A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Migration of Fluorinated Oligomers Towards HBP-Air Interface
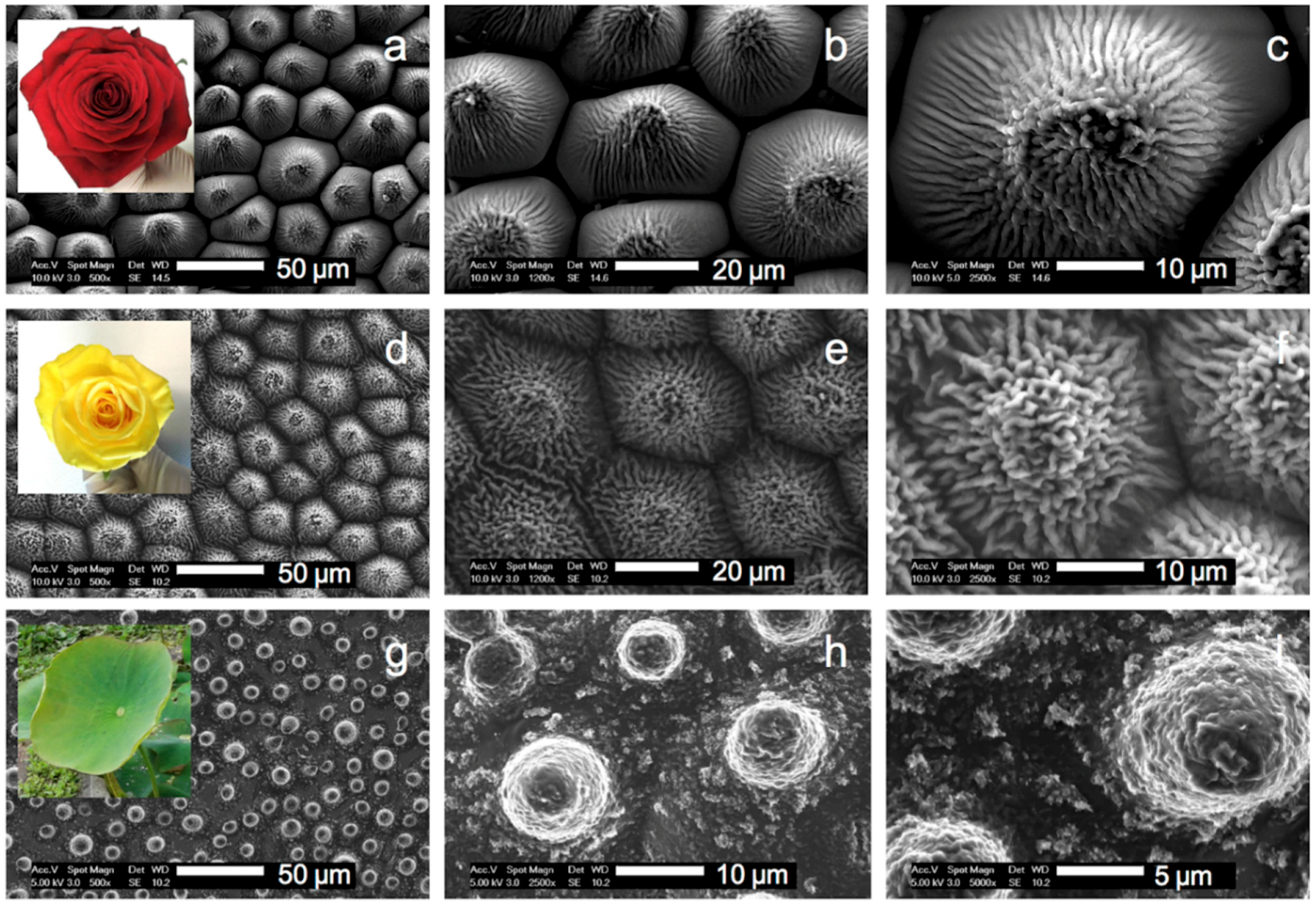
3.2. Replication of the Rose Petals and Lotus Leaf into HBP-F
3.3. Dilution of the Fluorinated Oligomer on the Texturized Surface and Reverse Migration
3.4. UV Flash Immobilization of Fluorinated Oligomer at the HBP-Air Interface
3.5. Self-cleaning Synthetic Plant Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Natural and biomimetic artificial surfaces for superhydrophobicity, self-cleaning, low adhesion, and drag reduction. Prog. Mater. Sci. 2011, 56, 1–108. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic surfaces. Curr. Opin. Coll. Interf. Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progress in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, V.A.; Raut, H.K.; Sreekumaran, A.; Ramakrishna, S. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- Nakajima, A. Design of hydrophobic surfaces for liquid droplet control. NPG Asia Mater. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent progress in antireflection and self-cleaning technology: From surface engineering to functional surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid. Interf. Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shirtcliffe, N.J.; McHale, G.; Newton, M.I.; Chabrol, G.; Perry, C.C. Dual-scale roughness produces unusually water-repellent surfaces. Adv. Mater. 2004, 16, 1929–1932. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Jung, Y.C.; Barthlott, W. Fabrication of artificial Lotus leaves and significance of hierarchical structure for superhydrophobicity and low adhesion. Soft Matter 2009, 5, 1386–1393. [Google Scholar] [CrossRef]
- Lepore, E.; Pugno, N. Superhydrophobic Polystyrene by Direct Copy of a Lotus Leaf. BioNanoScience 2011, 1, 136–143. [Google Scholar] [CrossRef]
- Park, S.; van Rijn, P.; Böker, A. Artificial leaves via reproduction of hierarchical structures by a fast molding and curing process. Macromol. Rapid Commun. 2012, 33, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Rasilainen, T.; Suvanto, M.; Pakkanen, T.A. Anisotropically microstructured and micro/nanostructured polypropylene surfaces. Surf. Sci. 2009, 603, 2240–2247. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, T.H. Effects of intrinsic hydrophobicity on wettability of polymer replicas of a superhydrophobic lotus leaf. J. Micromech. Microeng. 2007, 17, 687–692. [Google Scholar] [CrossRef]
- Sun, M.H.; Luo, C.X.; Xu, L.P.; Ji, H.; Qi, O.Y.; Yu, D.P.; Chen, Y. Artificial lotus leaf by nanocasting. Langmuir 2005, 21, 8978–8981. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, X.; Wang, C.; Zhang, B.; Wang, W. Rapid and low cost replication of complex microfluidic structures with PDMS double casting technology. Microsyst. Technol. 2014, 20, 1933–1940. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Jiang, G.; Mei, X.; Wang, Z.; Wang, K.; Cui, J. Study on hierarchical structured PDMS for surface super-hydrophobicity using imprinting with ultrafast laser structured models. Appl. Surf. Sci. 2016, 364, 528–538. [Google Scholar] [CrossRef]
- Migliaccio, C.P.; Lazarus, N. Fabrication of hierarchically structured superhydrophobic PDMS surfaces by Cu and CuO casting. Appl. Surf. Sci. 2015, 353, 269–274. [Google Scholar] [CrossRef]
- Shin, J.-H.; Han, K.-S.; Lee, H. Anti-reflection and hydrophobic characteristics of M-PDMS based moth-eye nano-patterns on protection glass of photovoltaic systems. Prog. Photovolt. Res. Appl. 2011, 19, 339–344. [Google Scholar] [CrossRef]
- Shang, H.M.; Wang, Y.; Limmer, S.J.; Chou, T.P.; Takahashi, K.; Cao, G.Z. Optically transparent superhydrophobic silica-based films. Thin Solid Films 2005, 472, 37–43. [Google Scholar] [CrossRef]
- Vourdas, N.; Tserepi, A.; Gogolides, E. Nanotextured super-hydrophobic transparent poly(methyl methacrylate) surfaces using high-density plasma processing. Nanotechnology 2007, 18, 125304. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Yang, B. Antireflective surfaces based on biomimetic nanopillared arrays. Nano Today 2010, 5, 117–127. [Google Scholar] [CrossRef]
- Ming, W.; Wu, D.; van Benthem, R.; de With, G. Superhydrophobic Films from Raspberry-like Particles. Nano Lett. 2005, 5, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Budunoglu, H.; Yildirim, A.; Guler, M.O.; Bayindir, M. Highly transparent, flexible and thermally stable superhydrophobic ORMOSIL aerogel thin films. ACS Appl. Mater. Interfaces 2011, 3, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongiovanni, R.; Beamson, G.; Mamo, A.; Priola, A.; Recca, A.; Tonelli, C. High resolution XPS investigation of photocured films containing perfluoropolyether acrylates. Polymer 2000, 41, 409–414. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Lombardi, V.; Priola, A.; Tonelli, C.; Di Meo, A. Surface properties of acrylic coatings containing perfluoropolyether chains. Surf. Coat. Int. Part B Coat. Trans. 2003, 86, 1–90. [Google Scholar] [CrossRef]
- Fabbri, P.; Messori, M.; Montecchi, M.; Nannarone, S.; Pasquali, L.; Pilati, F.; Tonelli, C.; Toselli, M. Perfluoropolyether-based organic-inorganic hybrid coatings. Polymer 2006, 47, 1055–1062. [Google Scholar] [CrossRef]
- Messori, M.; Toselli, M.; Pilati, F.; Fabbri, P.; Pasquali, L.; Montecchi, M.; Nannarone, S.; Tonelli, C. Perfluoropolyether-based organic-inorganic hybrid coatings: Preparation and surface characterisation. Surf. Coat. Int. Part B Coat. Trans. 2005, 88, 243–249. [Google Scholar] [CrossRef]
- Dalvi, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Schulte, A.J.; Droste, D.M.; Koch, K.; Barthlott, W. Hierarchically structured superhydrophobic flowers with low hysteresis of the wild pansy (Viola tricolor)—New design principles for biomimetic materials. Beilstein J. Nanotechnol. 2011, 2, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.E.; Schmäh, D.; Leterrier, Y.; Månson, J.-A.E. Time-intensity transformation and internal stress in UV-curable hyperbranched acrylates. Rheol. Acta 2007, 46, 693–701. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Yi, S.; Jin, Y.-H.; Leterrier, Y.; Cho, Y.-H.; Månson, J.-A.E. Acrylated hyperbranched polymer photoresist for ultra-thick and low-stress high aspect ratio micropatterns. J. Micromech. Microeng. 2008, 18, 045022. [Google Scholar] [CrossRef]
- Geiser, V.; Leterrier, Y.; Månson, J.-A.E. Rheological Behavior of Concentrated Hyperbranched Polymer/Silica Nanocomposite Suspensions. Macromolecules 2010, 43, 7705–7712. [Google Scholar] [CrossRef]
- Geiser, V.; Leterrier, Y.; Månson, J.-A.E. Low-Stress Hyperbranched Polymer/Silica Nanostructures Produced by UV Curing, Sol/Gel Processing and Nanoimprint Lithography. Macromol. Mater. Eng. 2012, 297, 155–166. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Malucelli, G.; Sangermano, M.; Priola, A. Fluorinated networks through photopolymerisation processes: Synthesis, characterisation and properties. J. Fluorine Chem. 2004, 125, 345–351. [Google Scholar] [CrossRef]
- Guo, X.; Hunter, A.; Chen, J. Preparation and Characterization of Acrylates and Polyacrylates Having Variable Fluorine Contents and Distributions. J. Polym. Sci. Pol. Chem. 1994, 32, 47–56. [Google Scholar] [CrossRef]
- Decker, C.; Zahouily, K.; Valet, A. Weathering performance of thermoset and photoset acrylate coatings. J. Coat. Technol. 2002, 74, 87–92. [Google Scholar] [CrossRef]
- Ping, T.; Zhou, Y.; He, Y.; Tang, Y.; Yang, J.; Akram, M.Y.; Nie, J. Preparation and characterization of yellowing resistance and low volume shrinkage of fluorinated polysiloxane urethane acrylate. Prog. Org. Coat. 2016, 97, 74–81. [Google Scholar] [CrossRef]
- González Lazo, M.A.; Teuscher, R.; Leterrier, Y.; Månson, J.-A.E.; Calderone, C.; Hessler-Wyser, A.; Couty, P.; Ziegler, Y.; Fischer, D. UV-nanoimprint lithography and large area roll-to-roll texturization with hyperbranched polymer nanocomposites for light-trapping applications. Sol. Energy Mater. Sol. Cells 2012, 103, 147–156. [Google Scholar] [CrossRef]
- Mikhaylova, Y.; Adam, G.; Haussler, L.; Eichhorn, K.J.; Voit, B. Temperature-dependent FTIR spectroscopic and thermoanalytic studies of hydrogen bonding of hydroxyl (phenolic group) terminated hyperbranched aromatic polyesters. J. Mol. Struct. 2006, 788, 80–88. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Di Meo, A.; Pollicino, A.; Priola, A.; Tonelli, C. New perfluoropolyether urethane methacrylates as surface modifiers: Effect of molecular weight and end group structure. React. Funct. Polym. 2008, 68, 189–200. [Google Scholar] [CrossRef]
- Krupers, M.; Slangen, P.-J.; Moller, M. Synthesis and properties of polymers based on oligo(hexafluoropropene oxide) containing methacrylates and copolymers with methyl methacrylate. Macromolecules 1998, 31, 2552–2558. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Brown, P.S.; Bain, C.D.; Badyal, J.P.S.; Sarkar, S. Topographical length scales of hierarchical superhydrophobic surfaces. Appl. Surf. Sci. 2014, 317, 1068–1074. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Bartlett, A.; Kozbial, A.; Li, L. A Cost-Effective Approach to Fabricate Superhydrophobic Coatings Using Hydrophilic Materials. Adv. Eng. Mater. 2016, 18, 567–571. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Wang, M.; Wang, C.; Jiang, L. Rose petals with a novel and steady air bubble pinning effect in aqueous media. Soft Matter 2012, 8, 2261–2266. [Google Scholar] [CrossRef]
- Varshneya, A.K.; Schaeffer, H.A.; Richardson, K.R.; Wightman, M.; Pye, L.D. Processing, Properties, and Applications of Glass and Optical Materials: Ceramic Transactions; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Geiser, V.; Leterrier, Y.; Månson, J.A.E. Conversion and Shrinkage Analysis of Acrylated Hyperbranched Polymer Nanocomposites. J. Appl. Polym. Sci. 2009, 114, 1954–1963. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Adhesion Mechanism of Water Droplets on Hierarchically Rough Superhydrophobic Rose Petal Surface. J. Nanomater. 2011, 2011, 818707. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Tamesue, S.; Takahashi, E.; Kosugi, S.; Fukami, K.; Mitsumata, T.; Tsubokawa, N.; Sakka, T.; Yamauchi, T. Fabrication of a poly(dimethylsiloxane) microstructured surface imprinted from patterned silicon wafer with a self-cleaning property. Polym. J. 2016, 48, 835–838. [Google Scholar] [CrossRef]






| Sample | Peak | Position Beam Energy (eV) | Full Width at Half Maximum (eV) | Raw Area (CPS) | Relative Sensitivity Factor | Atomic Mass | Atomic Conc. * (%) | Mass Conc. * (%) |
|---|---|---|---|---|---|---|---|---|
| HBP | F 1s | 687.3 | 0.000 | 0.0 | 1.000 | 18.998 | 0.00 | 0.00 |
| O 1s | 528.6 | 2.363 | 26,010.7 | 0.780 | 15.999 | 16.60 | 20.95 | |
| C 1s | 280.8 | 2.366 | 42,133.2 | 0.278 | 12.011 | 83.40 | 79.05 | |
| HBP-PFUA | F 1s | 685.3 | 2.363 | 3317.5 | 1.000 | 18.998 | 1.70 | 2.46 |
| O 1s | 528.7 | 2.431 | 35,280.0 | 0.780 | 15.999 | 24.19 | 29.56 | |
| C 1s | 281.9 | 2.773 | 34,850.6 | 0.278 | 12.011 | 74.11 | 67.98 |
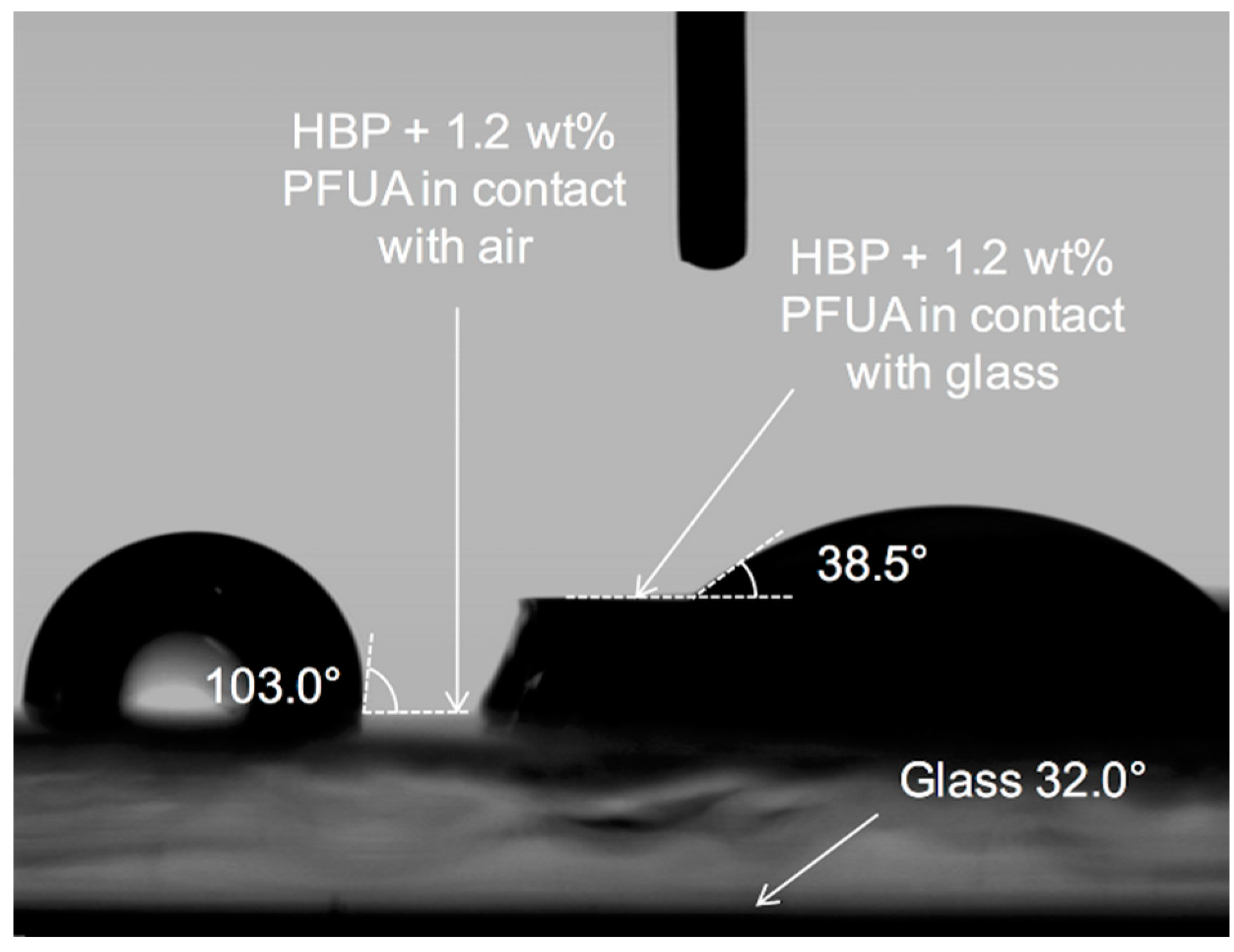
| Surface | Material | Air Exposure (min) | Template Material | WCA (°) |
|---|---|---|---|---|
| Flat | HBP | – | – | 68.7 ± 1.2 |
| HBP + 1 wt. % PFUA | 30 | – | 107.6 ± 2.6 | |
| Glass * | – | – | 32.0 | |
| PDMS * | – | – | 108.7 | |
| HBP * | – | Glass | 37.3 | |
| HBP | – | PDMS | 71.6 ± 1.7 | |
| HBP + 1 wt. % PFUA * | 0 | Glass | 38.5 | |
| HBP + 1 wt. % PFUA | 120 | PDMS | 97.5 ± 1.1 | |
| Red rose petal texture | HBP | – | PDMS | 120.4 ± 0.9 |
| HBP + 5 wt. % PFUA | 0 | PDMS | 117.8 ± 2.3 | |
| HBP + 5 wt. % PFUA | 120 | PDMS | 121.9 ± 1.1 | |
| Yellow rose petal texture | HBP | – | PDMS | 126.2 ± 1.5 |
| HBP + 5 wt. % PFUA | 0 | PDMS | 136.5 ± 2.0 | |
| HBP + 5 wt. % PFUA | 120 | PDMS | 137.6 ± 0.9 | |
| Lotus leaf texture | HBP | – | PDMS | 139.1 ± 1.8 |
| HBP + 5 wt. % PFUA | 0 | PDMS | 141.7 ± 2.0 | |
| HBP + 5 wt. % PFUA | 120 | PDMS | 140.1 ± 1.1 | |
| Negative red rose | PDMS | – | Red rose | 134.3 ± 5.3 |
| Negative yellow rose | PDMS | – | Yellow rose | 131.3 ± 4.1 |
| Negative lotus | PDMS | – | Lotus | 117.3 ± 2.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Lazo, M.A.; Katrantzis, I.; Dalle Vacche, S.; Karasu, F.; Leterrier, Y. A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces. Materials 2016, 9, 738. https://doi.org/10.3390/ma9090738
González Lazo MA, Katrantzis I, Dalle Vacche S, Karasu F, Leterrier Y. A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces. Materials. 2016; 9(9):738. https://doi.org/10.3390/ma9090738
Chicago/Turabian StyleGonzález Lazo, Marina A., Ioannis Katrantzis, Sara Dalle Vacche, Feyza Karasu, and Yves Leterrier. 2016. "A Facile in Situ and UV Printing Process for Bioinspired Self-Cleaning Surfaces" Materials 9, no. 9: 738. https://doi.org/10.3390/ma9090738







