Upscaling of a Batch De-Vulcanization Process for Ground Car Tire Rubber to a Continuous Process in a Twin Screw Extruder
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analyses
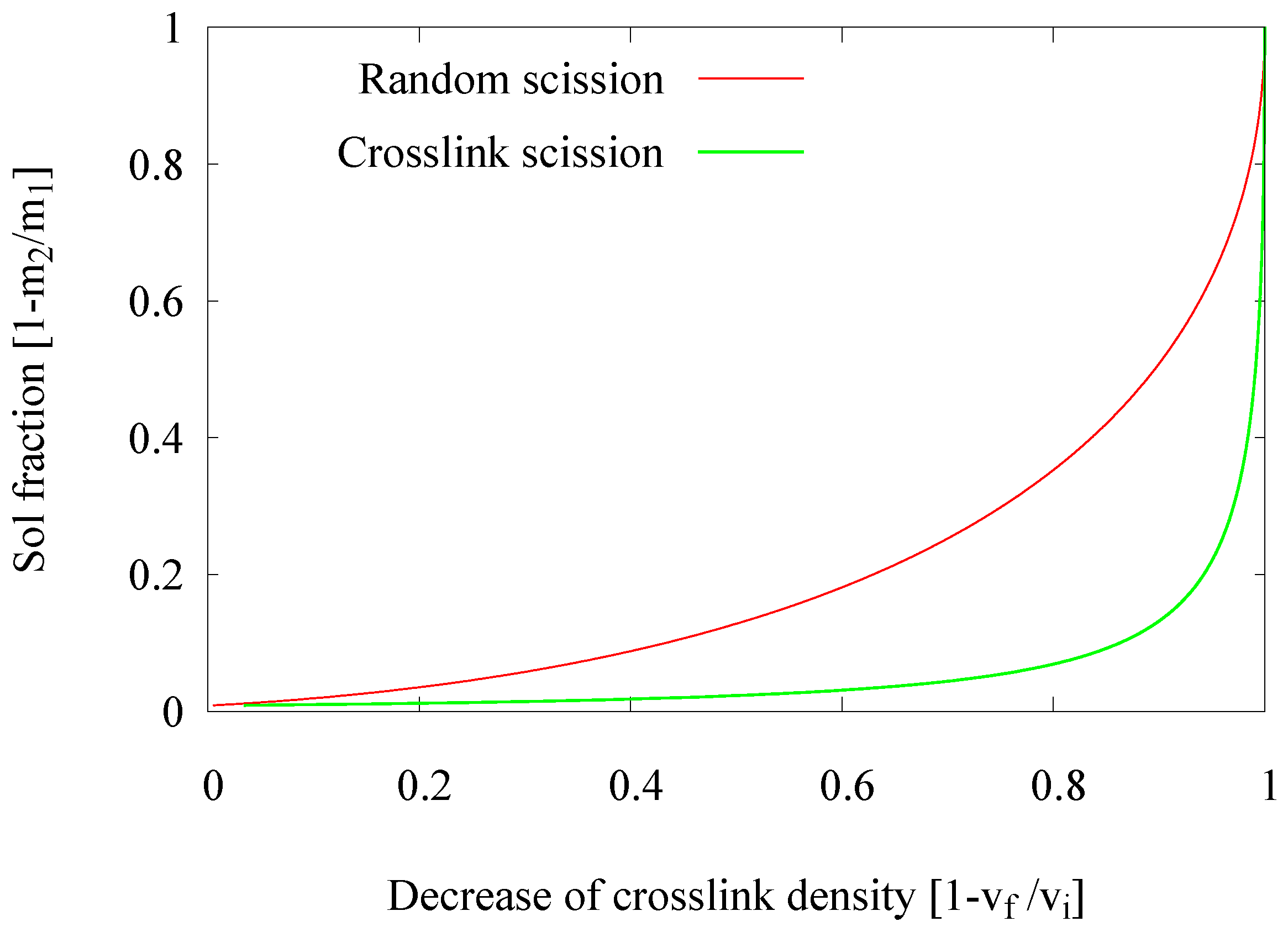
2.1.1. Decrease in Crosslink Density
2.1.2. Evaluation of the Visible Particle Size
2.2. Process Development
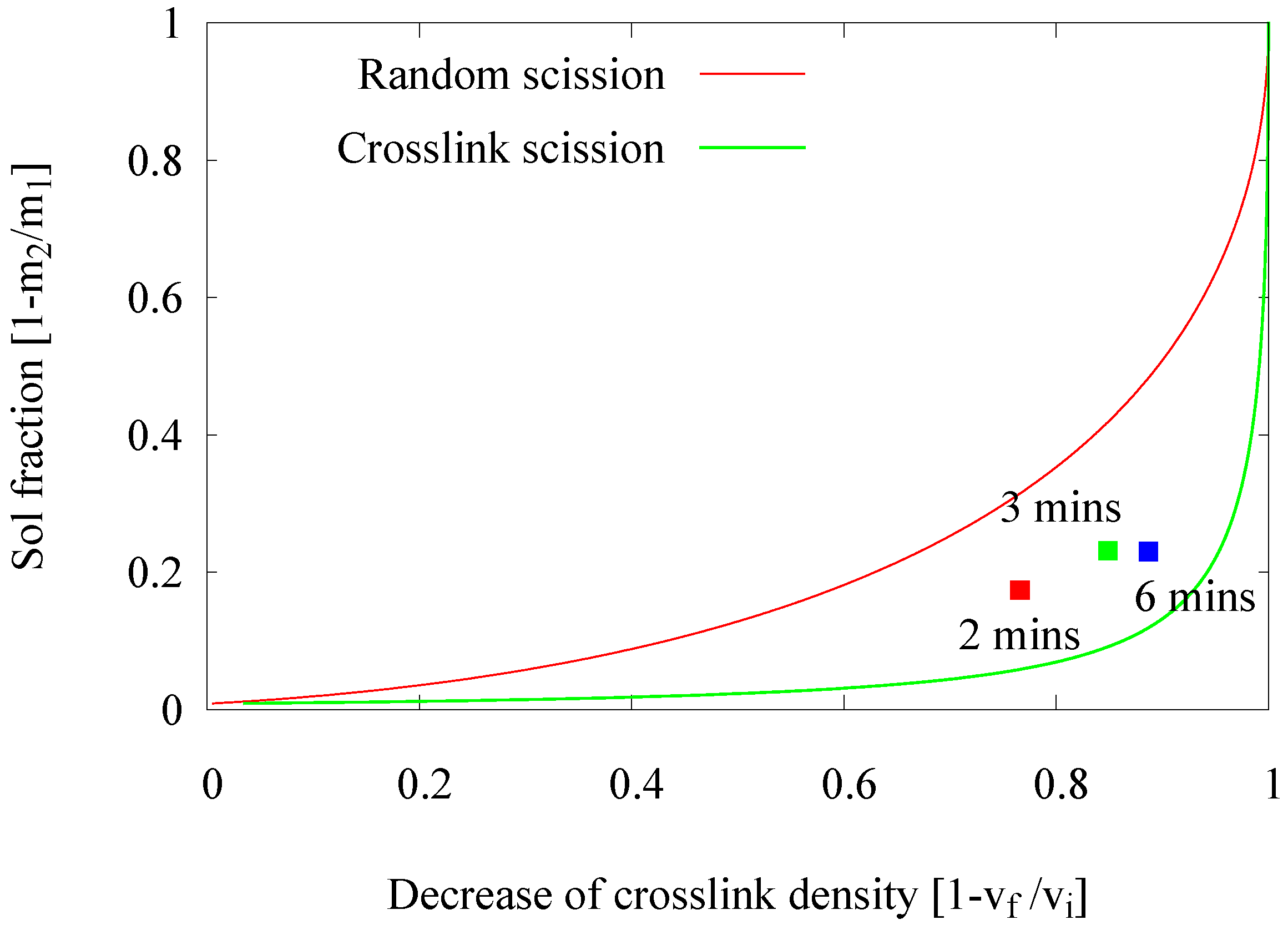
2.2.1. Influence of Devulcanization Time
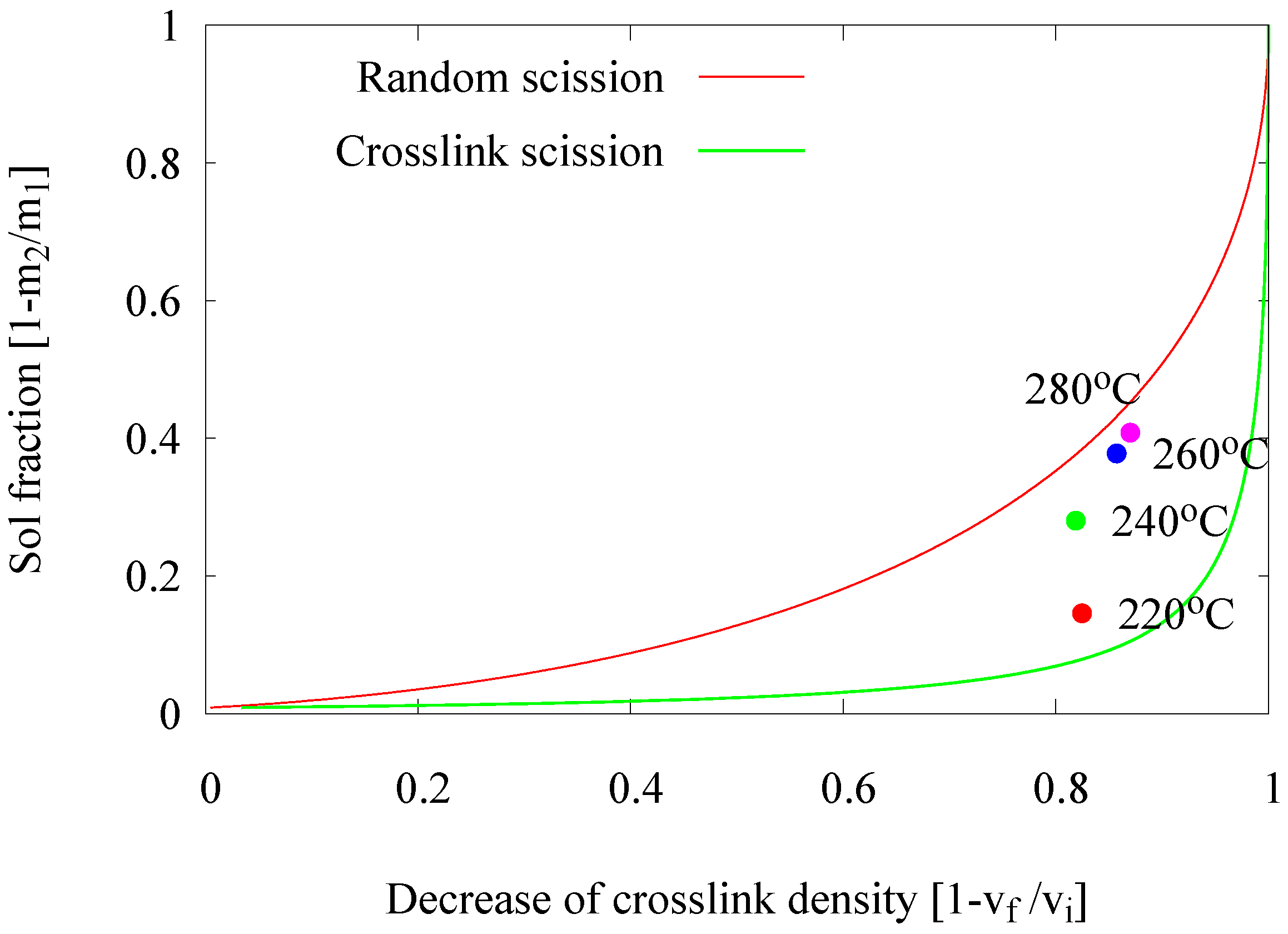
2.2.2. Influence of Devulcanization Temperature
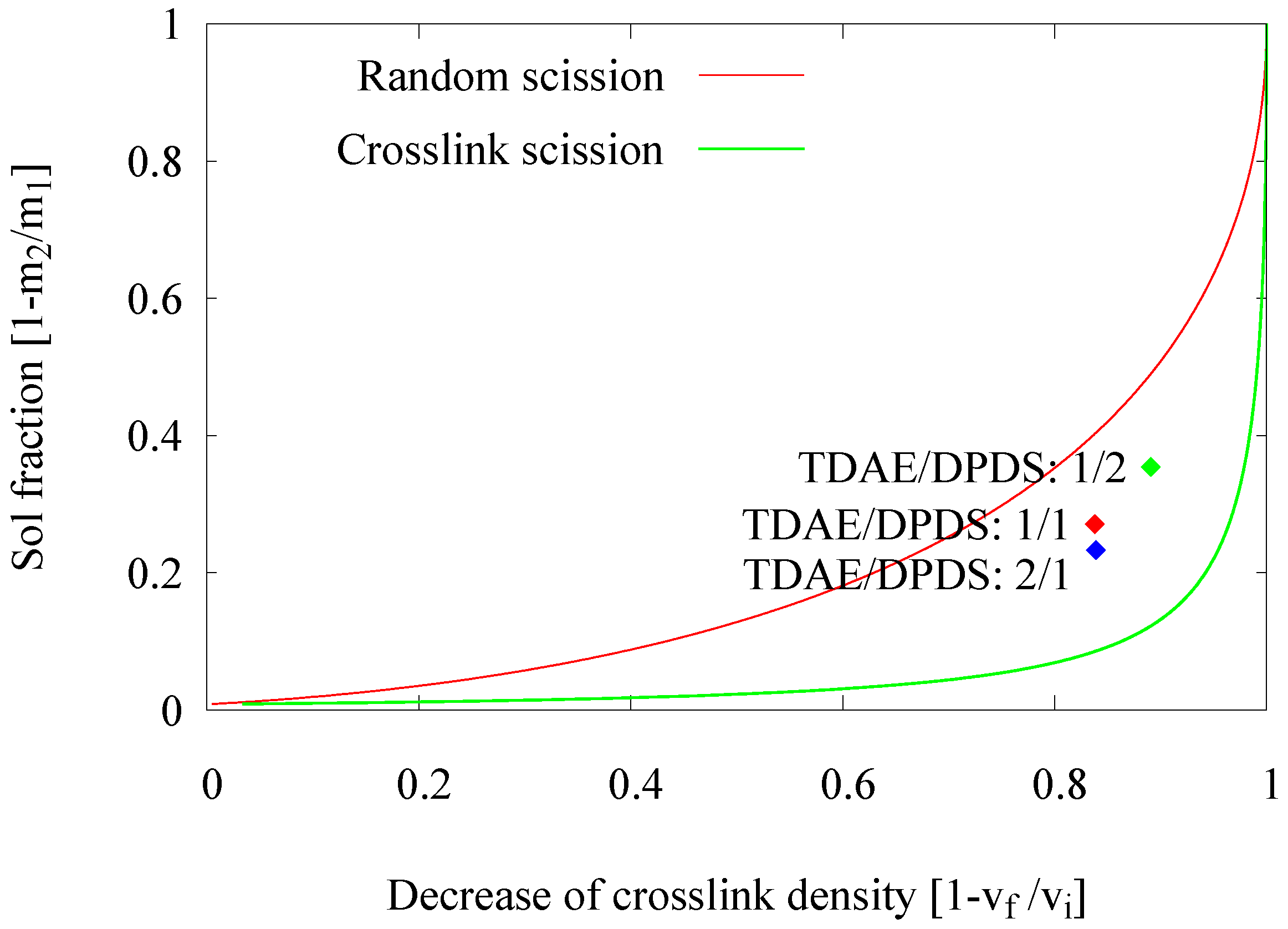
2.2.3. Influence of the Devulcanization Aid Concentration
2.3. Extruder Design and Parameter Optimization
2.3.1. The Influence of Screw Speed
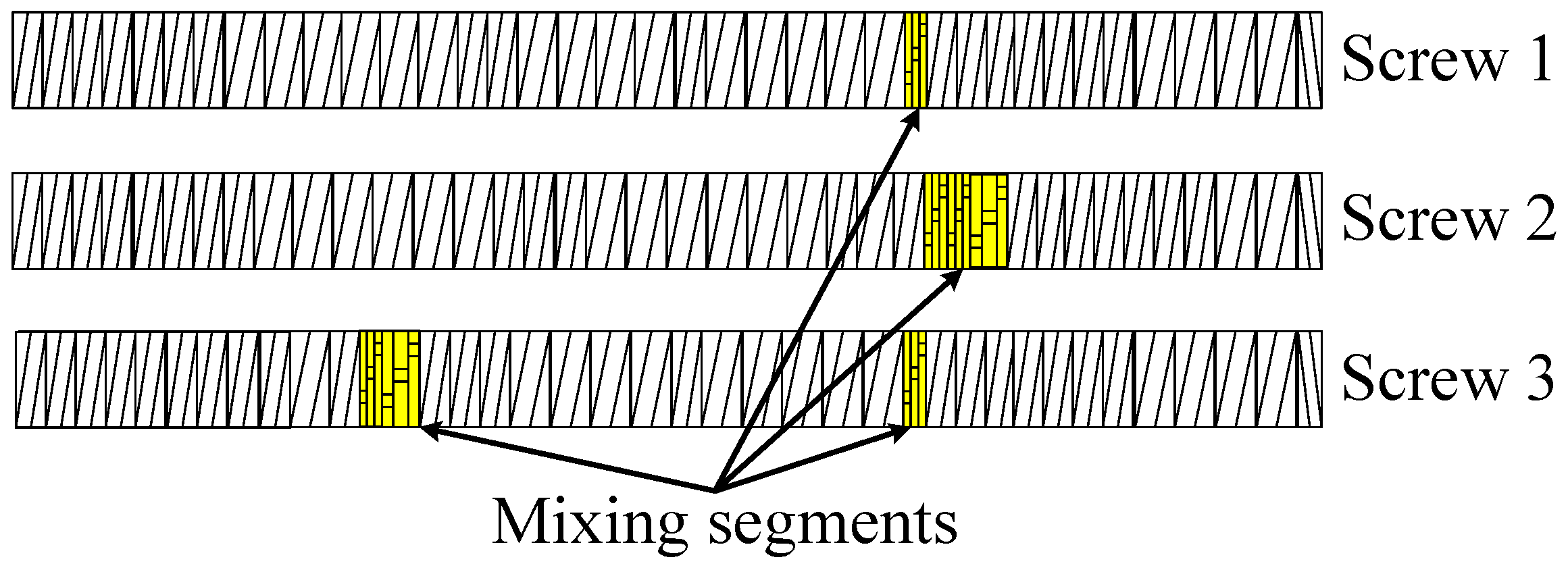
2.3.2. Influence of the Screw Configuration
- Screw 2 with additional mixing elements in the supply and mixing zone, with the intention to improve the distribution of the devulcanization aids into the GTR particles.
- Screw 3 with mixing elements added to the intermediate zone, following the observations of high shear during the milling process reducing the still visible particles in size and with the intention to have the inner, non-devulcanized parts of the GTR particles exposed to the temperatures necessary for devulcanization during a maximum residence period. This implies that the inner temperature of the larger particles might have been too low in screw Setups 1 and 2 for an effective devulcanization due to the low thermal conductivity of rubber.
- Devulcanization rate and quality: According to the Horikx graph, the screw with Configuration 1 shows the best results. The screw with Configuration 3 shows a slight decrease in the quality of the devulcanizate compared to Screw 1, indicated by the increasing amount of sol for a comparable amount of decrease in crosslink density. For the screw Configuration 2, both the quality and decrease in crosslink density are inferior: the results in the Horikx plot show a decrease in devulcanization quality, as indicated by a relative decrease in distance to the random scission line.
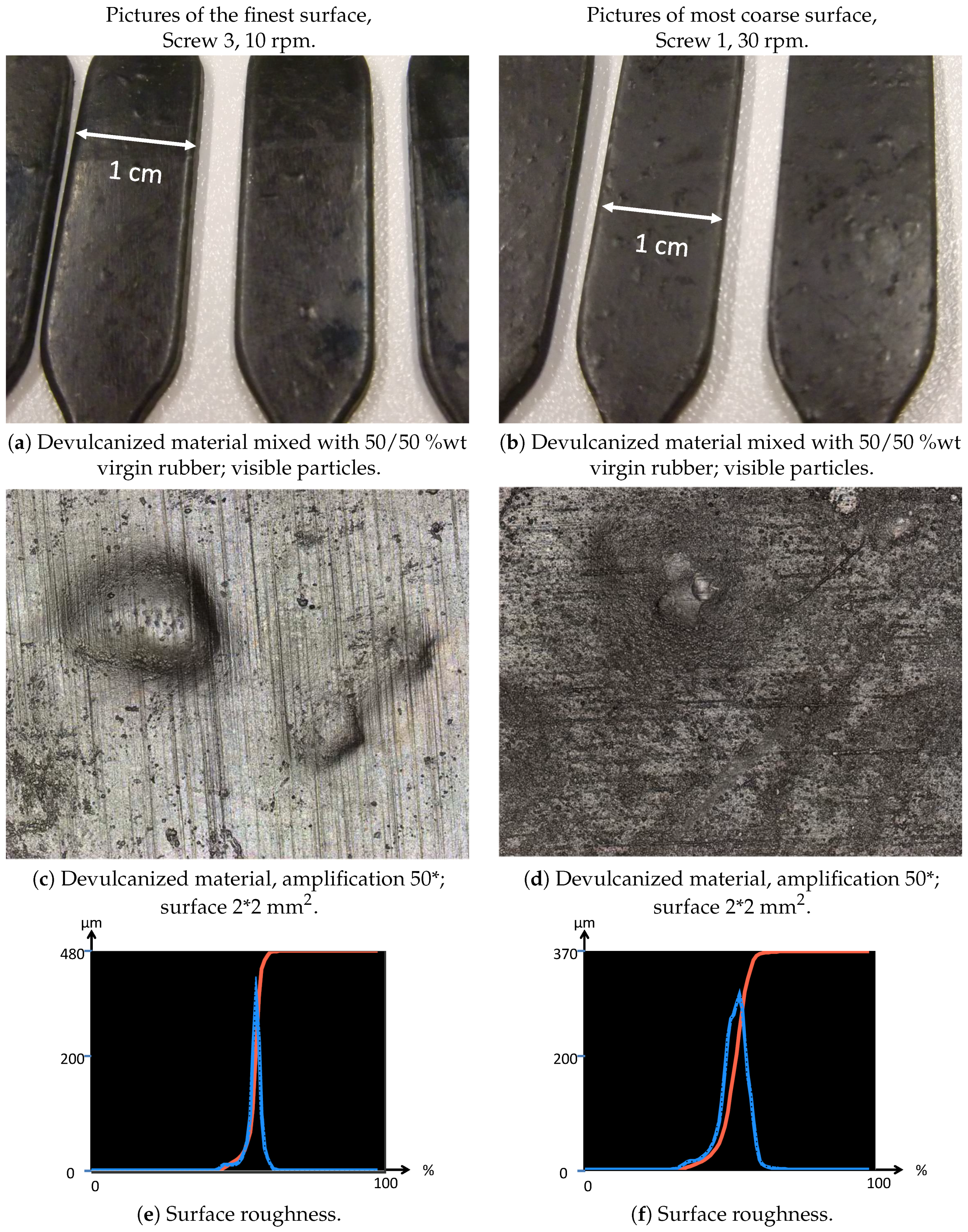
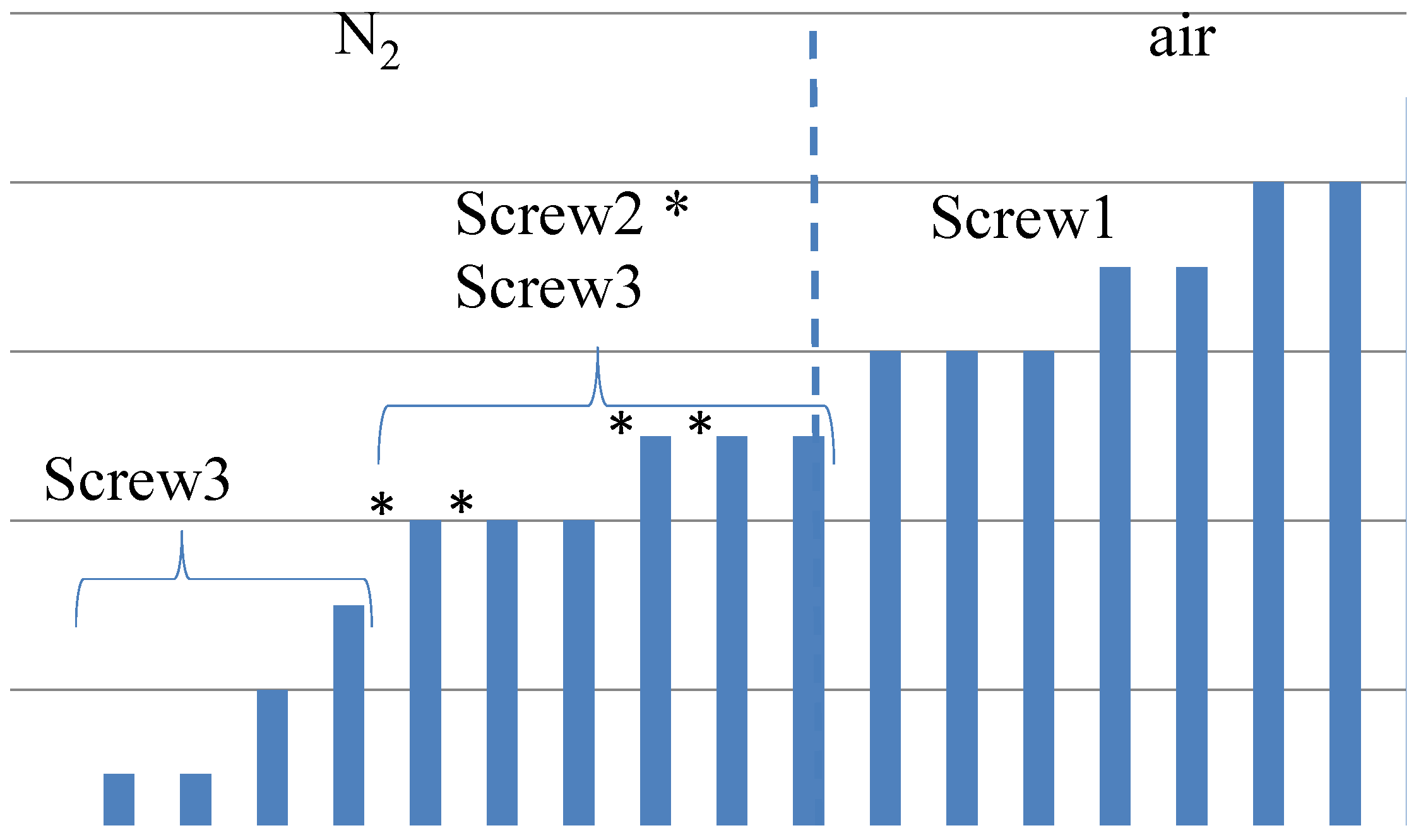
- Visible particles: The investigation of the particle size of the devulcanizates was done and evaluated by visual inspection, both with and without a microscope. Without instruments, it can clearly be observed that there are large differences in the amount and size of visible particles on the surface of the re-vulcanized rubber blends; see Figure 10. The pictures in Figure 10 show the most extreme cases: the most homogeneous blend of devulcanized and virgin rubber (Figure 10a) and the material with the coarsest surface morphology (Figure 10b). To further quantify the surface morphology of the materials, laser scanning microscope pictures were used. Figure 10c,d shows a typical surface morphology of a 2 2 mm2 area: they visualize the quantity and size differences of the particles for the samples. Figure 10e,f shows the quantitative evaluation of roughness of the surface: the more homogeneous sample has only a few, though still large particles with a height of 200 micrometer on the surface; the distribution is rather narrow. The sample with the coarse surface has a broader distribution and a higher number of particles.A count of the visible particles particles as given in Table 2 shows that there is a significant difference in the number of large, as well as small particles. The total number of particles increases by approximately a factor of four from Category 1–10. For the further comparison of the sample morphology, the interval between Categories 1 and 10 is separated into nine steps of increasing size and the number of visible particles. A clear relationship between screw configuration and the quality of the surface could be observed based on these characteristic numbers: with Screw 1, the particles were the largest and most numerous, as can be seen in Figure 10b and Figure 11; with Screw 3, the best material was produced with the smallest and lowest number of visible particles, as is shown in Figure 10a and Figure 11. Class 0 was assigned to a quality comparable to that of virgin material. The results for Screw 2 were in-between those of Screw 1 and Screw 3; see Figure 11.
3. Experimental Section
3.1. Materials
3.2. Preparation of Devulcanizates
3.2.1. Pre-Treatment
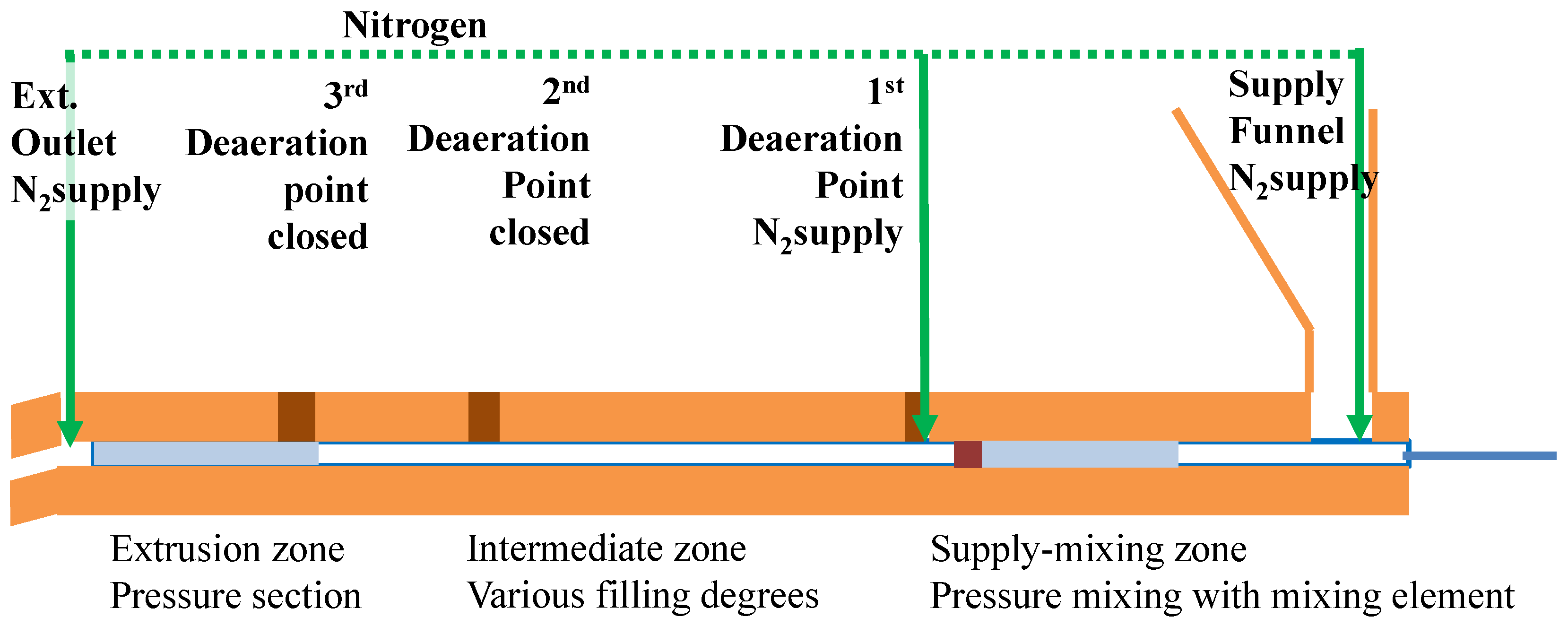
3.2.2. Extruder Setup
3.2.3. Additional Screw Designs
3.2.4. Experimental Conditions
3.2.5. Post-Treatment
3.2.6. Surface Roughness
4. Conclusions
- Devulcanization process conditions: The extruder conditions turned out to be comparable to the parameters found for the Brabender internal mixer, as shown in Table 1:
- -
- Temperature profile: The main factor determining the devulcanizate quality is the temperature: if it is too low, devulcanization is too slow; if it is too high, the polymer degrades. The optimum within the temperature window investigated here was 220 .
- -
- Time: The residence time in the extruder is another important influencing factor for the product quality: shorter times set by higher screw speeds result in insufficient devulcanization and uncontrolled breakdown of the polymer due to the higher shearing forces. The optimal condition for a screw configuration like Screw 3 is a 6-min residence time.
- -
- Devulcanization aid concentration: The optimum concentration of DPDS was found to be 18 mmol/100 g GTR. Increasing the concentration to 30 mmol/100 g GTR decreased the quality of the devulcanizate.
- Extruder parameters: The extruder parameters are interdependent: The screw configuration determines the amount of shear and the residence time, at a certain screw speed. For a small speed range, both residence time and shear rate can be assumed to have a linear relation with the speed; however, this is not valid for larger differences in screw speed.
- -
- Screw speed: For the given extruder and screw configuration and within a range of 10–120 rpm, 10 rpm leading to a residence time of 6 min was optimal.
- -
- Screw configuration: The low shear concept has been shown to be a useful approach. From the three screw variations tested, Screw 3, Figure 8, showed the best results in terms of devulcanization efficiency, quality and still visible particle size.
- -
- Fill factor: Being a paramount parameter for the amount of shear in an internal mixer, for the extruder screw, it appeared to be of less importance.
- Still visible particles: For non-optimal devulcanization parameters, large particles remain, which mostly consist of non-devulcanized material. However, even with the use of a small-sized passenger car tire powder, small visible particles remain in the devulcanized material. The first analysis indicates a high amount of silica in these particles, but additional investigations are necessary.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BR | Butadiene rubber |
| B.V. | Besloten Vennootschap, comparable with Ltd or Inc. |
| DPDS | Diphenyl disulfide , devulcanization aid |
| EPDM | Ethylene-propylene-diene rubber |
| GTR | Granulated car tire rubber |
| NR | Natural rubber |
| NWO | Nederlandse Organisatie voor Wetenschappelijk Onderzoek, The Netherlands Organization for Scientific Research |
| SBR | Styrene-butadiene rubber |
| TBBS | N-tert-butyl-2-benzothiazolesulfenamide, curative |
| TDAE | Treated distillate aromatic extract, processing oil |
| TDTBP | Tris(2,4-di-tert-butylphenyl)phosphite, antioxidant |
| THF | Tetrahydrofuran, solvent |
| TMQ | 2,2,4-trimethyl-1,2-dihydroquinoline, antioxidant |
| 6PPD | N-(1,3-dimethylbutyl)-N’-phenyl-p-phenylenediamine, antiozonant |
References
- Saiwari, S. Post-Consumer Tires Back into New Tires. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 23 May 2013. [Google Scholar]
- Madorsky, S.L. Thermal Degradation of Organic Polymers; Interscience Publicers: New York, NY, USA, 1964; p. 315. [Google Scholar]
- Golub, M.A. Thermal Rearrangements in 1,2-Poly(1,4-Hexadiene)s. J. Polym. Sci. Polym. Lett. Ed. 1978, 16, 253–260. [Google Scholar] [CrossRef]
- Golub, M.A. Thermal Rearrangements of Unsaturated Polymers. Rubber Chem. Technol. 1978, 51, 677–685. [Google Scholar] [CrossRef]
- Sarkar, M.D.; Mukunda, P.G.; De, P.P.; Bhowmick, A.K. Degradation of Hydrogenated Styrene—Butadiene Rubber at High Temperature. Rubber Chem. Technol. 1997, 70, 855–870. [Google Scholar] [CrossRef]
- Verbruggen, M.A.L. Devulcanization of EPDM Rubber. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 14 September 2007. [Google Scholar]
- Verbruggen, M.A.L.; van der Does, L.; Noordermeer, J.W.M.; van Duin, M.; Manuel, H.J. Mechanisms involved in the recycling of NR and EPDM. Rubber Chem. Technol. 1999, 72, 731–740. [Google Scholar] [CrossRef]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Devulcanization of Whole Passenger Care Tire Material. KGK Kautsch. Gummi Kunststoffe 2013, 66, 20–25. [Google Scholar]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative Investigation of the Devulcanization Parameters of Tire Rubbers. Rubber Chem. Technol. 2014, 87, 31–42. [Google Scholar] [CrossRef]
- Charlesby, A. Solubility and Molecular Size Distribution of Crosslinked Polystyrene. J. Polym. Sci. 1953, 11, 513–520. [Google Scholar] [CrossRef]
- Horikx, M.M. Chain scissions in a polymer network. Rubber Chem. Technol. 1956, 29, 1166–1173. [Google Scholar] [CrossRef]
- Porter, M. Structural Characterization of Filled Vulcanizates Part 1. Determination of the Concentration of Chemical Crosslinks in Natural Rubber Vulcanizates Containing High Abrasion Furnace Black. Rubber Chem. Technol. 1967, 40, 866–882. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of Filler-Reinforced Vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- NETZSCH-Gerätebau GmbH. Thermal Properties of Polymers; NETZSCH-Gerätebau GmbH: Vleuten, The Netherlands, 2013. [Google Scholar]
- Rajan, V.V.; Dierkes, W.K.; Noordermeer, J.W.M.; Joseph, R. Model compound studies on the devulcanization of natural rubber using 2,3-dimethyl-2-butene. Rubber Chem. Technol. 2005, 78, 572–587. [Google Scholar] [CrossRef]
- Sutanto, P. Development of a Continuous Process for EPDM Devulcanization in an Extruder. Ph.D. Thesis, Groningen University, Groningen, The Netherlands, 16 June 2006. [Google Scholar]
- Meijer, H.E.H.; Janssen, J.M.H.; Anderson, P.D. Mixing of Immiscible Liquids; Hanser Publications: Cincinnati, OH, USA, 2009. [Google Scholar]
- Raut, J.S.; Naik, V.M.; Jongen, T.R. Efficient simulation of time-dependent flows: Application to a twin screw extruder. AIChE J. 2003, 49, 1933–1946. [Google Scholar] [CrossRef]
- Wünsch, O.; Böhme, G. Highly viscous fluid flow in the kneading zone of a corotating twin-screw extruder. Forsch. Ingenieurwes. 2001, 66, 224–234. [Google Scholar]
- Dimensional and Geometrical Product Specifications and Verification; ISO/TC 213; International Organization for Standardization: Geneva, Switzerland, 1997.



| Factors | Optimized Brabender | Extruder Conditions |
|---|---|---|
| Internal Mixer Conditions | ||
| Devulcanization aid | DPDS, 15 mmol/100 g | DPDS 15, 18–30 mmol/100 g |
| compound | compound | |
| Devulcanization oil | TDAE 5 %wt | TDAE 5, 6.2–10 %wt |
| Anti-oxidant | TDTBP, 1 %wt | TDTBP 1 %wt |
| Swelling time | 30 min | 30 min and 24 h |
| Swelling temperature | 65 °C | 65 °C |
| Devulcanization time | 6 min | (<2), 2–6 min |
| Rotor speed/screw speed | 50 rpm | 10–120 rpm |
| Devulcanization temperature | 220 °C | 220–280 °C |
| Devulcanization atmosphere | Nitrogen gas purging | In air |
| nitrogen gas purging | ||
| Screw Configuration | High shear screw | Low shear screw with |
| (first transfer of internal | additional kneading elements | |
| mixer conditions) | in devulcanization zone | |
| Swelling equipment | Hot air oven | Heated mixer |
| Extrudate handling | Dumping in | Cooling in air |
| liquid nitrogen | Cooling in inert atmosphere | |
| Cooling with cooling calender | ||
| Ventilation | - | Ventilation system with scrubber |
| for cleaning and smell control |
| Category (See Figure 11) | Material | Size | Amount |
|---|---|---|---|
| 1 | Finest: | 1 mm | 2.5/cm2 |
| (s3-10-12) * | 0.1 mm, 1 mm | 5/cm2 | |
| 10 | Most coarse: | 1 mm | 8/cm2 |
| (s1-30-6) | 0.1 mm, 1 mm | 22/cm2 |
| Ingredients | Phr | Ingredients | Phr |
|---|---|---|---|
| Devulcanizate, | – | Zinc oxide | 3.0 |
| based on polymer content | 50.0 | Stearic acid | 2.0 |
| SBR | 44.7 | Sulfur | 2.5 |
| BR | 17.5 | TBBS | 1.5 |
| Carbon black N375 | 80.0 | 6PPD | 1.0 |
| TDAE oil | 9.8 | TMQ | 2.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiwari, S.; Van Hoek, J.W.; Dierkes, W.K.; Reuvekamp, L.E.A.M.; Heideman, G.; Blume, A.; Noordermeer, J.W.M. Upscaling of a Batch De-Vulcanization Process for Ground Car Tire Rubber to a Continuous Process in a Twin Screw Extruder. Materials 2016, 9, 724. https://doi.org/10.3390/ma9090724
Saiwari S, Van Hoek JW, Dierkes WK, Reuvekamp LEAM, Heideman G, Blume A, Noordermeer JWM. Upscaling of a Batch De-Vulcanization Process for Ground Car Tire Rubber to a Continuous Process in a Twin Screw Extruder. Materials. 2016; 9(9):724. https://doi.org/10.3390/ma9090724
Chicago/Turabian StyleSaiwari, Sitisaiyidah, Johannes W. Van Hoek, Wilma K. Dierkes, Louis E.A.M. Reuvekamp, Geert Heideman, Anke Blume, and Jacques W.M. Noordermeer. 2016. "Upscaling of a Batch De-Vulcanization Process for Ground Car Tire Rubber to a Continuous Process in a Twin Screw Extruder" Materials 9, no. 9: 724. https://doi.org/10.3390/ma9090724






