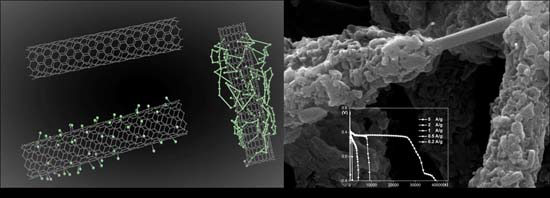
New Supercapacitors Based on the Synergetic Redox Effect between Electrode and Electrolyte
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Modification of MWCNTs
2.3. Preparation of PANI/F-MWCNTs
3. Characterization
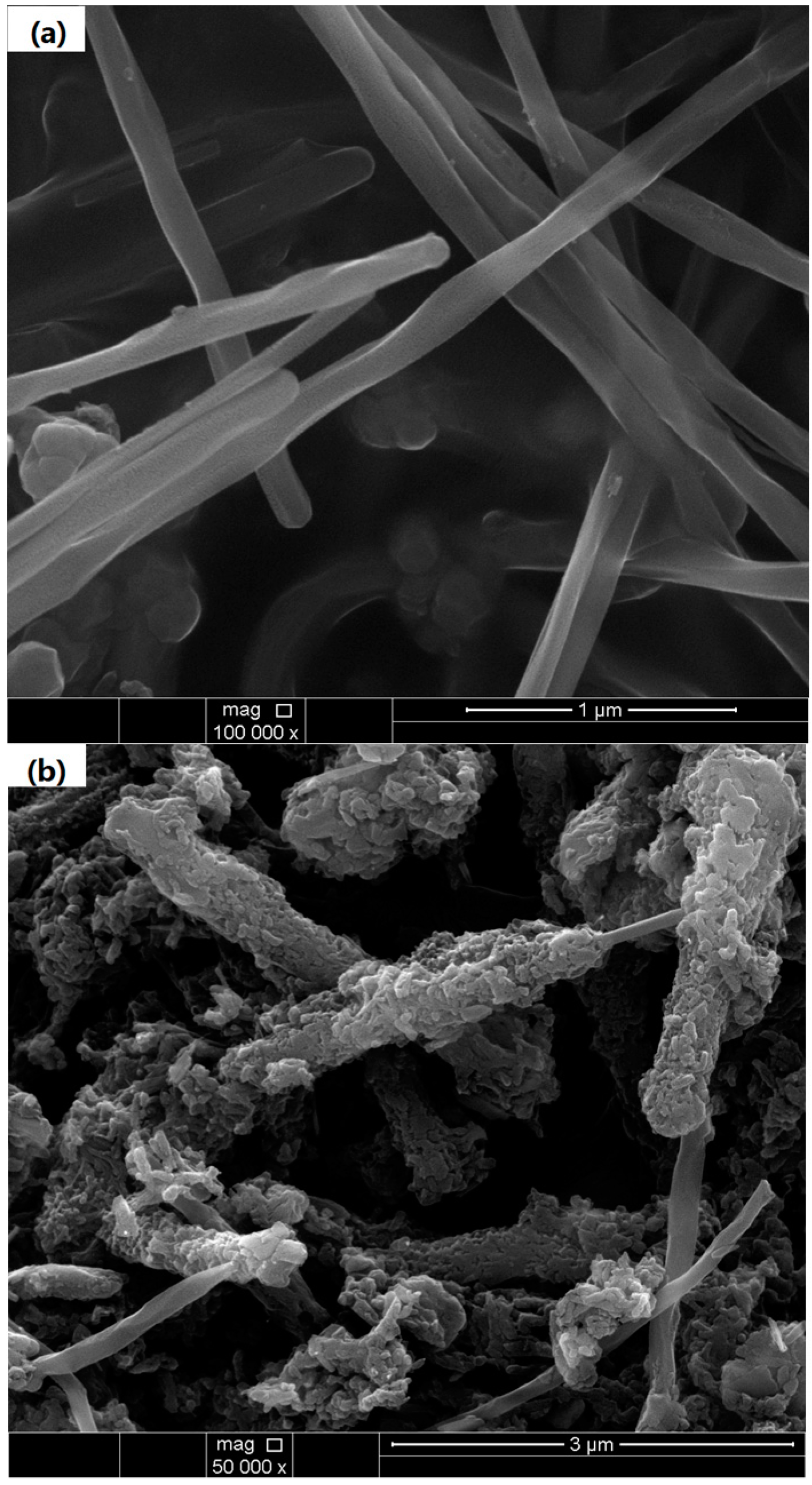
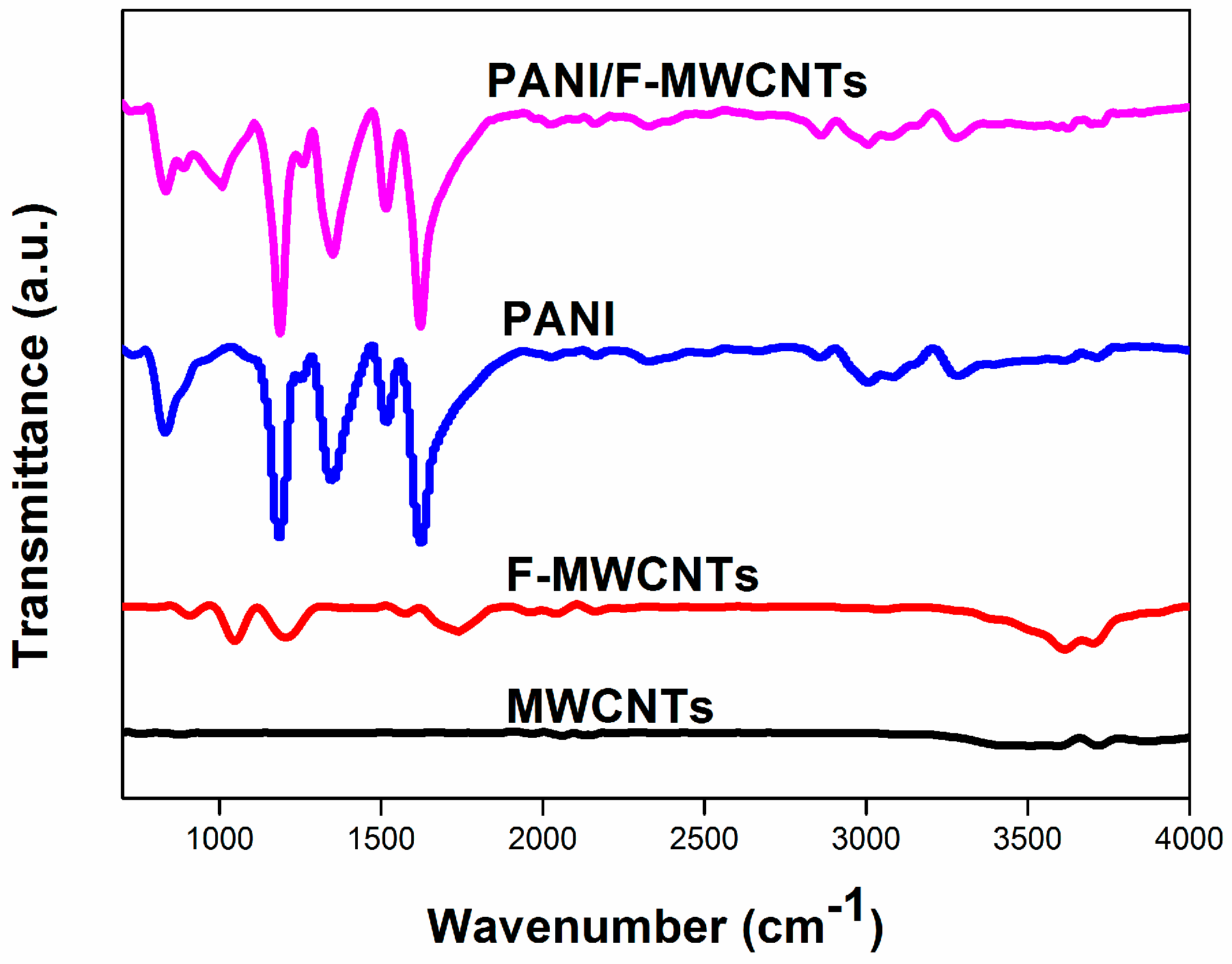
3.1. Structure and Composition Characterization
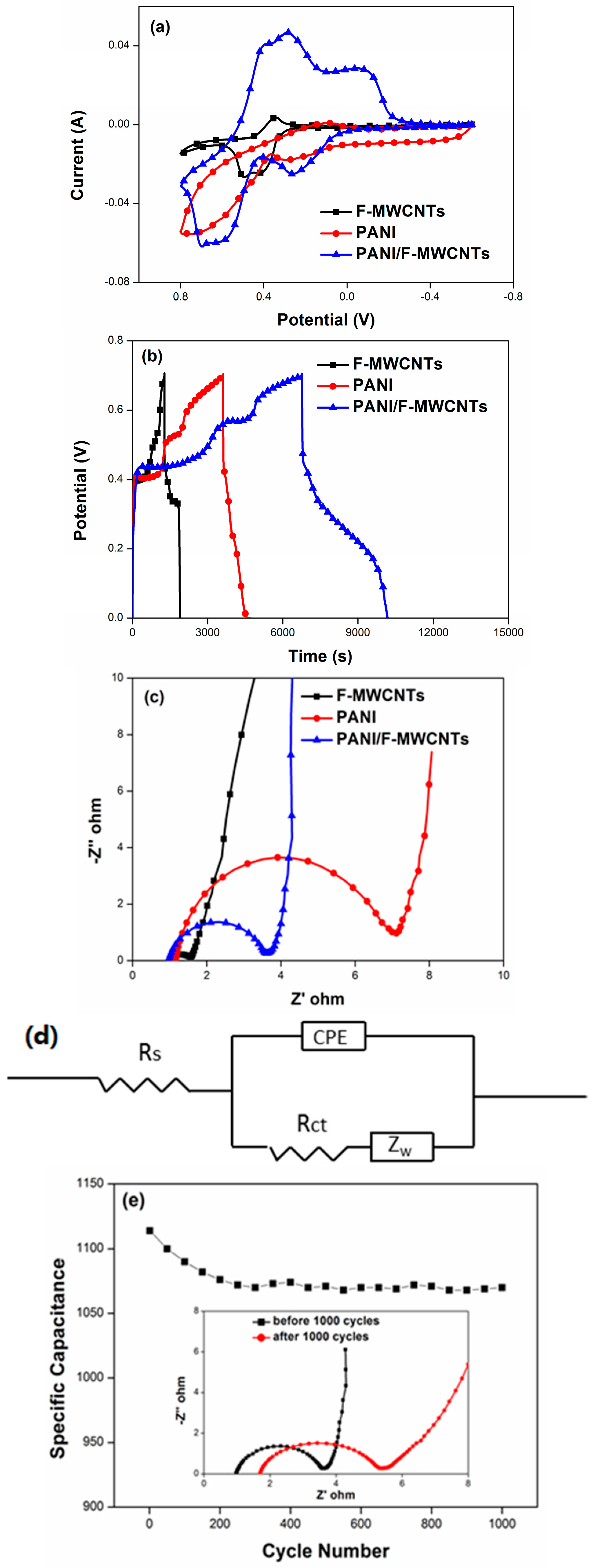
3.2. Electrochemical Characterization
4. Results and Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Z.; Ren, J.; Zhang, Z.; Chen, X.; Guan, G.; Qiu, L.; Zhang, Y.; Peng, H. Recent Advancement of Nanostructured Carbon for Energy Applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Park, H.H.; Jin, H.-J. Pseudocapacitive Effects of N-Doped Carbon Nanotube Electrodes in Supercapacitors. Materials 2012, 5, 1258–1266. [Google Scholar] [CrossRef]
- Lee, H.-M.; Lee, K.; Kim, C.-K. Electrodeposition of Manganese-Nickel Oxide Films on a Graphite Sheet for Electrochemical Capacitor Applications. Materials 2014, 7, 265–274. [Google Scholar] [CrossRef]
- Zu, L.; Cui, X.; Jiang, Y.; Hu, Z.; Lian, H.; Liu, Y.; Jin, Y.; Li, Y.; Wang, X. Preparation and Electrochemical Characterization of Mesoporous Polyaniline-Silica Nanocomposites as an Electrode Material for Pseudocapacitors. Materials 2015, 8, 1369–1383. [Google Scholar] [CrossRef]
- Wang, J.-G.; Kang, F.; Wei, B. Engineering of MnO2-based nanocomposites for high-performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B. A perspective: Carbon nanotube macro-films for energy storage. Energy Environ. Sci. 2013, 6, 3183–3201. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, V.; Gnanaraj, J.; Lee, Y.-S.; Madhavi, S. Insertion-Type Electrodes for Nonaqueous Li-Ion Capacitors. Chem. Rev. 2014, 114, 11619–11635. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Yang, W.-D.; Lee, K.-C.; Huang, C.-M. An Effective Electrodeposition Mode for Porous MnO2/Ni Foam Composite for Asymmetric Supercapacitors. Materials 2016, 9, 246. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Tang, J.; Kamachi, Y.; Nakato, T.; Kim, J.H.; Yamauchi, Y. Asymmetric Supercapacitors Using 3D Nanoporous Carbon and Cobalt Oxide Electrodes Synthesized from a Single Metal-Organic Framework. ACS Nano 2015, 9, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Lota, G.; Frackowiak, E. Striking capacitance of carbon/iodide interface. Electrochem. Commun. 2009, 11, 87–90. [Google Scholar] [CrossRef]
- Li, Q.; Li, K.; Sun, C.; Li, Y. An investigation of Cu2+ and Fe2+ ions as active materials for electrochemical redox supercapacitors. J. Electroanal. Chem. 2007, 611, 43–50. [Google Scholar] [CrossRef]
- Wang, X.; Chandrabose, R.S.; Chun, S.-E.; Zhang, T.; Evanko, B.; Jian, Z.; Boettcher, S.W.; Stucky, G.D.; Ji, X. High Energy Density Aqueous Electrochemical Capacitors with a KI-KOH Electrolyte. ACS Appl. Mater. Interfaces 2015, 7, 19978–19985. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923. [Google Scholar] [CrossRef] [PubMed]
- Roldán, S.; Blanco, C.; Granda, M.; Menéndez, R.; Santamaría, R. Towards a Further Generation of High-Energy Carbon-Based Capacitors by Using Redox-Active Electrolytes. Angew. Chem. Int. Ed. 2011, 50, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Roldán, S.; Granda, M.; Menéndez, R.; Santamaría, R.; Blanco, C. Mechanisms of Energy Storage in Carbon-Based Supercapacitors Modified with a Quinoid Redox-Active Electrolyte. J. Phys. Chem. C 2011, 115, 17606–17611. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, X.; Zu, L.; Hu, Z.; Gan, J.; Lian, H.; Liu, Y.; Xing, G. High Rate Performance Nanocomposite Electrode of Mesoporous Manganese Dioxide/Silver Nanowires in KI Electrolytes. Nanomaterials 2015, 5, 1638–1653. [Google Scholar] [CrossRef]
- Hu, Z.; Zu, L.; Jiang, Y.; Lian, H.; Liu, Y.; Li, Z.; Chen, F.; Wang, X.; Cui, X. High Specific Capacitance of Polyaniline/Mesoporous Manganese Dioxide Composite Using KI-H2SO4 Electrolyte. Polymers 2015, 7, 1939–1953. [Google Scholar] [CrossRef]
- Hu, Z.; Zu, L.; Jiang, Y.; Lian, H.; Liu, Y.; Wang, X.; Cui, X. High performance nanocomposite electrodes of mesoporous silica platelet-polyaniline synthesized via impregnation polymerization. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly sensitive, room temperature gas sensor based on polyaniline-multiwalled carbon nanotubes (PANI/MWCNTs) nanocomposite for trace-level ammonia detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Wu, H.; Wu, Y.; Shang, Y.; Pan, J.; Xiong, X. Fabrication of TiO2@PANI nanobelts with the enhanced absorption and photocatalytic performance under visible light. Mater. Lett. 2016, 172, 52–55. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y.; Jena, R.K. Non-covalent interactions and supercapacitance of pseudo-capacitive composite electrode materials (MWCNTCOOH/MnO2/PANI). Synth. Met. 2015, 208, 2–12. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Youssef, A.M.; Mohamed, S.A.; Abdel-Aziz, M.S.; Abdel-Aziz, M.E.; Turky, G.; Kamel, S. Biological studies and electrical conductivity of paper sheet based on PANI/PS/Ag-NPs nanocomposite. Carbohydr. Polym. 2016, 147, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Hu, Y.; Wang, L.; Guo, Q.; Chen, S.; Chen, S.; Hou, H.; Song, Y. Macroporous Carbon/Nitrogen-doped Carbon Nanotubes/Polyaniline Nanocomposites and Their Application in Supercapacitors. Electrochim. Acta 2016, 189, 158–165. [Google Scholar] [CrossRef]
- Potphode, D.D.; Sivaraman, P.; Mishra, S.P.; Patri, M. Polyaniline/partially exfoliated multi-walled carbon nanotubes based nanocomposites for supercapacitors. Electrochim. Acta 2015, 155, 402–410. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, J.-I.; Park, S.-J. Activated carbon nanotubes/polyaniline composites as supercapacitor electrodes. Energy 2014, 78, 298–303. [Google Scholar] [CrossRef]
- Bavio, M.A.; Acosta, G.G.; Kessler, T. Synthesis and characterization of polyaniline and polyaniline—Carbon nanotubes nanostructures for electrochemical supercapacitors. J. Power Sources 2014, 245, 475–481. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Polyaniline/single-wall carbon nanotube (PANI/SWCNT) composites for high performance supercapacitors. Electrochim. Acta 2006, 52, 1721–1726. [Google Scholar] [CrossRef]
- Tran, V.C.; Nguyen, V.H.; Nguyen, T.T.; Lee, J.H.; Huynh, D.C.; Shim, J.-J. Polyaniline and multi-walled carbon nanotube-intercalated graphene aerogel and its electrochemical properties. Synth. Met. 2016, 215, 150–157. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Yoon, E.-H.; Kim, K.-B. Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J. Power Sources 2011, 196, 10791–10797. [Google Scholar] [CrossRef]
- Dong, B.; He, B.-L.; Xu, C.-L.; Li, H.-L. Preparation and electrochemical characterization of polyaniline/multi-walled carbon nanotubes composites for supercapacitor. Mater. Sci. Eng. B 2007, 143, 7–13. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, L.-B.; Wang, B.; Luo, Y.-C.; Kang, L. In-situ electrochemical polymerization of multi-walled carbon nanotube/polyaniline composite films for electrochemical supercapacitors. Synth. Met. 2009, 159, 260–266. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, Z.-Y.; Li, L.; Zhang, Y.; Wei, Y.-L.; Wang, L.-F.; Zhu, M.-F. Polyaniline/multi-walled carbon nanotube composites with core–shell structures as supercapacitor electrode materials. Electrochim. Acta 2010, 55, 3904–3908. [Google Scholar] [CrossRef]
- Dhibar, S.; Das, C.K. Silver Nanoparticles Decorated Polyaniline/Multiwalled Carbon Nanotubes Nanocomposite for High-Performance Supercapacitor Electrode. Ind. Eng. Chem. Res. 2014, 53, 3495–3508. [Google Scholar] [CrossRef]
- Kotal, M.; Thakur, A.K.; Bhowmick, A.K. Polyaniline-carbon nanofiber composite by a chemical grafting approach and its supercapacitor application. ACS Appl. Mater. Interfaces 2013, 5, 8374–8386. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, G.; Li, X.; Cheng, Q.; Li, C. Interfacial Synthesis and Supercapacitive Performance of Hierarchical Sulfonated Carbon Nanotubes/Polyaniline Nanocomposites. Ind. Eng. Chem. Res. 2012, 51, 3981–3987. [Google Scholar] [CrossRef]
- Singh, C.; Paul, A. Physisorbed Hydroquinone on Activated Charcoal as a Supercapacitor: An Application of Proton-Coupled Electron Transfer. J. Phys. Chem. C 2015, 119, 11382–11390. [Google Scholar] [CrossRef]
- Gastol, D.; Walkowiak, J.; Fic, K.; Frackowiak, E. Enhancement of the carbon electrode capacitance by brominated hydroquinones. J. Power Sources 2016, 326, 587–594. [Google Scholar] [CrossRef]
- Zhong, J.; Fan, L.-Q.; Wu, X.; Wu, J.-H.; Liu, G.-J.; Lin, J.-M.; Huang, M.-L.; Wei, Y.-L. Improved energy density of quasi-solid-state supercapacitors using sandwich-type redox-active gel polymer electrolytes. Electrochim. Acta 2015, 166, 150–156. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, E.; Luo, Z.; Hu, T.; Liu, T.; Li, Z.; Zhao, Q. A hydroquinone redox electrolyte for polyaniline/SnO2 supercapacitors. Electrochim. Acta 2014, 118, 106–111. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, Y.; Wu, Y.; Wu, Z.; Liu, E. The effect of hydroquinone as an electrolyte additive on electrochemical performance of the polyaniline supercapacitor. Mater. Res. Bull. 2014, 50, 303–306. [Google Scholar] [CrossRef]
- Yu, H.; Wu, J.; Fan, L.; Xu, K.; Zhong, X.; Lin, Y.; Lin, J. Improvement of the performance for quasi-solid-state supercapacitor by using PVA–KOH–KI polymer gel electrolyte. Electrochim. Acta 2011, 56, 6881–6886. [Google Scholar] [CrossRef]


| Materials | Electrolytes | Test Condition | Specific Capacitance (F/g) | References |
|---|---|---|---|---|
| KSC/NCNTs/PANI | 2 M H2SO4 | 0.1 A/g | 1090 | [28] |
| Px-MWCNT | 1 M H2SO4 | 20 mA/cm2 | 118 | [29] |
| PANI/MWCNT | 1 M NaNO3 | 0.5 A/g | 176.5 | [30] |
| PANI/CNTf | 1 M H2SO4 | 2 A/g | 1744 | [31] |
| PANI/SWCNT | 1 M H2SO4 | 5 mA/cm2 | 485 | [32] |
| PANI/MWCNTs/rGO | 1 M H2SO4 | 0.5 A/g | 987 | [33] |
| PANI/MWNT | 1 M H2SO4 | 5 mV/s | 139 | [34] |
| PANI/MWNTs | 1 M NaNO3 | 5 mA/cm2 | 328 | [35] |
| MWCNT/PANI | 0.5 M H2SO4 | 5 mA/cm2 | 500 | [36] |
| PANI/MWCNT | 0.1 M H2SO4 | 5 mV/s | 560 | [37] |
| Ag-PANI/MWCNTs | 1 M KCl | 1 A/g | 528 | [38] |
| PANI-g-TCNF | 1 M H2SO4 | 0.3 A/g | 550 | [39] |
| PANI/sMWCNT | 1 M H2SO4 | 1 A/g | 431.3 | [40] |
| Activated Charcoal/HQ | 1 M H2SO4 | 5 mV/s | 207 | [41] |
| Activated carbon | C6H4Br2O2-2 M KOH | 5 mV/s | 314 | [42] |
| Activated carbon | PVA-H2SO4-HQ/ PVA-H2SO4-MB | 0.5 A/g | 563.7 | [43] |
| PANI/SnO2 | 0.4 M HQ-1 M H2SO4 | 0.5 A/g | 857 | [44] |
| PANI | 0.4 M HQ-1 M H2SO4 | 0.5 A/g | 584 | [45] |
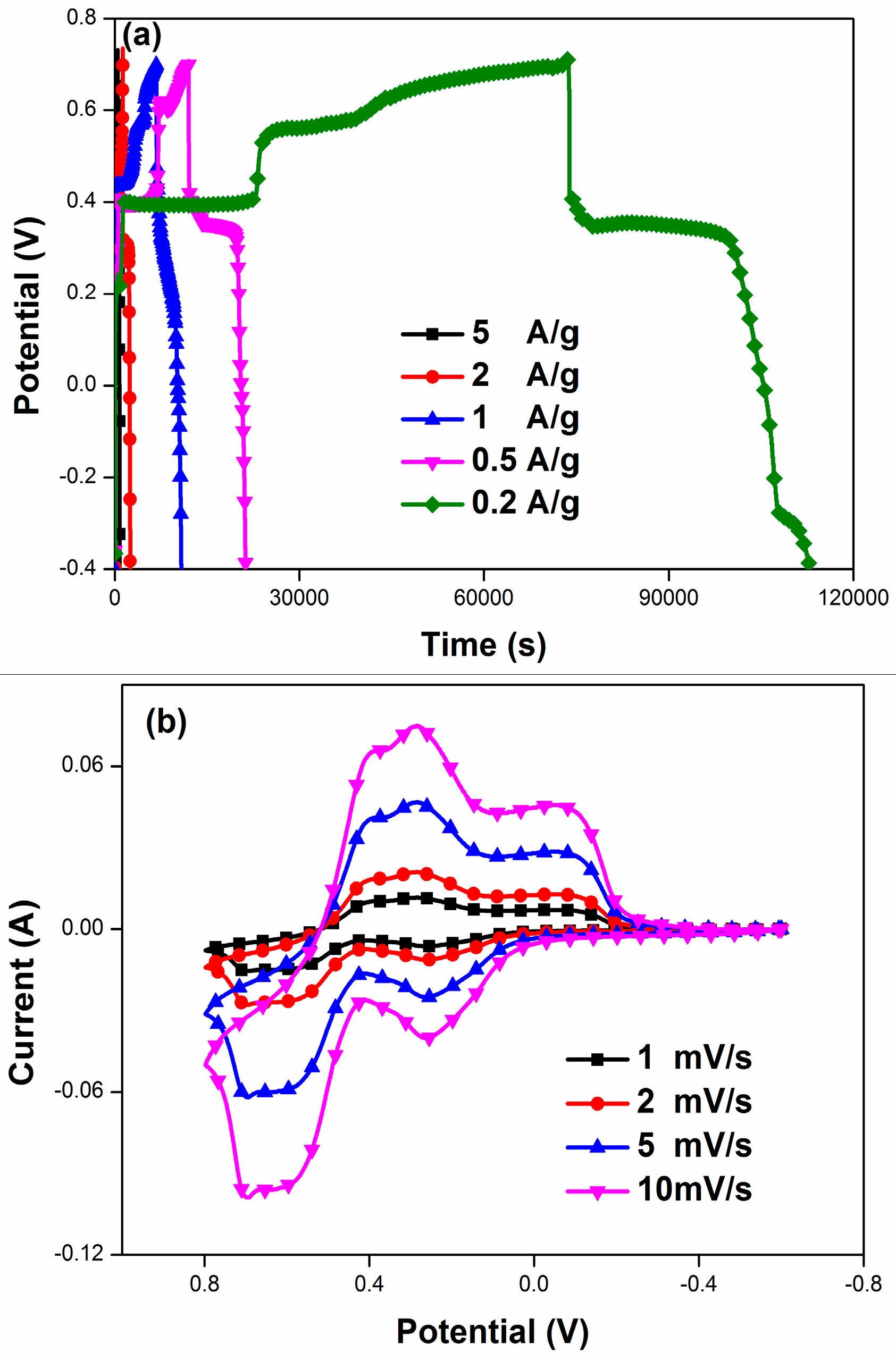
| PANI/F-MWCNTs | 1 M H2SO4-2 M HQ | 5 A/g | 1620 | Present work |
| 2 A/g | 2596 | |||
| 1 A/g | 4002 | |||
| 0.5 A/g | 5375 | |||
| 0.2 A/g | 7926 |
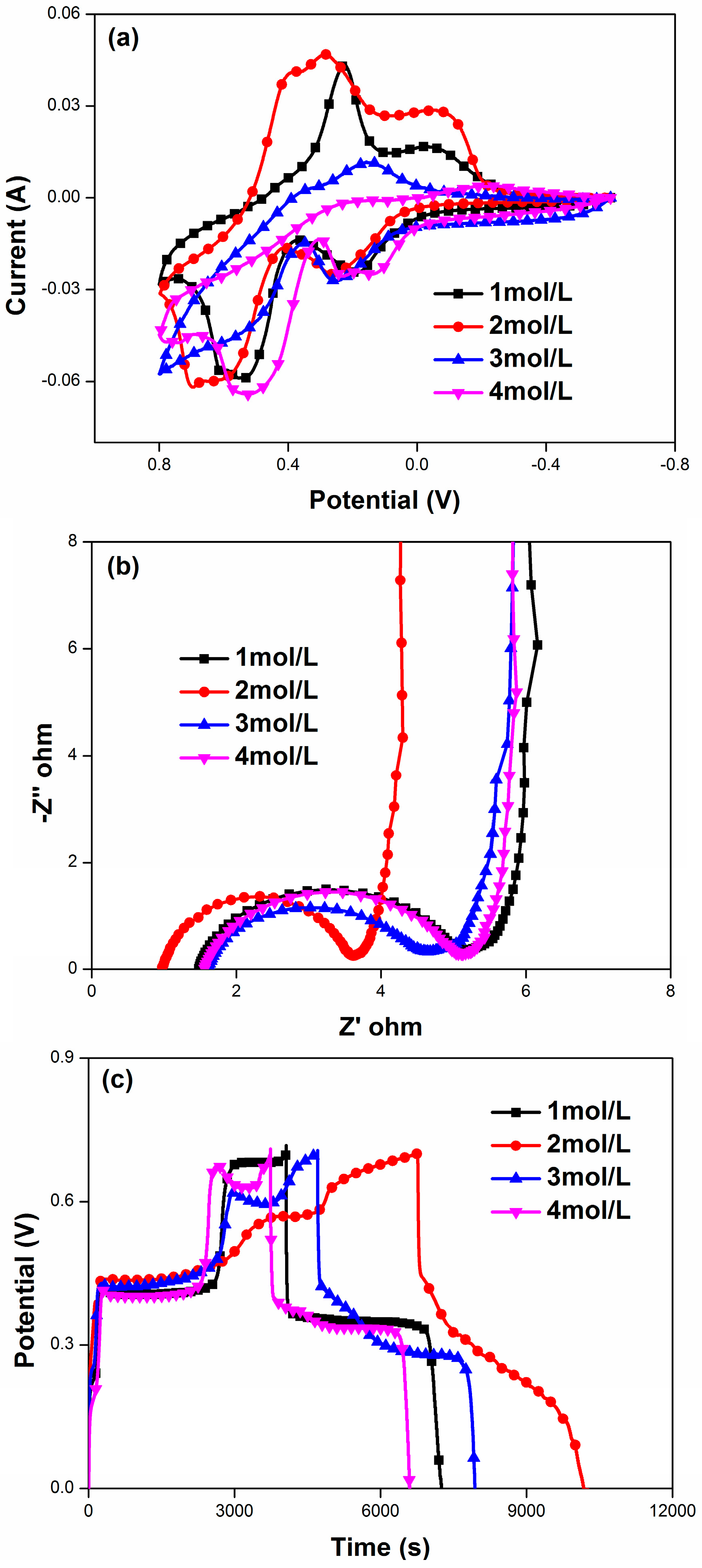
| HQ Concentration | C (F/g) | Rs (Ω·cm2) | Rct (Ω·cm2) |
|---|---|---|---|
| 1 | 3927 | 1.48 | 3.76 |
| 2 | 4002 | 0.975 | 2.642 |
| 3 | 3846 | 1.61 | 3.04 |
| 4 | 3559 | 1.56 | 3.61 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cui, X.; Zu, L.; Cai, X.; Liu, Y.; Wang, X.; Lian, H. New Supercapacitors Based on the Synergetic Redox Effect between Electrode and Electrolyte. Materials 2016, 9, 734. https://doi.org/10.3390/ma9090734
Zhang Y, Cui X, Zu L, Cai X, Liu Y, Wang X, Lian H. New Supercapacitors Based on the Synergetic Redox Effect between Electrode and Electrolyte. Materials. 2016; 9(9):734. https://doi.org/10.3390/ma9090734
Chicago/Turabian StyleZhang, You, Xiuguo Cui, Lei Zu, Xiaomin Cai, Yang Liu, Xiaodong Wang, and Huiqin Lian. 2016. "New Supercapacitors Based on the Synergetic Redox Effect between Electrode and Electrolyte" Materials 9, no. 9: 734. https://doi.org/10.3390/ma9090734