Carbon Supported Engineering NiCo2O4 Hybrid Nanofibers with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
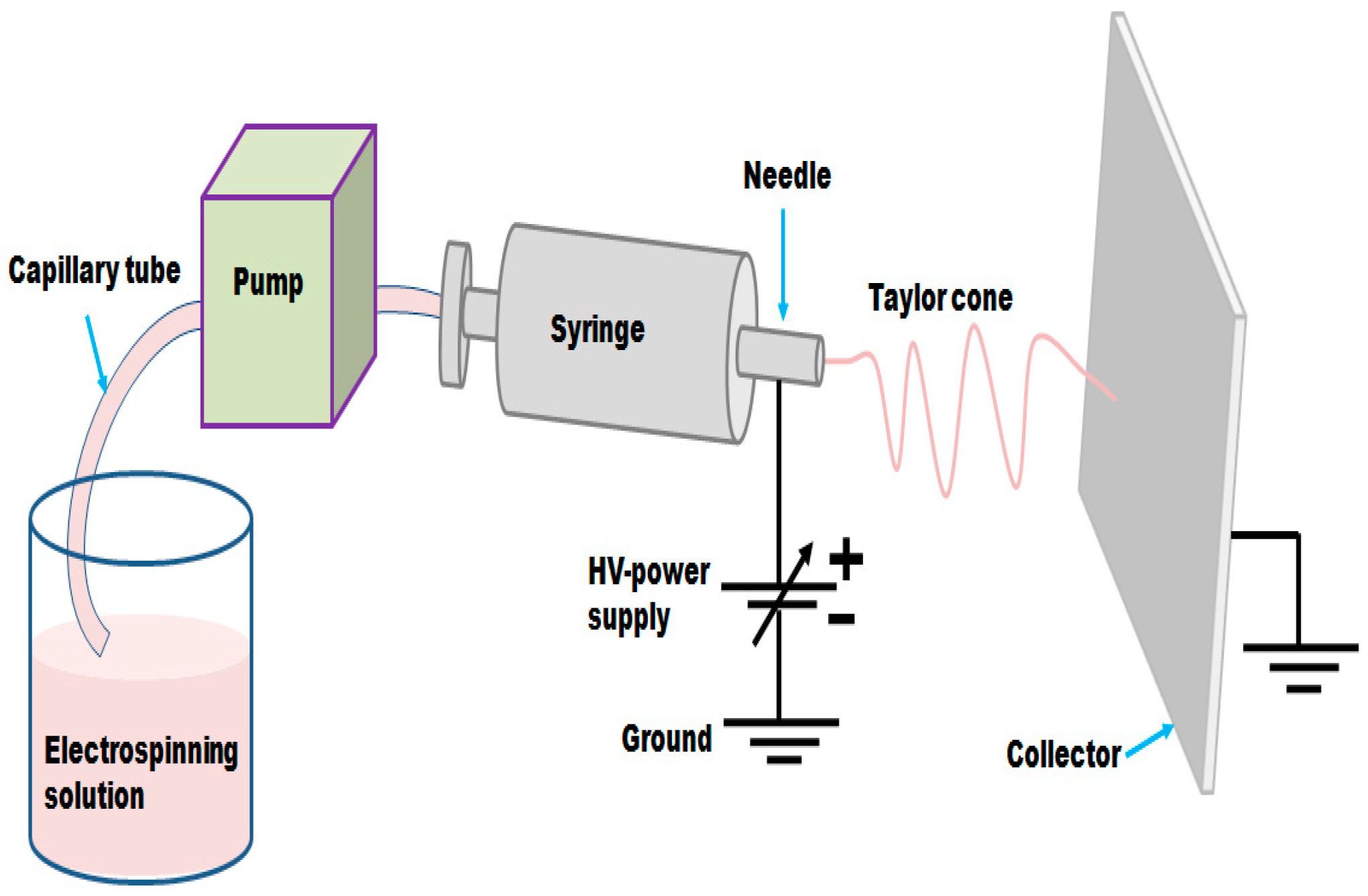
2.2. Preparation of C/NiCo2O4 and NiCo2O4 Nanofibers by Electrospinning Method
2.3. Electrochemical Measurements
2.4. Characterization of the Catalysts
3. Results and Discussion
3.1. Synthesis
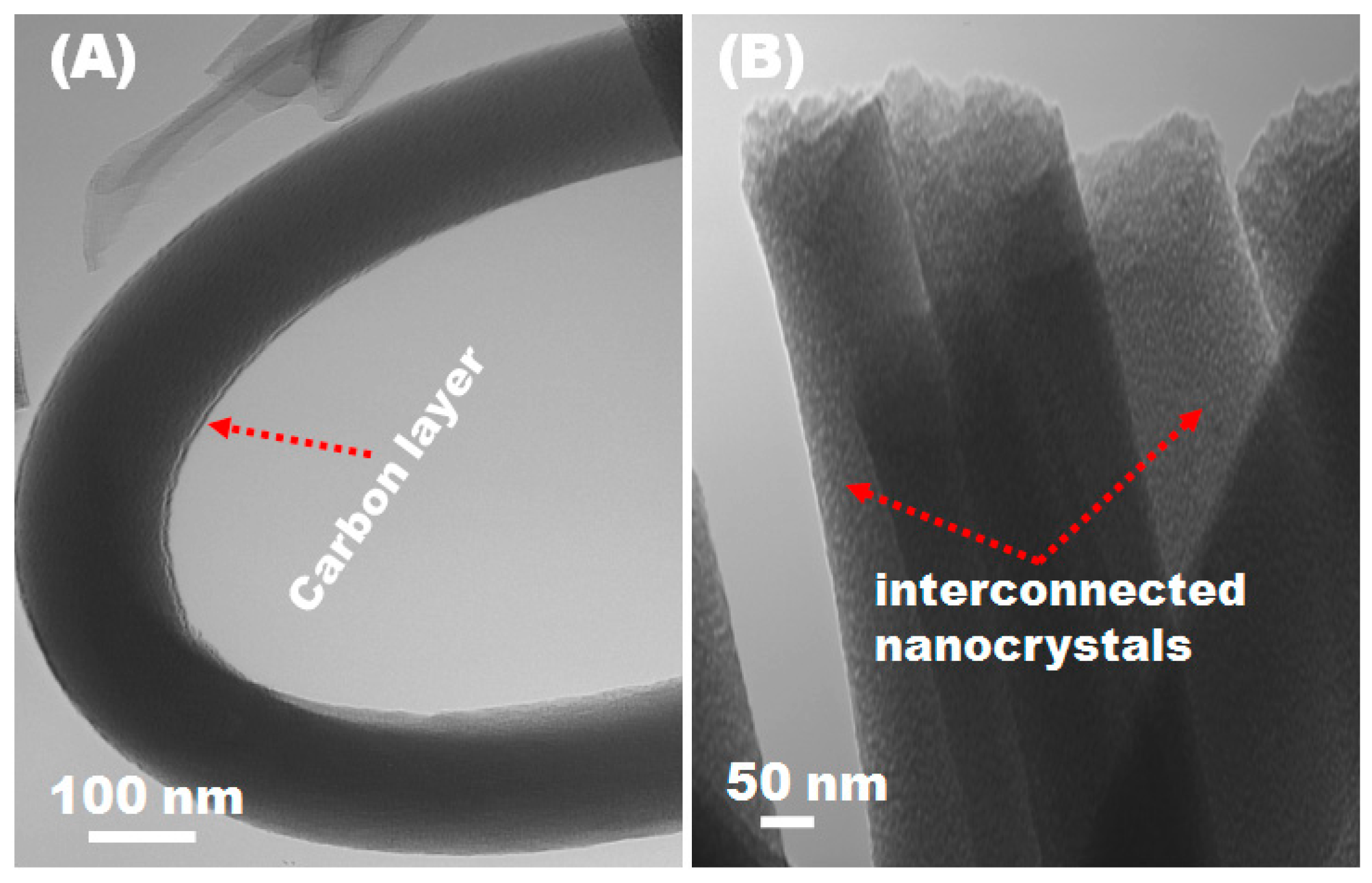
3.2. Morphology and Structure Analyses
3.3. Crystallographic and Chemical Composition of Synthesized Nanofibers
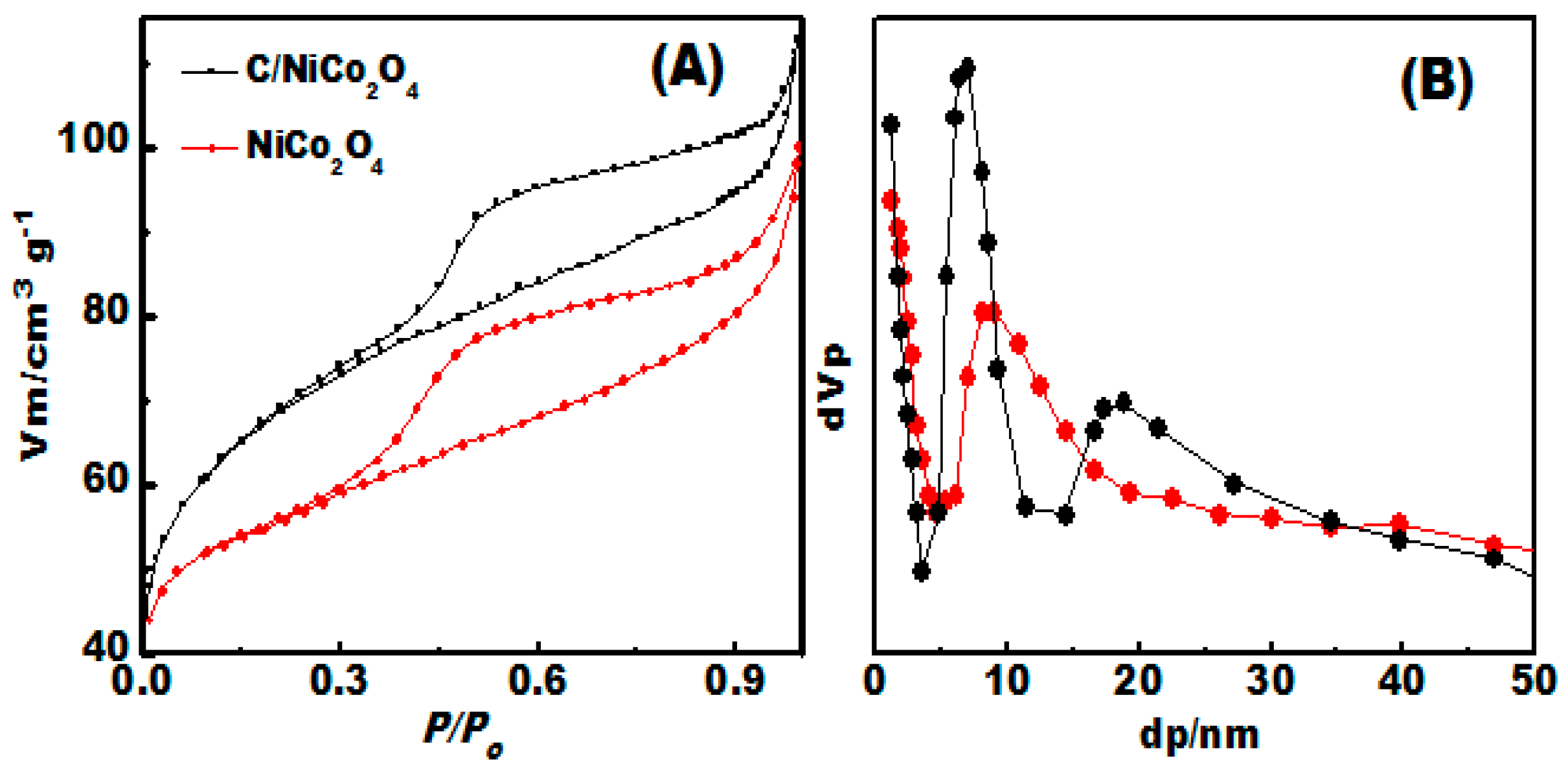
3.4. Surface Area and Porous Structure Investigation
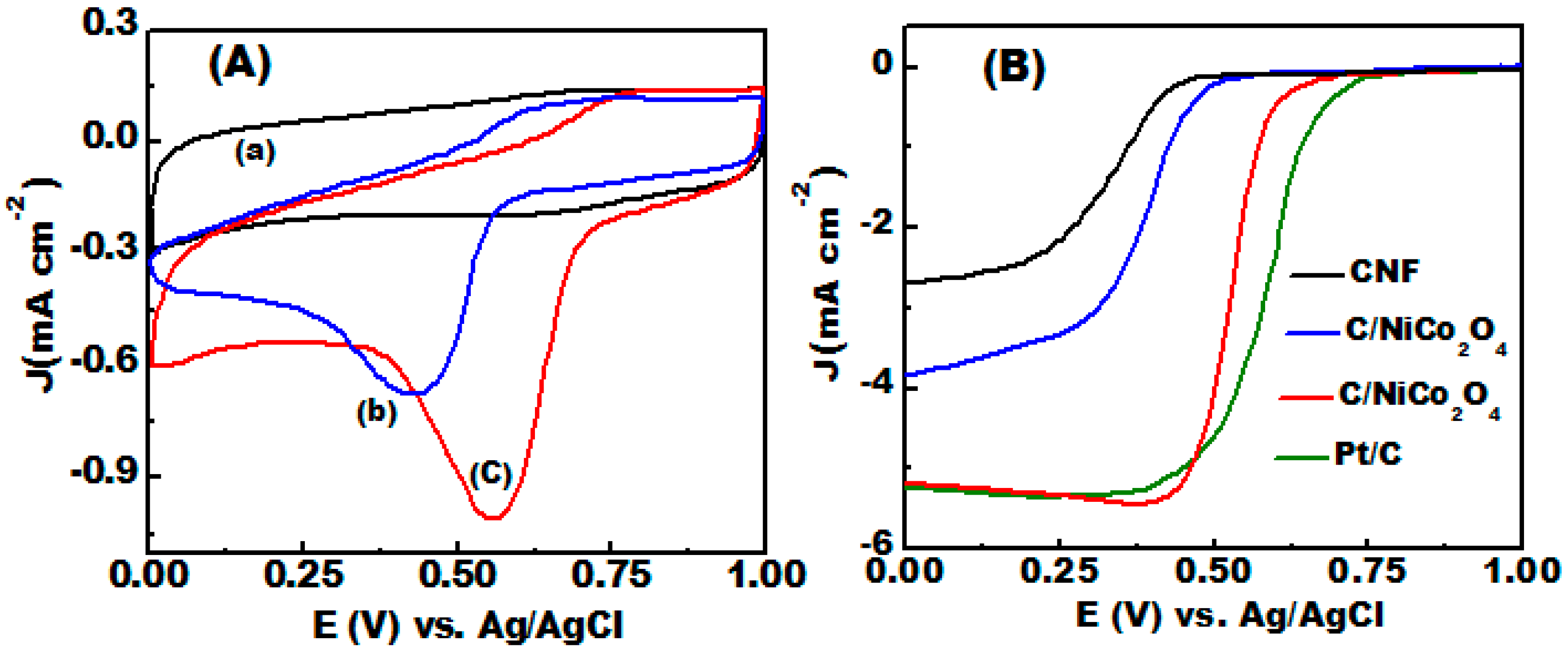
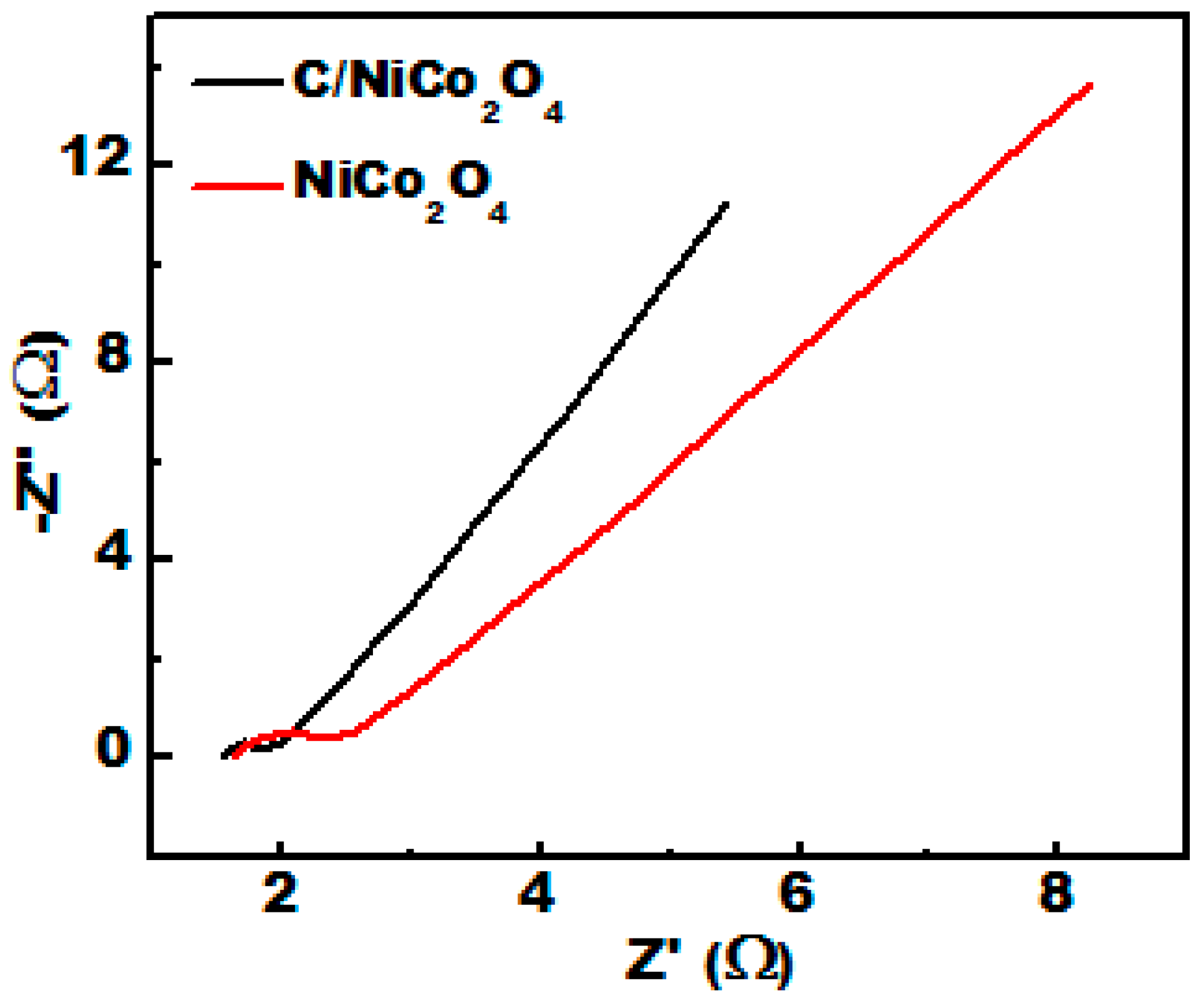
3.5. ORR Electrocatalytic Activity
- (i)
- Fast electron transport to the catalytically active sites due to improved conductivity.
- (ii)
- Synergetic contact between the carbon matrices and homogeneously distributed Ni and Co species which enhances the accessible active sites and thus lead to better utilization of the electroactive material.
- (iii)
- Richness of electroactive sites can efficiently contribute to the high electrocatalytic activity.
- (iv)
- Well-developed mesoporous structure which can significantly facilitate the diffusion of ions and electrons, adsorption of O2-molecules, and subsequently improve the reaction kinetics.
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Respinis, M.; Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 2014, 6, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors. Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Debe, M.K. Electrocatalyst approaches and challenges for automotive. Nature 2012, 486, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Hassen, D.; El-Safty, S.A.; Tsuchiya, K.; Chatterjee, A.; Elmarakbi, A.; Shenashen, M.A.; Sakai, M. Longitudinal hierarchy Co3O4mesocrystals with high-dense exposure facets and anisotropic interfaces for direct-ethanol fuel cells. Sci. Rep. 2016, 6, 24330. [Google Scholar] [CrossRef] [PubMed]
- Sa, Y.J.; Kwon, K.; Cheon, J.Y.; Kleitz, F.; Joo, S.H. Ordered mesoporous Co3O4spinels as stable, bifunctional, noble metal-free oxygen electrocatalysts. J. Mater. Chem. A 2013, 1, 9992–10001. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.Y.; Li, Y.G.; Wang, H.L.; Zhou, J.G.; Wang, J.; Regier, T.; Dai, H.J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xia, B.Y.; Wang, X.; Lou, X.W.D. Strongly coupled NiCo2O4-rGO hybrid nanosheets as a methanol-tolerant electrocatalyst for the oxygen reduction reaction. Adv. Mater. 2014, 26, 2408–2412. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B 2005, 56, 9–35. [Google Scholar] [CrossRef]
- Gasteiger, H.A.; Markovic, N.M. Just a dream—Or future reality. Science 2009, 324, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zheng, Y.; Chen, J.; Li, J.; Hulicova, J.D.; Jaroniec, M.; Qiao, S.Z. Facile oxygen reduction on a three-dimensionally ordered macroporous graphitic C3N4/carbon composite electrocatalyst. Angew. Chem. Int. Ed. 2012, 51, 3958–3962. [Google Scholar] [CrossRef]
- Louie, M.W.; Bell, A.T. An investigation of thin-film Ni−Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese−cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Chen, C.; Xi, J.; Xu, Y.; Xiao, F.; Wang, S.; Yang, S. Core–shell Co@Co3O4 nanoparticle-embedded bamboo-like nitrogen-doped carbon nanotubes (BNCNTs) as a highly active electrocatalyst for the oxygen reduction reaction. Nanoscale 2015, 7, 7056–7064. [Google Scholar] [PubMed]
- Xie, G.; Chen, B.; Jiang, Z.; Niu, X.; Cheng, S.; Zhen, Z.; Jiang, Y.; Rong, H.; Jiang, Z.-J. High catalytic activity of Co3O4 nanoparticles encapsulated in a graphene supported carbon matrix for oxygen reduction reaction. RSC Adv. 2016, 6, 50349–50357. [Google Scholar] [CrossRef]
- He, Q.; Li, Q.; Khene, S.; Renll, X.; López-Suárez, F.E.; Lozano-Castelló, D.; Bueno-López, A.; Wu, G. High-loading cobalt oxide coupled with nitrogen-doped graphene for oxygen reduction in anion-exchange-membrane alkaline fuel cells. J. Phys. Chem. C 2013, 117, 8697–8707. [Google Scholar] [CrossRef]
- Huang, Y.; Miao, Y.; Lu, H.; Liu, T. Hierarchical ZnCo2O4@NiCo2O4 core−sheath nanowires: Bifunctionality towards high performance supercapacitors and the oxygen-reduction reaction. Chem. Eur. J. 2015, 21, 10100–10108. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Chen, X.; Evans, D.G.; Yang, W.S. Well-dispersed Co3O4/Co2MnO4 nanocomposites as a synergistic bifunctional catalyst for oxygen reduction and oxygen evolution reactions. Nanoscale 2013, 5, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ma, J.; Li, C. Hierarchical porous NiCo2O4 nanowires for high-rate supercapacitors. Chem. Commun. 2012, 48, 4465–4467. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, Q.; Jiang, L. Facile approach to prepare nickel cobaltite nanowire materials for supercapacitors. Small 2011, 7, 2454–2459. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol–gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.Z.; Li, J.Y.; Hou, L.R.; Zhang, X.G.; Shen, L.F.; Lou, X.W.-D. Ultrathinmesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.P.; Wan, Y.L.; Liu, W.; Ma, Z.S.; Ji, S.M.; Wang, J.B.; Zhou, Y.C.; Hodgson, P.; Li, Y.C. Facile synthesis of NiCo2O4 nanorod arrays on Cu conductive substrates as superior anode materials for high-rate Li-ion batteries. CrystEngComm 2013, 15, 1578–1585. [Google Scholar] [CrossRef]
- Li, J.F.; Xiong, S.L.; Liu, Y.R.; Ju, Z.C.; Qian, Y.T. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Windisch, C.F.; Exarhos, G.J.; Sharma, S.K. Influence of temperature and electronic disorder on the Raman spectra of nickel cobalt oxides. J. Appl. Phys. 2002, 92, 5572–5574. [Google Scholar] [CrossRef]
- Lei, Q.; Li, G.; Li, Y.; Hongyan, Y.; Dan, X. Direct growth of NiCo2O4 nanostructures on conductive substrates with enhanced electrocatalytic activity and stability for methanol oxidation. Nanoscale 2013, 5, 7388–7396. [Google Scholar]
- Zhan, J.; Cai, M.; Zhang, C.; Wang, C. Synthesis of mesoporous NiCo2O4 fibers and their electrocatalytic activity on direct oxidation of ethanol in alkaline media. J. Electrochim. Acta 2015, 154, 70–76. [Google Scholar] [CrossRef]
- Prathap, M.A.; Srivastava, R. Synthesis of NiCo2O4 and its application in the electrocatalytic oxidation of methanol. Nano Energy 2013, 2, 1046–1053. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, H.; He, K.; Wang, S.; Tang, Y.; Chen, J. NiCo2O4/N-doped graphene as an advanced electrocatalyst for oxygen reduction reaction. J. Power Sources 2015, 280, 640–648. [Google Scholar] [CrossRef]
- Li, L.L.; Peng, S.J.; Cheah, Y.L.; Teh, P.; Wang, J.; Wee, G.; Ko, Y.; Wong, C.; Srinivasan, M. Electrospun porous NiCo2O4 nanotubes as advanced electrodes for electrochemical capacitors. Chem. Eur. J. 2013, 19, 5892–5898. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, C.G.; Qu, L.T.; Hu, C.W.; Cao, M.H. Three-dimensional macroporous NiCo2O4 sheets as a non-noble catalyst for efficient oxygen reduction reactions. Chem. Eur. J. 2013, 42, 14271–14278. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, S.; Guo, C.; Xia, X.; Guo, X.-Y. Mesoporous NiCo2O4 nanoplates on three-dimensional graphene foam as an efficient electrocatalyst for the oxygen reduction reaction. ACS Appl. Mater. Interfaces 2016, 8. [Google Scholar] [CrossRef]
- Lapham, D.P.; Colbeck, I.; Schoonman, J.; Kamlag, Y. The preparation of NiCo2O4 films by electrostatic spray deposition. Thin Solid Films 2001, 391, 17–20. [Google Scholar] [CrossRef]
- Nkeng, P.; Koenig, J.F.; Gautier, J.L.; Chartier, P.; Poillerat, G. Enhancement of surface areas of Co3O4 and NiCo2O4 electrocatalysts prepared by spray pyrolysis. J. Electroanal. Chem. 1996, 402, 81–89. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Samuel, S.; Chartier, P. Active thin NiCo2O4 film prepared on nickel by spray pyrolysis for oxygen evolution. Int. J. Hydrogen Energy 1995, 20, 9–15. [Google Scholar] [CrossRef]
- Tseung, A.C.C.; Jasem, S. Oxygen evolution on semiconducting oxides. Electrochim. Acta 1977, 22, 31–34. [Google Scholar] [CrossRef]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A facile technique for fabricating polymeric nanofibers doped with carbon nanotubes and metallic nanoparticles for sensor applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Li, L.L.; Peng, S.J.; Cheah, Y.L.; Wang, J.; Teh, P.F.; Ko, Y.W.; Wong, C.L.; Srinivasan, M. Electrospun eggroll-like CaSnO3 nanotubes with high lithium storage performance. Nanoscale 2013, 5, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Viswanathamurthi, P.; Bhattarai, N.; Kim, H.Y.; Lee, D.R. Vanadium pentoxidenanofibers by electrospinning. Scr. Mater. 2003, 49, 577–581. [Google Scholar] [CrossRef]
- Yang, X.; Shao, C.; Guan, H.; Li, X.; Gong, J. Preparation and characterization of ZnO nanofibers by using electrospun PVA/zinc acetate composite fiber as precursor. Inorg. Chem. Commun. 2004, 7, 176–178. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, S.; Zhang, Y.; Cao, M. Engineering hybrid between nickel oxide and nickel cobaltate to achieve exceptionally high activity for oxygen reduction reaction. J. Power Sources 2014, 272, 808–815. [Google Scholar] [CrossRef]
- Zhang, P.; Zhao, X.; Zhang, X.; Lai, Y.; Wang, X.; Li, J.; Wei, G.; Su, Z. Electrospun doping of carbon nanotubes and platinum nanoparticles into the β‑phase polyvinylidene difluoride nanofibrous membrane for biosensor and catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.Y.; Ahn, C.; You, D.J.; Pak, C.; Hur, S.H.; Kim, J.; Joo, S.H. Ordered mesoporous carbon–carbon nanotube nanocomposites as highly conductive and durable cathode catalyst supports for polymer electrolyte fuel cells. J. Mater. Chem. A 2013, 1, 1270–1283. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.P.; Jiang, W.-J.; Wan, S.; Zhang, X.; Hu, J.-S.; Wei, Z.-D.; Wan, L.-J. Self-deposition of Ptnanocrystals on Mn3O4 coated carbon nanotubes for enhanced oxygen reduction electrocatalysis. J. Mater. Chem. A 2013, 1, 7463–7468. [Google Scholar] [CrossRef]
- Zhu, C.; Yu, Y.; Gu, L.; Weichert, K.; Maier, J. Electrospinning of highly electroactive carbon-coated single-crystalline LiFePO4 nanowires. Angew. Chem. Int. Ed. 2011, 50, 6278–6282. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.A.M.; Abdelkareem, M.A.; Shin, G.; Kim, H.Y. Pd-doped Co nanofibers immobilized on a chemically stable metallic bipolar plate as novel strategy for direct formic acid fuel cells. Int. J. Hydrogen Energy 2013, 38, 7438–7447. [Google Scholar] [CrossRef]
- Kime, H.Y. Titanium oxide nanofibers attached to zinc oxide nanobranches as a novel nano-structure for lithium ion batteries applications. J. Ceram. Process. Res. 2010, 11, 437–442. [Google Scholar]
- Thomas, J.M.; Jie, X.; Sait, E.; Yatin, J.M.; William, M.S.; Haolan, X.; Thomas, N. NiO nanofibers as a candidate for a nanophotocathode. Nanomaterials 2014, 4, 256–266. [Google Scholar]
- Khalid, S.; Cao, C.; Wang, L.; Zhu, Y. Microwave assisted synthesis of porous NiCo2O4 microspheres: Application as high performance asymmetric and symmetric supercapacitors with large areal capacitance. Sci. Rep. 2016, 6, 22699. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Ren, W.; Cheng, C.; Wang, Y.; Huang, Z.; Yang, H.Y. Three-dimensional NiCo2O4@ polypyrrole coaxial nanowire arrays on carbon textiles for high-performance flexible asymmetric solid-state supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 21334–21346. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, D.C.; Ding, Y.; Wang, Z.L.; Zeng, Z.Z.; Liu, M.L. Hybrid composite Ni(OH)2@NiCo2O4 grown on carbon fiber paper for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 11159–11162. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Gong, Y.; Zhang, Y.; Luo, C.; Li, W.; Fu, Q.; Pan, C. Facile synthesis of carbon nanosphere/NiCo2O4 core-shell sub-microspheres for high performance supercapacitor. Sci. Rep. 2015, 5, 12903. [Google Scholar] [CrossRef] [PubMed]
- Koninck, M.D.; Marsan, B. MnxCu1−xCo2O4 used as bifunctional electrocatalyst in alkaline medium. Electrochim. Acta 2008, 53, 7012–7021. [Google Scholar] [CrossRef]
- Koninck, M.D.; Poirier, S.C.; Marsan, B. CuxCo3−xO4 used as bifunctional electrocatalyst physicochemical properties and electrochemical characterization for the oxygen evolution reaction. J. Electrochem. Soc. 2006, 153, A2103–A2110. [Google Scholar]
- Han, C.; Bo, X.; Zhang, Y.; Li, M.; Wang, A.; Guo, L. Dicobalt phosphide nanoparticles encased in boron and nitrogen co-doped graphitic layers as novel non-precious metal oxygen reduction electrocatalysts in alkaline media. Chem. Commun. 2015, 51, 15015–15018. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, D.; Wang, Y.; Wan, Y.; Hou, B. Catalytic activity of graphene–cobalt hydroxide composite for oxygen reduction reaction in alkaline media. J. Power Sources 2012, 198, 122–126. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Wu, Q.; Li, X.; Zhao, Y.; Lai, H.; Yang, L.; Sun, T.; Li, Y.; Wang, X. Advanced non-precious electrocatalyst of the mixed valence CoOx nanocrystals supported on N-doped carbon nanocages for oxygen reduction. Sci. China Chem. 2015, 58, 180–186. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Q.; Li, Y.; Lai, Z.; Yu, H.; Wang, H.; Peng, F. Nitrogen-doped graphene-supported cobalt carbonitride@oxide core–shell nanoparticles as a non-noble metal electrocatalyst for an oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 1142–1151. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Wu, L.; Sun, S. Co/CoO nanoparticles assembled on graphene for electrochemical reduction of oxygen. Angew. Chem. Int. Ed. 2012, 124, 11940–11943. [Google Scholar] [CrossRef]
- Gong, K.P.; Du, F.; Xia, Z.H.; Durstock, M.; Dai, L.M. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.L.; Wu, D.Q.; Feng, X.L.; Mullen, K. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction. Angew. Chem. Int. Ed. 2010, 122, 2619–2623. [Google Scholar] [CrossRef]
- Gochi-Ponce, Y.; Alonso-Nunez, G.; Alonso-Vante, N. Synthesis and electrochemical characterization of a novel platinum chalcogenideelectrocatalyst with an enhanced tolerance to methanol in the oxygen reduction reaction. Electrochem. Commun. 2006, 8, 1487–1491. [Google Scholar] [CrossRef]
- Pattabi, M.; Castellanos, R.H.; Castillo, R.; Ocampo, A.L.; Moreira, J.; Sebastian, P.J.; McClure, J.C.; Mathew, X. Electrochemical characterization of tungsten carbonyl compound for oxygen reduction reaction. Int. J. Hydrogen Energy 2001, 26, 171–174. [Google Scholar] [CrossRef]
- Gao, Z.; Song, N.; Li, X. Microstructural design of hybrid CoO@NiO and graphene nano-architectures for flexible high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14833–14844. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.; Zhao, D.; Wang, G. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef] [PubMed]





| Material | Half-Wave Potential (E1/2, V vs. Ag/AgCl) | Limited Current Density (JL) (mA cm−2) | Onset Potential (V vs. Ag/AgCl) | Ref |
|---|---|---|---|---|
| BNC/Co2P-2 | −0.15 | 4.85 | −0.07 | [57] |
| NiCo2O4-rGO | about −0.35 | 2.0 | −0.088 | [8] |
| Co(OH)2/graphene | about −0.186 | 0.61 | −0.05 | [58] |
| CoOx/NCNCs | −0.174 | about 5.28 | −0.10 | [59] |
| CoCN@CoOx(18)/NG | −0.16 | 5.62 | about −0.1 | [60] |
| G–Co/CoO NPs | −0.176 | about 4.6 | about −0.13 | [61] |
| C/NiCo2O4 | 0.59 | 5.4 | 0.53 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, D.; El-safty, S.; Khalil, K.A.; Dewidar, M.; Abu El-magd, G. Carbon Supported Engineering NiCo2O4 Hybrid Nanofibers with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. Materials 2016, 9, 759. https://doi.org/10.3390/ma9090759
Hassan D, El-safty S, Khalil KA, Dewidar M, Abu El-magd G. Carbon Supported Engineering NiCo2O4 Hybrid Nanofibers with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction. Materials. 2016; 9(9):759. https://doi.org/10.3390/ma9090759
Chicago/Turabian StyleHassan, Diab, Sherif El-safty, Khalil Abdelrazek Khalil, Montasser Dewidar, and Gamal Abu El-magd. 2016. "Carbon Supported Engineering NiCo2O4 Hybrid Nanofibers with Enhanced Electrocatalytic Activity for Oxygen Reduction Reaction" Materials 9, no. 9: 759. https://doi.org/10.3390/ma9090759





