université de Montréal, Montreal, QC, Canada. Presence of crown damage by the tar spot disease on A. platanoides, one year after the study took place. 2008; Personal observation. [Google Scholar]
- Cincotta, C.L.; Adams, J.M.; Holzapfel, C. Testing the enemy release hypothesis: A comparison of foliar insect herbivory of the exotic Norway maple (Acer platanoides L.) and the native sugar maple (A. saccharum L.). Biol. Invasions 2009, 11, 379–388. [Google Scholar] [CrossRef]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trend Ecol. Evol. 2005, 20, 441–448. [Google Scholar]
- Reinhart, K.O.; Callaway, R.M. Soil biota facilitate exotic Acer invasions in Europe and North America. Ecol. Appl. 2004, 14, 1737–1745. [Google Scholar] [CrossRef]
- Richardson, D.M.; Allsopp, N.; D’Antonio, C.M.; Milton, S.J.; Rejmanek, M. Plant invasions—The role of mutualisms. Biol. Rev. Cambridge Phil. Soc. 2000, 75, 65–93. [Google Scholar]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar]
- Agrawal, A.A.; Kotanen, P.M.; Mitchell, C.E.; Power, A.G.; Godsoe, W.; Klironomos, J. Enemy release? An experiment with congeneric plant pairs and diverse above- and belowground enemies. Ecology 2005, 86, 2979–2989. [Google Scholar]
- Hudler, G.W.; Jensen-Tracy, S.; Banik, M.T. Rhytisma americanum sp. nov.: A previously undescribed species of Rhytisma on Maples (Acer spp.). Mycotaxon 1998, 68, 405–416. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lapointe, M.; Brisson, J. A Comparison of Invasive Acer platanoides and Native A. saccharum First-Year Seedlings: Growth, Biomass Distribution and the Influence of Ecological Factors in a Forest Understory. Forests 2012, 3, 190-206. https://doi.org/10.3390/f3020190
Lapointe M, Brisson J. A Comparison of Invasive Acer platanoides and Native A. saccharum First-Year Seedlings: Growth, Biomass Distribution and the Influence of Ecological Factors in a Forest Understory. Forests. 2012; 3(2):190-206. https://doi.org/10.3390/f3020190
Chicago/Turabian StyleLapointe, Marie, and Jacques Brisson. 2012. "A Comparison of Invasive Acer platanoides and Native A. saccharum First-Year Seedlings: Growth, Biomass Distribution and the Influence of Ecological Factors in a Forest Understory" Forests 3, no. 2: 190-206. https://doi.org/10.3390/f3020190




