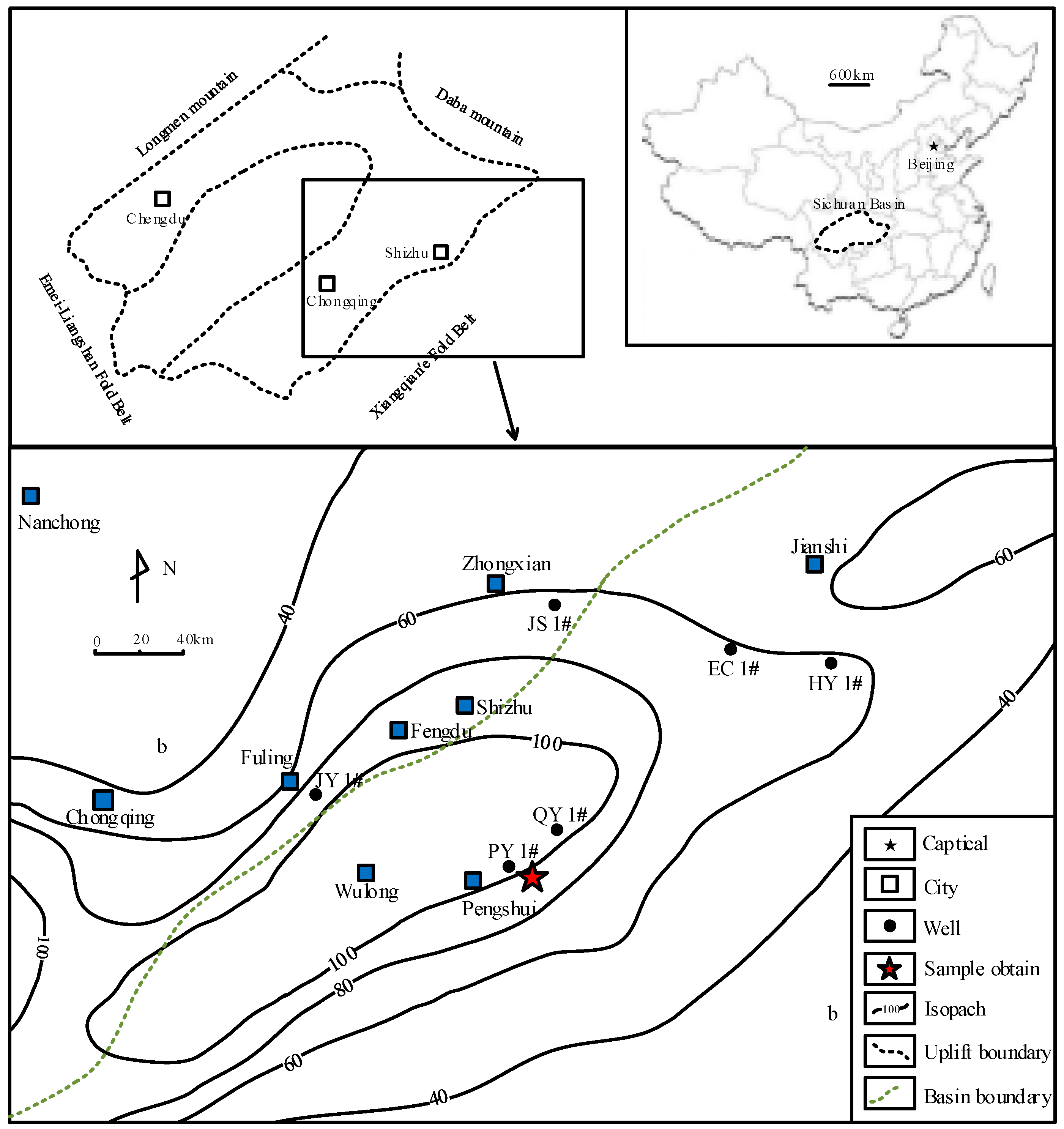
4.1. Microstructure Alteration after CO2-Water-Interaction
Mineralogical variations associated with microstructure alteration induced by water with gaseous/super-critical CO
2 was investigated by SEM together with EDS analysis.
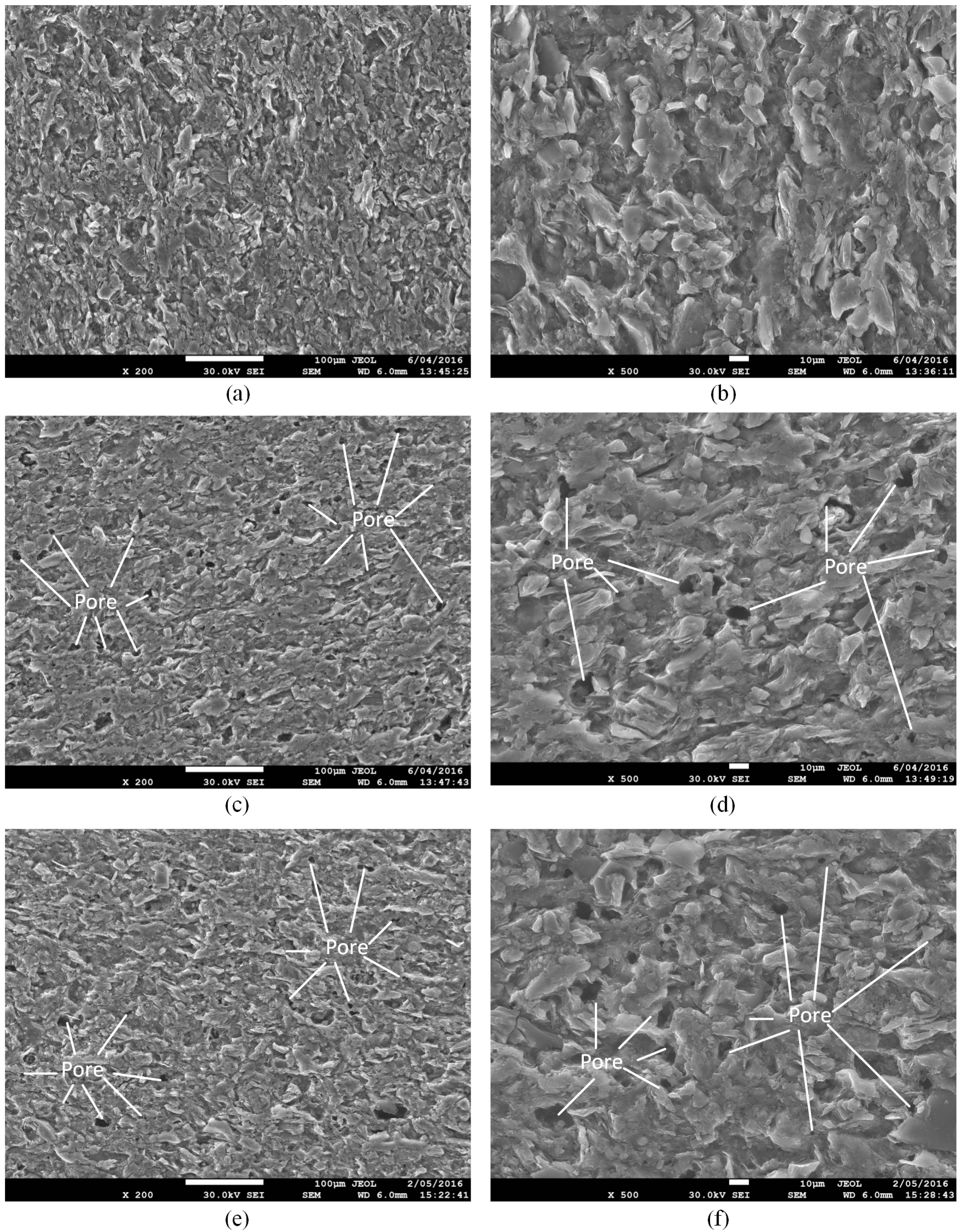
Figure 3 shows the microstructure of shale samples without any fluid saturation (a,b), samples with water + gaseous CO
2 (c,d) and water + SC-CO
2 saturation (e,f). It is clear that the saturation of water with both gaseous and super-critical CO
2 creates many pores on the surface of the shale slice, possibly because the dissolution of CO
2 in water decreases the pH of the fluid (Equation (1)), which may accelerate the chemical reactions for mineral dissolution (Equations (2) and (3)) and carbonate precipitation (Equations (4) and (5)). Although SEM can only observe the surface of the sample, we can deduce that, with long-term saturation, water, CO
2 and ions in the fluids will penetrate into the matrix of the shale and create more pores inside. These pores will create a secondary porosity system, which decreases the strength of the natural pore structure, and the strength of the sample therefore decreases after saturation [
37,
38]. As SEM analysis can only concentrate on an ultra-small area of the slice’s surface, the difference of the effect of gaseous CO
2 and SC-CO
2 on the microstructure of shale samples is minor in the SEM images.
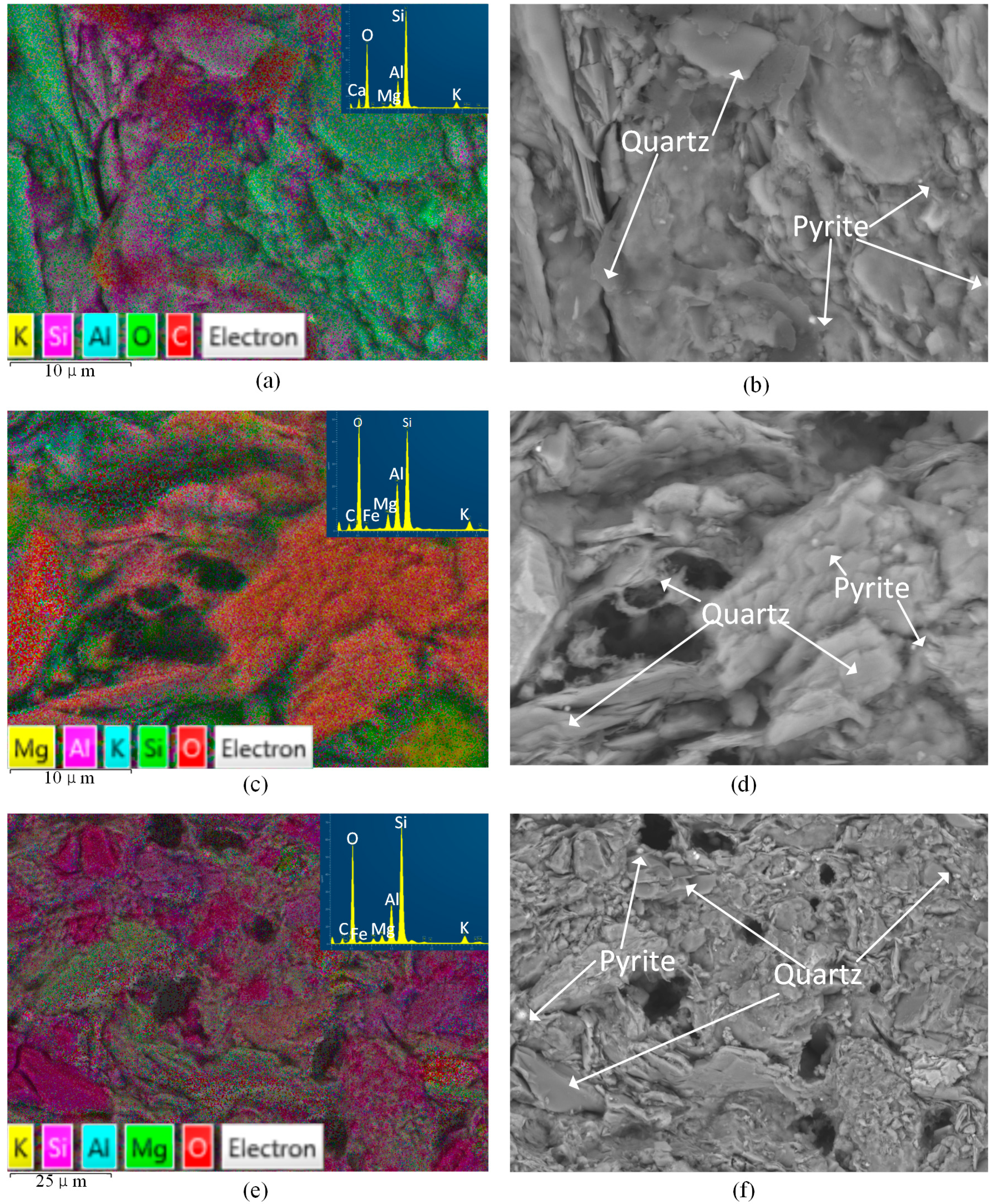
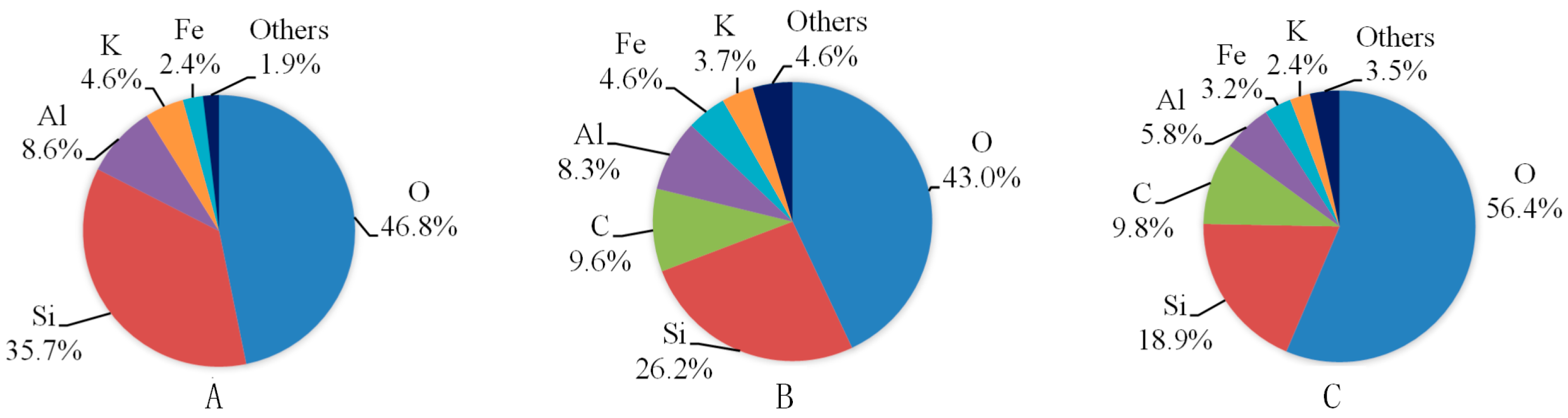
Figure 4 shows the EDS results of shale samples with or without fluid saturation. The X-ray spectra results are shown in
Figure 5. For each slice, we observed three different areas and here present the one with a moderate percentage of carbon. From
Figure 4 and
Figure 5 we can see that oxygen and silica are the first and second highest proportion of all the elements of the three kinds of shale slices. For slices after saturation with water with gaseous CO
2 and SC-CO
2, carbon accounts for the third highest percentage of the elements, which are 9.6% and 9.8%, respectively. However, for samples without saturation, the percentage of carbon is negligible. This is mainly because the precipitation of carbonates attaches on the surface of the shale slice. As the gaseous and super-critical CO
2 applied in the study have similar solubility and pH in water (shown in Table 4), they present similar percentages of carbon. Compared to the spectra of samples without saturation, ions like Al and K decrease and Fe increase in samples with water and CO
2 saturation. This is caused by the dissolution of minerals, such as K-feldspar.
4.2. Effects of CO2-Water-Rock Interaction on Mechanical Behaviors
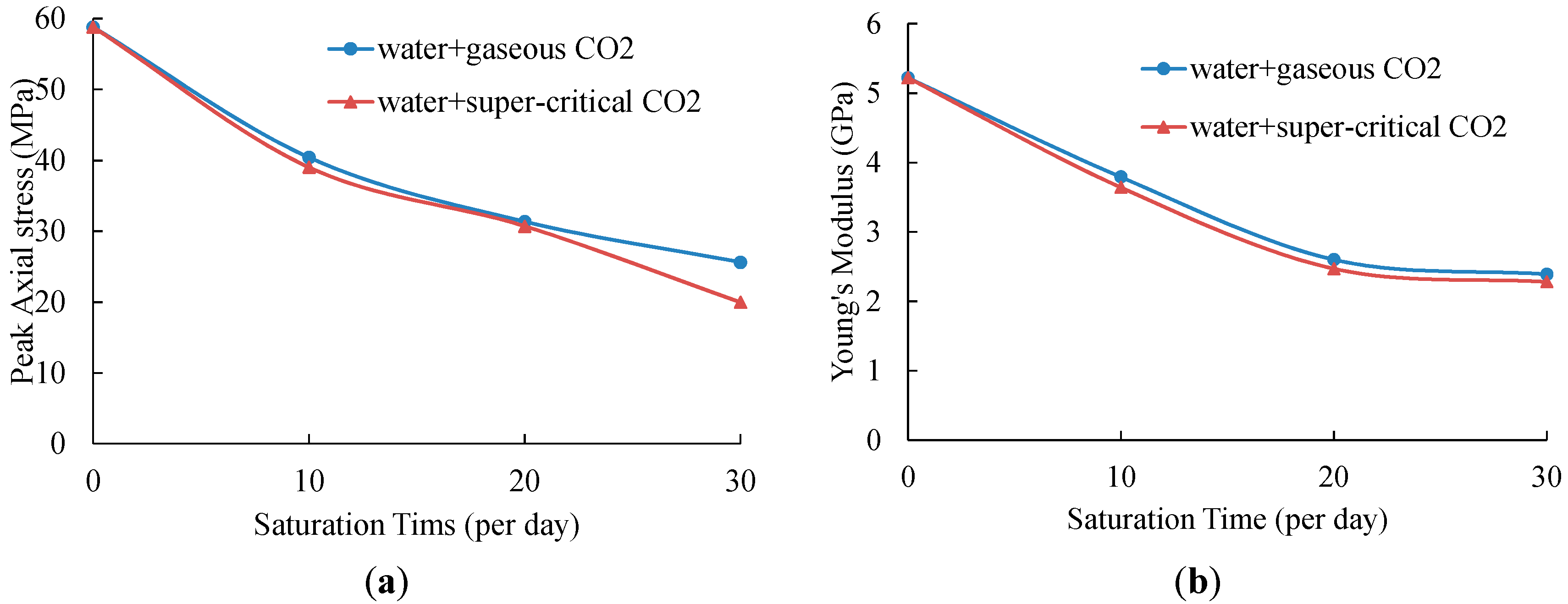
The variation of UCS and Young’s modulus of samples without saturation and samples saturated in water absorbed with gaseous and super-critical CO
2 are shown in
Table 3 and
Figure 6. The standard deviations of UCS values and Young’s modulus for each saturation condition are minor, except for the two samples saturated in water with SC-CO
2 for 30 days, which show large UCS variation. Considering the change of strength with saturation time, we chose the higher one for the purposes of discussion. For other groups, the average values are used in all discussions.
According to
Table 3 and
Figure 6, of all the tested samples, samples without saturation have the highest UCS and Young’s modulus values, of 58.82 MPa and 5.22 GPa, respectively. After 10-day saturation in water with gaseous CO
2, the UCS value decreases to 40.42 MPa, and the Young’s modulus also shows a reduction of 27.39% to 3.79 GPa. When the saturation time is 20 days, samples show only 31.36 MPa strength and 2.60 GPa Young’s modulus, which is 46.48% and 49.81% respectively lower than samples with no saturation. For 10 more days of saturation, the UCS and Young’s modulus keeps decreasing and the values are smaller than half of that of samples without saturation, at 25.63 MPa and 2.39 GPa, respectively. For shale samples saturated in water with SC-CO
2, the UCS values show reductions of 33.66%, 47.77% and 66.05% with saturation times of 10 days, 20 days and 30 days, respectively. The Young’s modulus also decreases dramatically from 5.22 GPa to 3.64 GPa (10-day saturation), 2.47 GPa (20-day saturation) and 2.28 GPa (30-day saturation), respectively.
The considerable reductions of strength and Young’s modulus for saturated samples are due to the CO
2-water-rock interactions coupled with chemical and mechanical effects. When shale samples are saturated in gaseous/super-critical CO
2 with the medium of fresh water, clays in the rock absorb water resulting in the shale swelling, which causes the decrease of strength and Young’s modulus [
39]. According to Heller and Zoback [
40] and Luo et al. [
41], shale gas, which exists in natural fractures, porous matrices and kerogen, is easier to be replaced by CO
2 as CO
2 has better adsorption ability in shale [
42]. Therefore, the adsorption of CO
2 in shale samples will also cause shale swelling and strength decrease [
43]. More importantly, the dissolution of CO
2 in water leads to the chemical reactions for mineral dissolution (Equations (2) and (3)) and carbonate precipitation (Equations (4) and (5)). The dissolution and precipitation process creates pores in the rock, as shown in
Figure 3, which changes the microstructure of shale samples. This phenomenon will contribute to the reduction of strength and Young’s modulus. Meanwhile, longer saturation time will cause greater damage on the shale sample, and the strength and Young’s modulus will therefore be lower. This is in accordance with the experimental results.
From
Figure 6, we can see that the UCS and Young’s modulus values of samples saturated in water with gaseous and super-critical CO
2 have the same variation trend with saturation time. However, with the same saturation time, both of the values of samples soaked in water + SC-CO
2 fluids are smaller than those of samples soaked in water + gaseous CO
2 fluids. The small discrepancies of strength and Young’s modulus are mainly caused by the difference of properties between gaseous CO
2 and SC-CO
2, as shown in
Table 4. SC-CO
2 has higher density, viscosity, thermal conductivity and dissolution ability in water than gaseous CO
2. The pH of water dissoluted with these two fluids is similar, that of SC-CO
2 based water being 2.83 and that of gaseous CO
2 based water being 2.84. These differences overall contribute to the difference in results. Moreover, water, CO
2 and ions under super-critical saturation conditions will more easily penetrate into shale samples than under gaseous saturation conditions because of the 2 MPa higher confining pressure.
Another important mechanical characteristic of reservoir rock is the brittleness index (
BI). The brittleness index can be obtained by many methods, including mechanical analysis, energy analysis and mineral composition analysis [
46]. In the present study, mechanical analysis was used for the calculation of the brittleness index (
BI). It is defined by the following equation [
47].
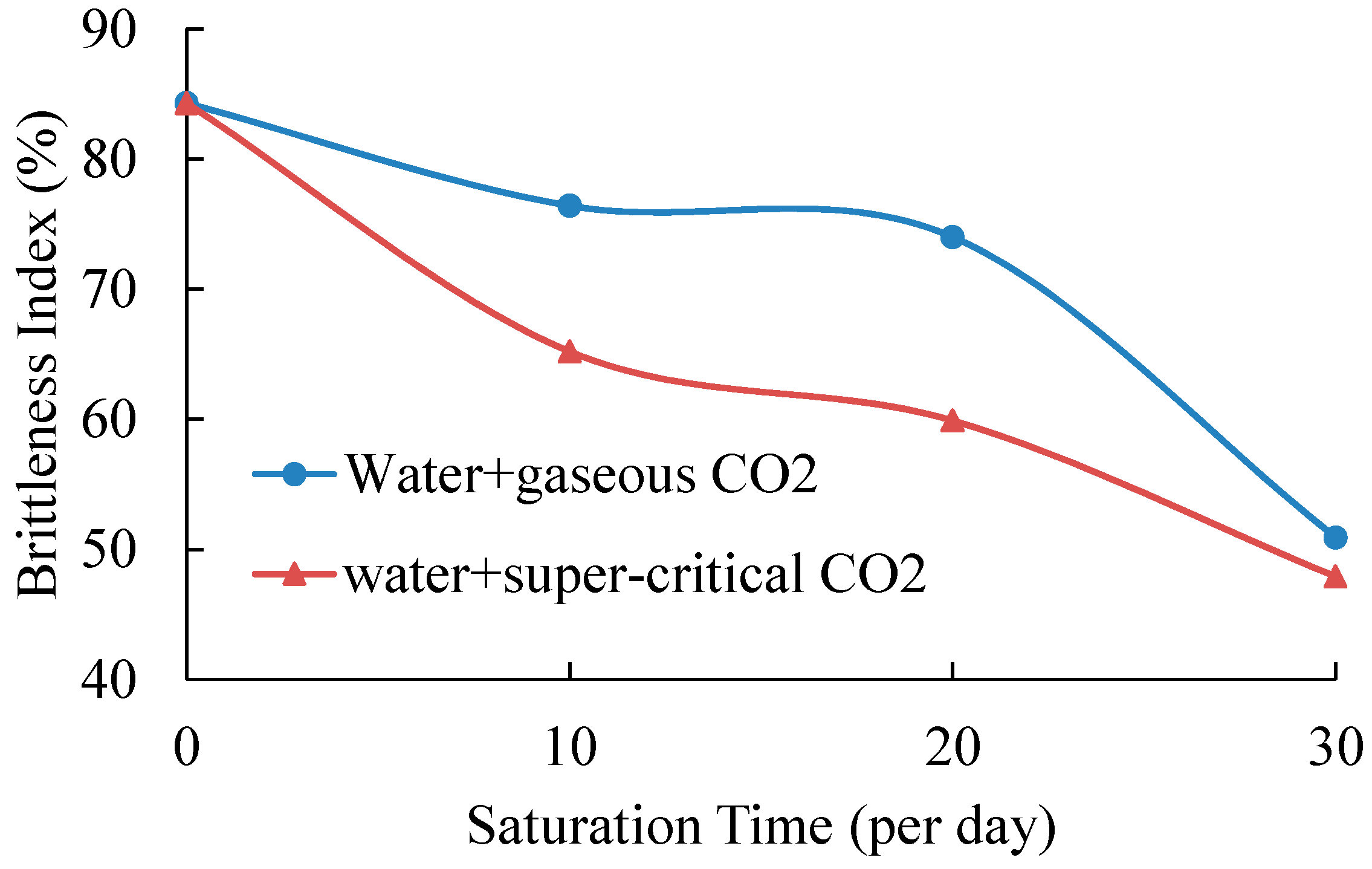
Table 5 shows the brittleness index of all tested samples and
Figure 7 presents the variations of brittleness index with saturation time. The standard deviations of samples without saturation and samples saturated in gaseous/super-critical CO
2 for 10 and 20 days are minor, varying from 1.2% to 3.8%. However, when the saturation time is 30 days, the samples saturated under gaseous conditions have a high standard deviation of 7.5%, and samples saturated under super-critical conditions have only one value (the other one is excluded because of the ultra-low UCS value). This means that samples with a longer saturation time will have larger variations in the brittleness index, which is caused by chemical-mechanical effects. However, the average value is still more reasonable for analysis.
According to
Table 5 and
Figure 7, samples without saturation have the highest brittleness index of 84.3%, which is consistent with the mineralogical analysis of samples that contain a high percentage of rigid components. After 10-day saturation, the values of samples in water with gaseous/super-critical CO
2 decrease to 76.4% and 65.2%, respectively. This is mainly because the adsorption of water and CO
2 and the chemical reactions of shale and fluids increase the plasticity and toughness. When the saturation time is 20 days, the brittleness index of both the two saturation conditions continues to increase. For samples saturated under gaseous conditions, the brittleness is 74.0%, slightly smaller than that of samples saturated under the same conditions for 10 days. Samples with SC-CO
2 and water saturation present a much lower brittleness index of 59.9%. Compared to the results of 10-day saturation, 20-day saturation may create more cracks and pores in shale samples, which cause shale samples to have higher plasticity. When the saturation time is extended to 30 days, both kinds of saturated samples present higher axial strains than those with a shorter saturation time before failure, and their brittleness index decreases to 50.9% and 47.9%, respectively. The discrepancy of physical properties (
Table 4) between gaseous and super-critical CO
2 has less effect on shale strength and Young’s modulus. However, their influence on shale’s brittleness index is considerable. Samples with SC-CO
2 and water saturation present a lower brittleness index than that of samples saturated in gaseous CO
2 and water fluids for all three saturation times. This means that the beneficial properties of SC-CO
2 decrease shale’s brittleness and increase its plasticity.
4.3. Effects of CO2-Water-Rock Interaction on Crack Propagation
With the benefits of high sensitivity and non-destructive monitoring, AE analysis is a widely used method to investigate the stages of crack closure, crack initiation and crack damage in rock mechanics studies and engineering applications [
37,
48]. The crack closure stage is characterized by very small AE energy, which is released by seating and loading adjustment. With the increase of axial load, stable crack growth or dilation occurs and the AE energy increases gradually, leading to the beginning of crack initiation. When rock samples reach the crack damage point, the AE energy increases drastically and the unstable crack growth creates considerable damage and samples finally fail. Hence, AE analysis provides an additional way to manifest rock mechanics during UCS tests.
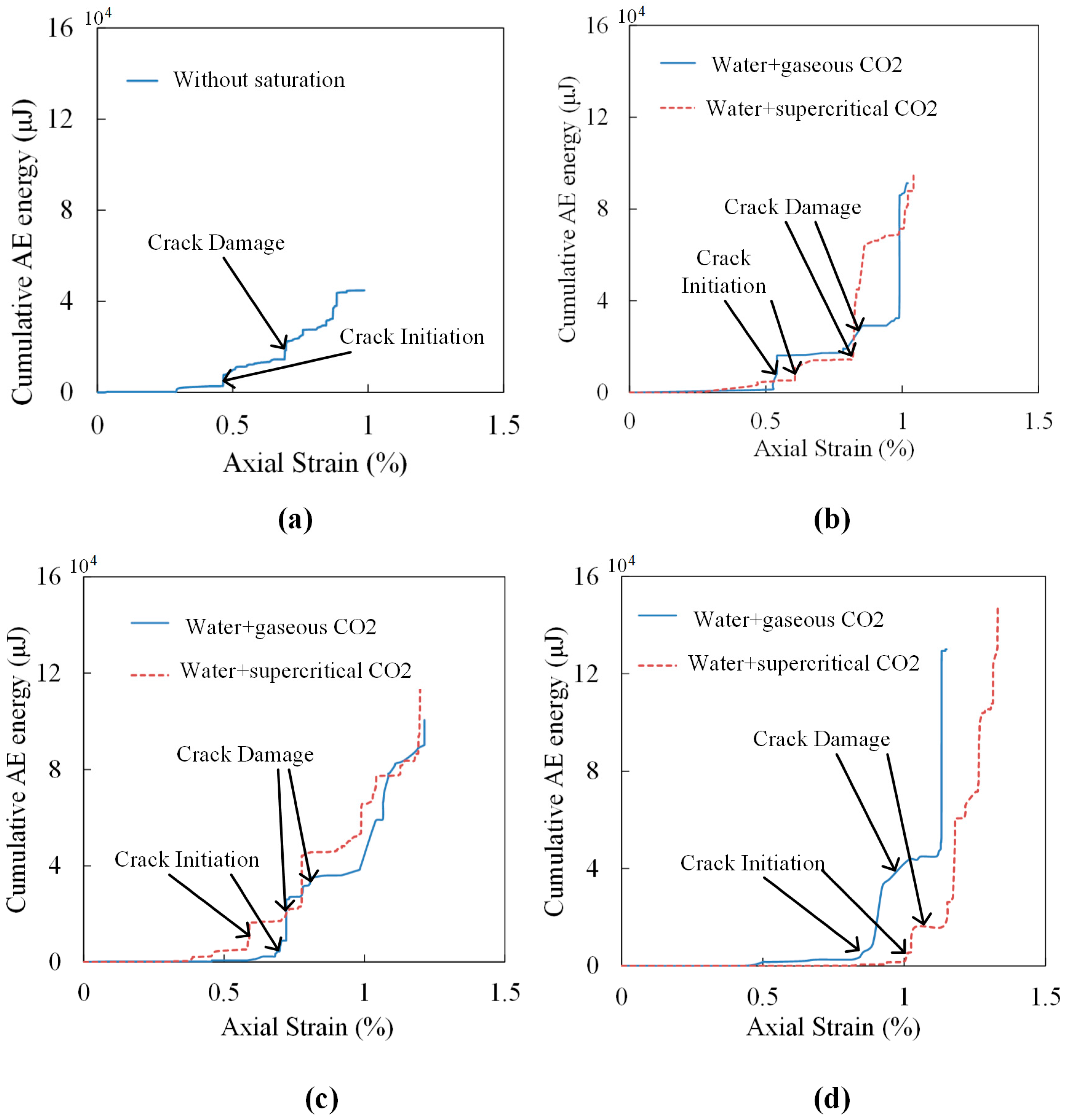
Figure 8 shows the variation of cumulative AE energy with axial strain for all kinds of saturated samples. As each group has two samples, here we present only one of the AE results for each saturation condition. The cumulative AE energy and axial stress for samples on both crack initiation and crack damage and the peak cumulative AE energy are listed in
Table 6.
According to
Figure 8 and
Table 6, samples with longer saturation time show higher axial strain when failure occurs. This is mainly because of the swelling of samples caused by water and CO
2 adsorption, and the occurrence of pores and cracks causes samples to have higher strain variations. This also indicates that CO
2 saturation decreases sample brittleness and increases its plasticity. When samples reach the crack initiation point, their values of cumulative AE energy are low, accounting for a small amount of the peak value ranging from 1.4% to 6.4%. A sample without saturation has the highest axial stress at this point, which is 23.36 MPa. The axial stress for samples saturated in gaseous CO
2 and water increase with the increase of saturation time, and the corresponding proportion of peak axial stress increases from 31.6% to 77.2%. For samples saturated under super-critical conditions, the axial stress decreases with the extension of saturation time. However, the proportion of axial stress on peak stress increases with increased saturation time, meaning that samples after gaseous/super-critical CO
2 and water saturation require a higher percentage of peak UCS value to reach the crack initiation when the saturation time is longer. From
Figure 8, we can also observe that samples saturated under gaseous conditions reach crack initiation faster than under super-critical conditions when the saturation durations are 10 days and 30 days. In contrast, for 20-day saturation, the trend is reversed. Samples saturated in water with SC-CO
2 reach the crack damage point earlier than samples saturated in water with gaseous CO
2 in 20 days. However, when saturation durations are 10 days and 30 days, the crack damage points are almost the same for both kinds of saturated samples. The reason for this difference is mainly because shale samples are anisotropic. When samples reach to the crack damage point, the cumulative AE energy increases to a higher level, ranging from 10.6%–38.2% on peak cumulative AE energy.
According to
Figure 8,
Table 3 and
Table 6, peak cumulative AE energy shows a negative correlation with the UCS values. A sample without saturation has the lowest peak cumulative AE energy of 44,802 μJ. For samples saturated in water with gaseous/super-critical CO
2, the peak cumulative AE energy increases with increasing saturation time. Specifically, with 10 days’ saturation, the values for samples under gaseous and super-critical conditions doubled and reached 91,227 μJ and 94,840 μJ, respectively. After 20- and 30-day saturation, the values of peak cumulative AE energy were 100,564 μJ and 130,037 μJ for samples under gaseous conditions, and 116,068 μJ and 147,862 μJ for samples under super-critical conditions, respectively. This phenomenon is mainly caused by several reasons. Firstly, the adsorption of water and CO
2 increases the conductivity of AE emissions. Meanwhile, the swelling caused by water and CO
2 adsorption creates more artificial fractures. The propagation of these fractures creates more AE energy. In addition, carbonates created by the precipitation process are crushed during the UCS tests and generate acoustic emissions. More importantly, according to the SEM results in
Figure 3, the numerous pores produced by chemical reactions decrease the brittleness and increase the plasticity of samples. Therefore, after the peak strength point, samples with CO
2 saturation can still bear load and create AE energy.