Microfabrication for Drug Delivery
Abstract
:1. Introduction
2. Microfabrication: Technologies and Materials
2.1. Microfabrication and MEMS
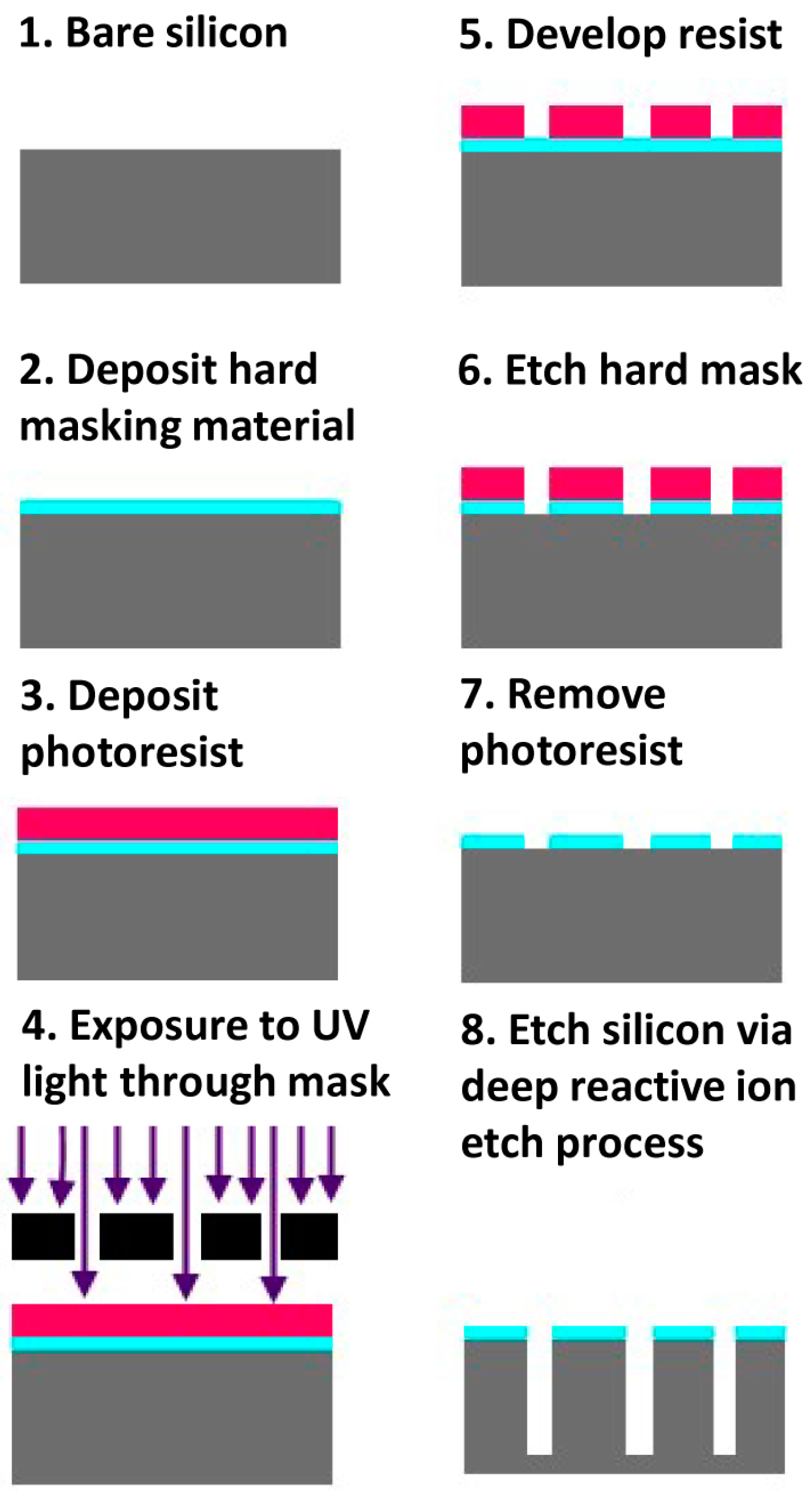
2.1.1. Silicon-Based Fabrication
2.1.2. Polymer-Based Fabrication
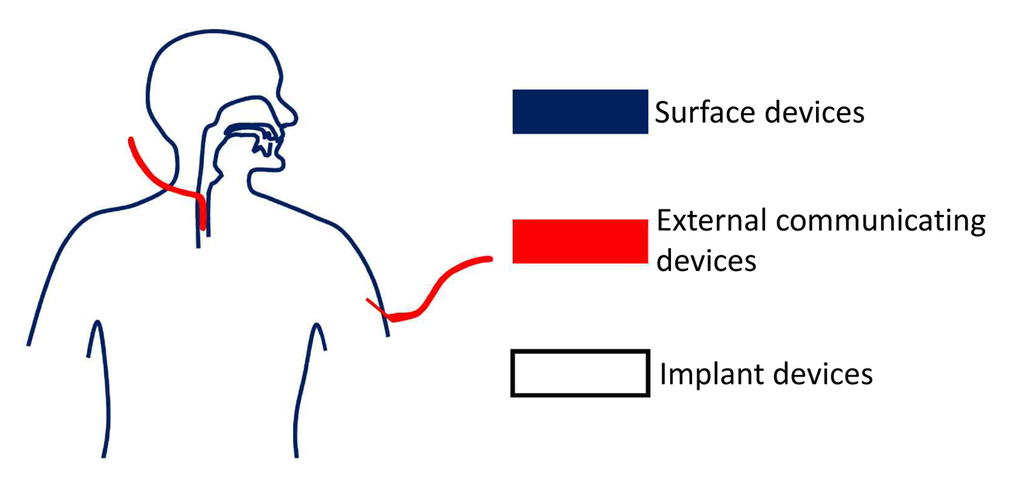
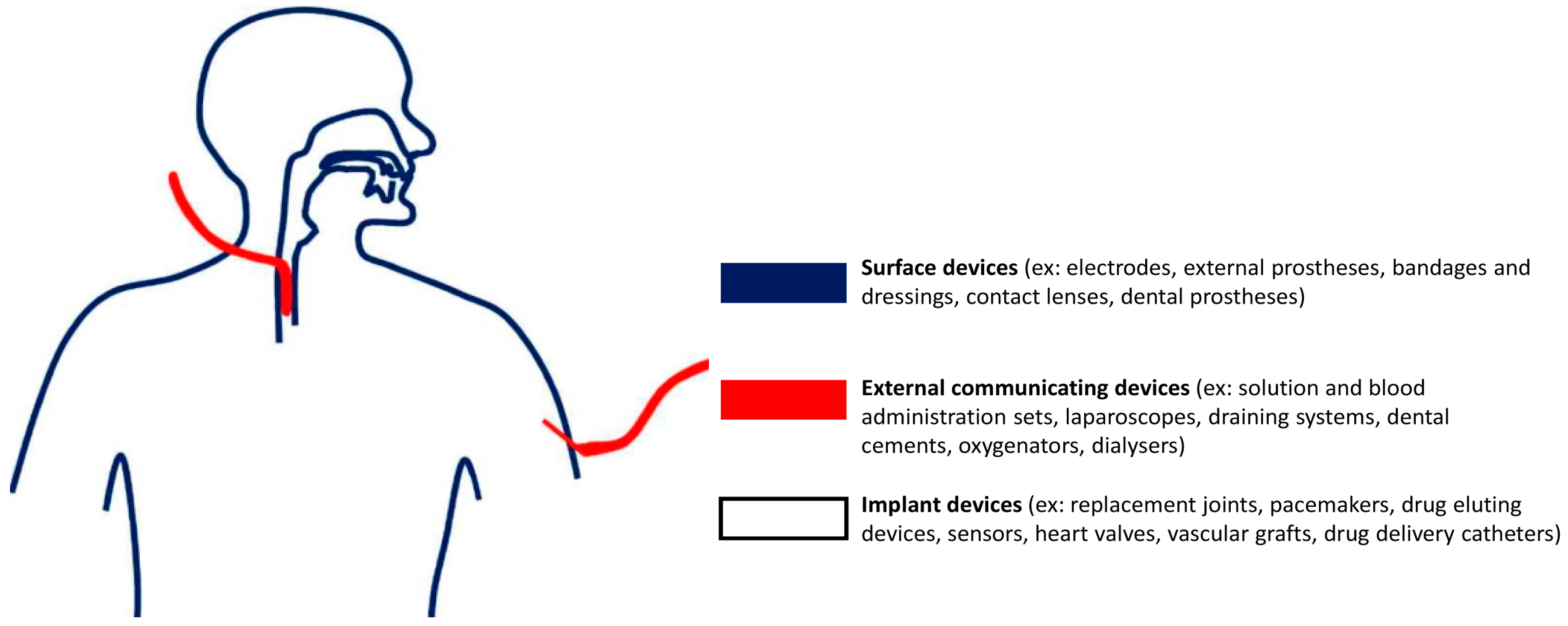
2.2. Biocompatibility of Materials Used in Microfabrication
3. Microfabricated Drug Delivery Systems
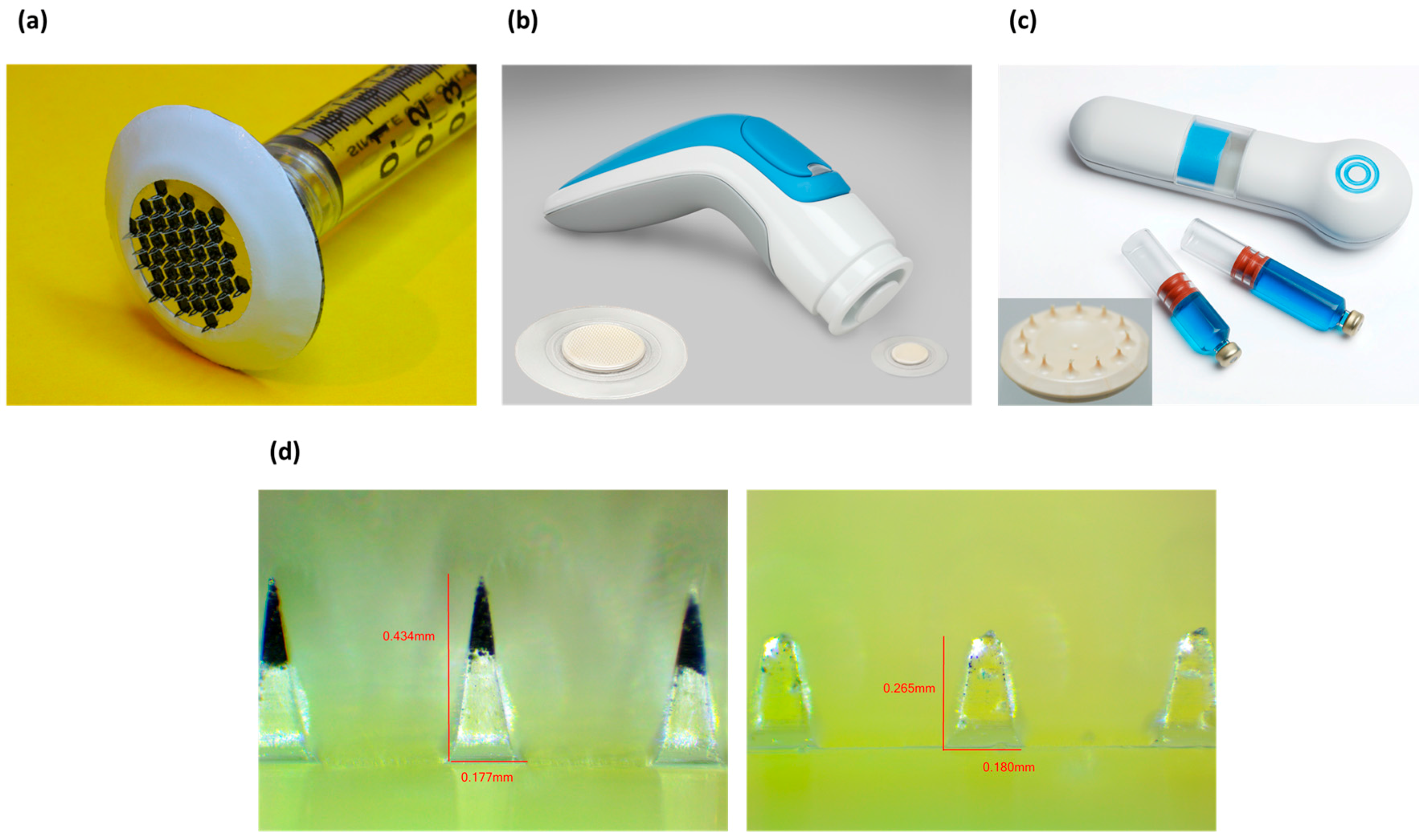
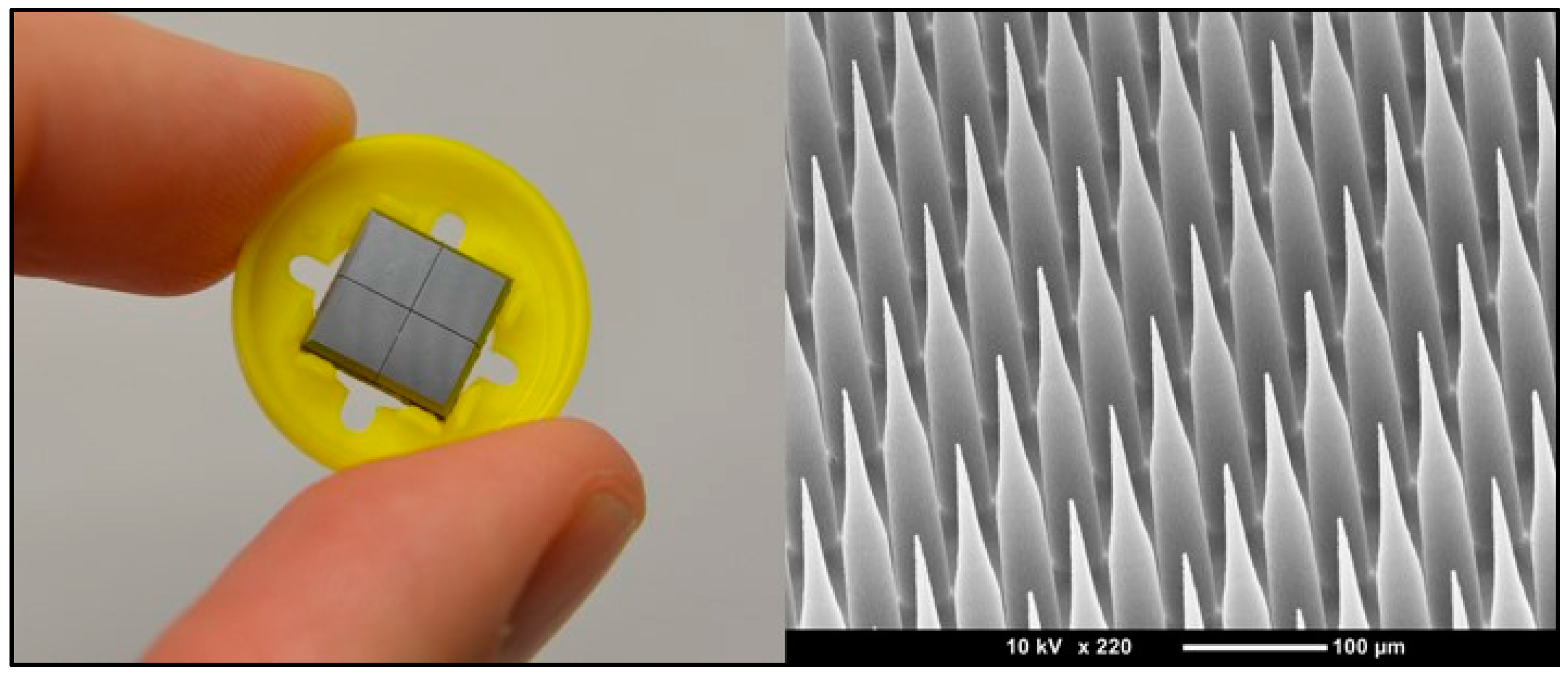
3.1. Microneedles
3.1.1. Background
3.1.2. Status
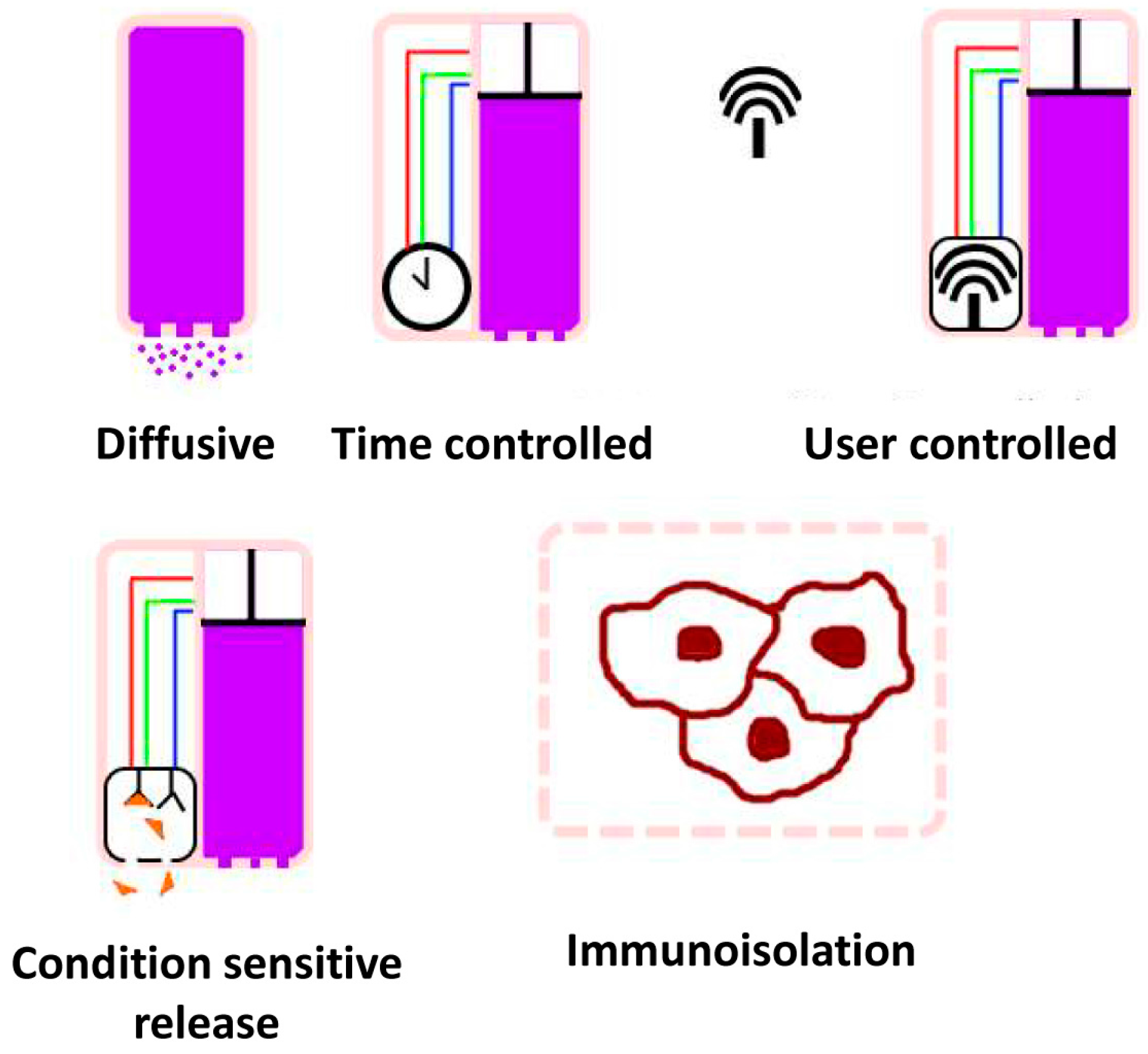
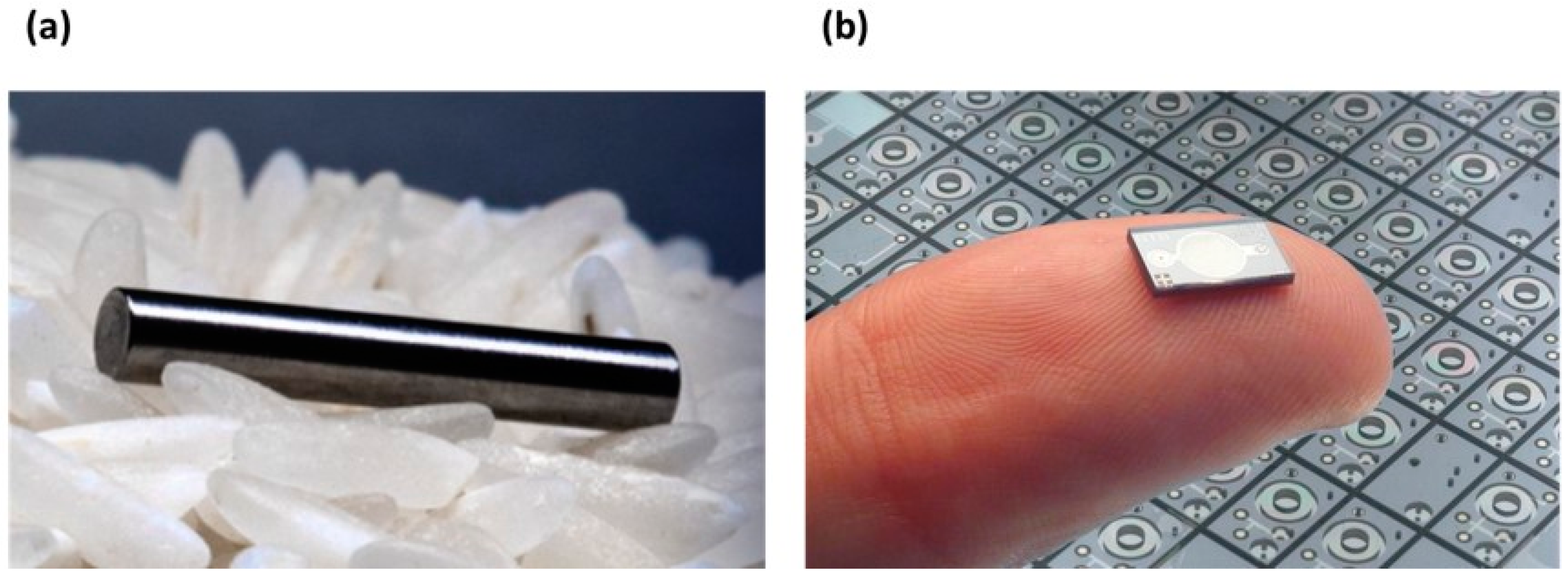
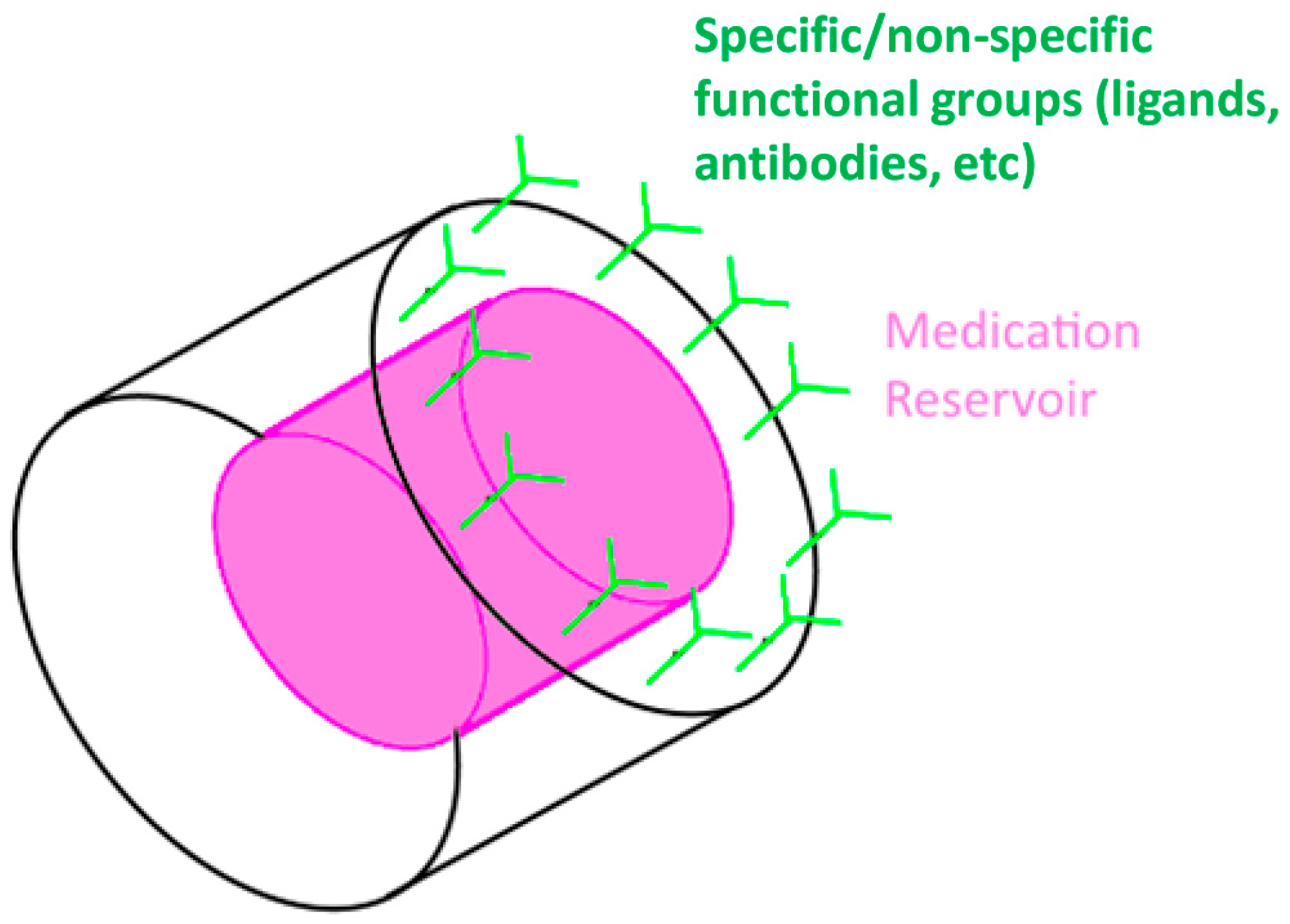
3.2. Implants
3.2.1. Background
3.2.2. Status
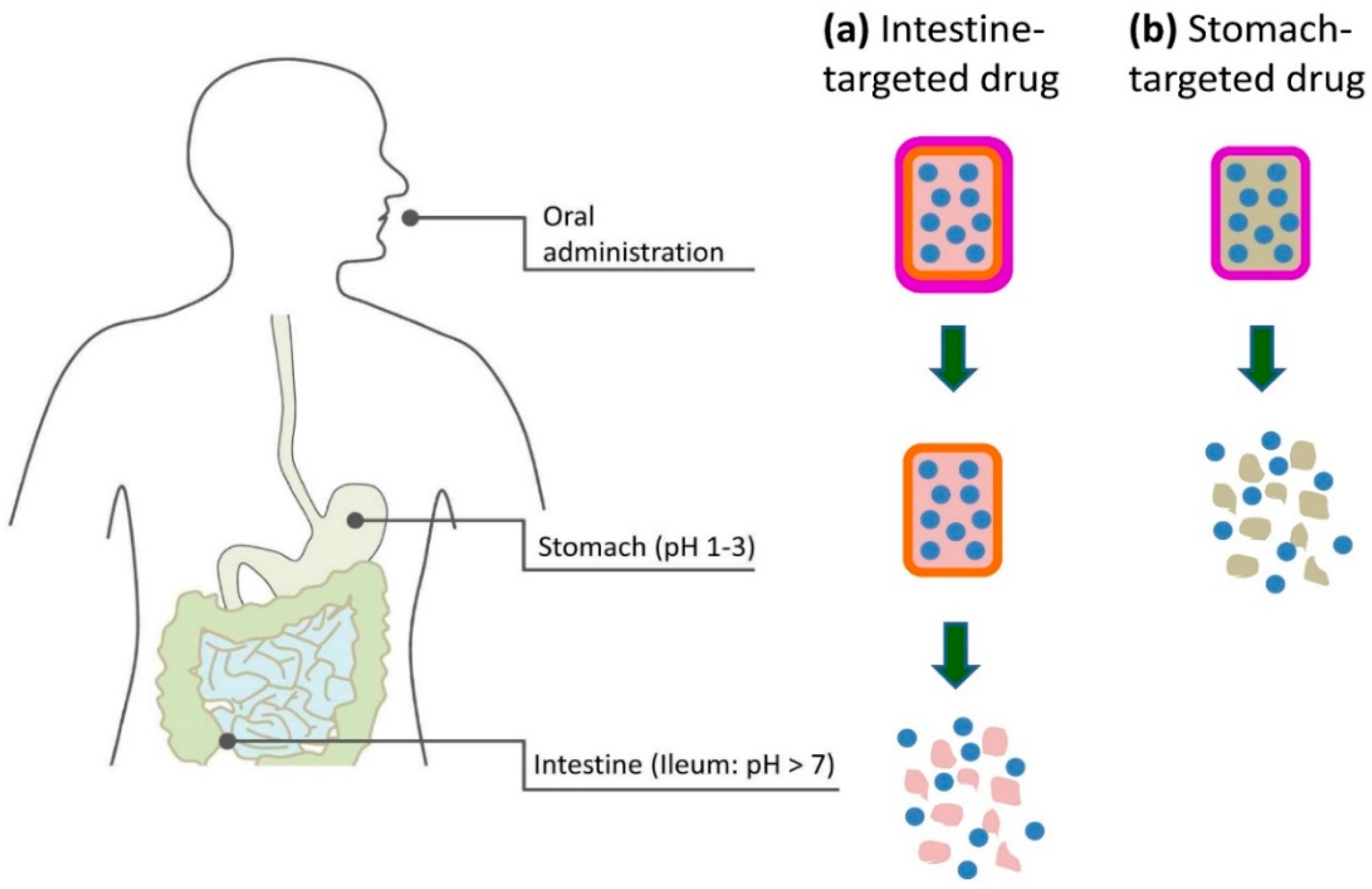
3.3. Oral Delivery and Detection
3.3.1. Background
3.3.2. Status
3.4. Vaccine Delivery Systems
3.4.1. Background
3.4.2. Status
4. Prospects
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Choi, H.-J.; Montemagno, C.D. Convergence of nanotechnology and biotechnology. In Handbook of Science and Technology Convergence; Bainbridge, S.W., Roco, C.M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–20. [Google Scholar]
- Lamos, E.M.; Younk, L.M.; Davis, S.N. Concentrated insulins: The new basal insulins. Ther. Clin. Risk Manag. 2016, 12, 389–400. [Google Scholar] [PubMed]
- Hrabakova, R.; Kollareddy, M.; Tyleckova, J.; Halada, P.; Hajduch, M.; Gadher, S.J.; Kovarova, H. Cancer cell resistance to aurora kinase inhibitors: Identification of novel targets for cancer therapy. J. Proteome Res. 2013, 12, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Nir, Y.; Paz, A.; Sabo, E.; Potasman, I. Fear of injections in young adults: Prevalence and associations. Am. J. Trop. Med. Hyg. 2003, 68, 341–344. [Google Scholar] [PubMed]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Choonara, B.F.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014, 32, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Wang, W.; Soper, S.A. Bio-Mems: Technologies and Applications; CRC Press: Bocaraton, FL, USA, 2006. [Google Scholar]
- Hughes, G.A. Nanostructure-mediated drug delivery. Nanomedicine 2005, 1, 22–30. [Google Scholar] [PubMed]
- Grayson, A.C.R.; Shawgo, R.S.; Johnson, A.M.; Flynn, N.T.; Li, Y.; Cima, M.J.; Langer, R. A biomems review: MEMS technology for physiologically integrated devices. IEEE Proc. 2004, 92, 6–21. [Google Scholar] [CrossRef]
- Moga, K.A.; Bickford, L.R.; Geil, R.D.; Dunn, S.S.; Pandya, A.A.; Wang, Y.; Fain, J.H.; Archuleta, C.F.; O'Neill, A.T.; Desimone, J.M. Rapidly-dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Adv. Mater. 2013, 25, 5060–5066. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.T., Jr.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335–338. [Google Scholar] [PubMed]
- Santini, J.J.T.; Richards, A.C.; Scheidt, R.; Cima, M.J.; Langer, R. Microchips as controlled drug-delivery devices. Angew. Chem. Int. Ed. 2000, 39, 2396–2407. [Google Scholar] [CrossRef]
- Reed, M.L.; Wu, C.; Kneller, J.; Watkins, S.; Vorp, D.A.; Nadeem, A.; Weiss, L.E.; Rebello, K.; Mescher, M.; Smith, A.J.; et al. Micromechanical devices for intravascular drug delivery. J. Pharm. Sci. 1998, 87, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Rickman, A. The commercialization of silicon photonics. Nat. Photonics 2014, 8, 579–582. [Google Scholar] [CrossRef]
- Morris, M.A. Directed self-assembly of block copolymers for nanocircuitry fabrication. Microelectron. Eng. 2015, 132, 207–217. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Zorman, C.A.; Mehregany, M. Materials for microelectro-mechanical systems. In MEMS: Design and Fabrication; Gad-el-Hak, M., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wang, L.Y.; Park, H.Y.; Lim, S.I.I.; Schadt, M.J.; Mott, D.; Luo, J.; Wang, X.; Zhong, C.J. Core@shell nanomaterials: Gold-coated magnetic oxide nanoparticles. J. Mater. Chem. 2008, 18, 2629–2635. [Google Scholar] [CrossRef]
- Tilli, M.; Motooka, T.; Airaksinen, V.-M.; Franssila, S.; Paulasto-Krockel, M.; Lindroos, V. Handbook of Silicon Based Mems Materials and Technologies; William Andrew: San Diego, CA, USA, 2015. [Google Scholar]
- Hupert, M.L.; Guy, W.J.; Llopis, S.D.; Situma, C.; Rani, S.; Nikitopoulos, D.E.; Soper, S.A. High-precision micromilling for low-cost fabrication of metal mold masters. Proc. SPIE 2006, 6112. [Google Scholar] [CrossRef]
- Zavracky, P.M.; Earles, T.; Pokrovskiy, N.L.; Green, J.A.; Burns, B.E. Fabrication of vertical sidewalls by anisotropic etching of silicon (100) wafers. J. Electrochem. Soc. 1994, 141, 3182–3188. [Google Scholar] [CrossRef]
- Laermer, F.; Schilp, A. Method of Anisotropically Etching Silicon. U.S. Patent US5501893, 26 March 1996. [Google Scholar]
- Pease, R.F.W. Electron-beam lithography. Contemp. Phys. 1981, 22, 265–290. [Google Scholar] [CrossRef]
- Guerin, L.; Bossel, M.; Demierre, M.; Calmes, S.; Renaud, P. Simple and low cost fabrication of embedded micro-channels by using a new thick-film photoplastic. In Proceedings of the 1997 International Conference on Solid-State Sensors and Actuators Chicago, Chicago, IL, USA, 16–19 June 1997; pp. 1419–1422.
- Zhang, N.; Chu, J.S.; Byrne, C.J.; Browne, D.J.; Gilchrist, M.D. Replication of micro/nano-scale features by micro injection molding with a bulk metallic glass mold insert. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Casey, B.G.; Monaghan, W.; Wilkinson, C.D.W. Embossing of nanoscale features and environments. Microelectron. Eng. 1997, 35, 393–396. [Google Scholar] [CrossRef]
- Jo, B.H.; Van Lerberghe, L.M.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81. [Google Scholar] [CrossRef]
- Folch, A.; Toner, M. Microengineering of cellular interactions. Annu. Rev. Biomed. Eng. 2000, 2, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Delamarche, E.; Schmid, H.; Michel, B.; Bosshard, H.R.; Biebuyck, H. Printing patterns of proteins. Langmuir 1998, 14, 2225–2229. [Google Scholar] [CrossRef]
- Branch, D.W.; Corey, J.M.; Weyhenmeyer, J.A.; Brewer, G.J.; Wheeler, B.C. Microstamp patterns of biomolecules for high-resolution neuronal networks. Med. Biol. Eng. Comput. 1998, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.F.; Zheng, M.L.; Duan, X.M. Two-photon polymerization microfabrication of hydrogels: An advanced 3D printing technology for tissue engineering and drug delivery. Chem. Soc. Rev. 2015, 44, 5031–5039. [Google Scholar] [CrossRef] [PubMed]
- Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ISO 10993-1:2009; ISO: Vernier, Swtzerland, 2009.
- Use of International Standard ISO 10993, Biological Evaluation of Medical Devices Part 1: Evaluation and Testing; FDA: Silver Spring, MD, USA, 2015.
- Greene, W. The Emergence of India’s Pharmaceutical Industry and Implications for the US Generic Drug Market; U.S. International Trade Commission: Washington, DC, USA, 2007.
- Moynihan, R.; Cassels, A. Selling Sickness: How the World’s Biggest Pharmaceutical Companies Are Turning US All into Patients; Nation Books: New York, NY, USA, 2006. [Google Scholar]
- Anderson, J.M. Biological responses to materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- FDA. Zimmer M/L Taper with Kinectiv Technology Prosthesis Femoral Stems and Necks Higher than Expected Levels of Manufacturing Residues. Available online: http://www.fda.gov/MedicalDevices/Safety/ucm451936.htm (accessed on 15 June 2016).
- Hsu, T.R. MEMS and Microsystems: Design, Manufacture, and Nanoscale Engineering; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bertsch, A.; Renaud, P. Special issue: 15 years of SU8 as MEMS material. Micromachines 2015, 6, 790–792. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Rogers, J.A.; Jackman, R.J.; Zhao, X.-M.; Whitesides, G.M. Microfabrication, microstructures and microsystems. In Microsystem Technology in Chemistry and Life Science; Manz, A., Becker, H., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1998; pp. 1–20. [Google Scholar]
- Belanger, M.C.; Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 2001, 58, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Voskerician, G.; Shive, M.S.; Shawgo, R.S.; von Recum, H.; Anderson, J.M.; Cima, M.J.; Langer, R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003, 24, 1959–1967. [Google Scholar] [CrossRef]
- Geninatti, T.; Bruno, G.; Barile, B.; Hood, R.L.; Farina, M.; Schmulen, J.; Canavese, G.; Grattoni, A. Impedance characterization, degradation, and in vitro biocompatibility for platinum electrodes on bioMEMS. Biomed. Microdevices 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Veiseh, M.; Zhang, M. Surface modification of silicon and gold-patterned silicon surfaces for improved biocompatibility and cell patterning selectivity. Biosens. Bioelectron. 2005, 20, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Mayer, I.; De Pasquale, G.; Maier, W.; Dirsch, O.; De Groot, P.; Wu, Y.P.; Noll, G.; Leskosek, B.; Meier, B.; et al. Stent coating with titanium-nitride-oxide for reduction of neointimal hyperplasia. Circulation 2001, 104, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Milburn, C.; Chen, J.; Cao, Y.; Oparinde, G.M.; Adeoye, M.O.; Beye, A.; Soboyejo, W.O. Investigation of effects of argenine–glycine–aspartate (rgd) and nano-scale titanium coatings on cell spreading and adhesion. Mater. Sci. Eng. C 2009, 29, 306–314. [Google Scholar] [CrossRef]
- Kotzar, G.; Freas, M.; Abel, P.; Fleischman, A.; Roy, S.; Zorman, C.; Moran, J.M.; Melzak, J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials 2002, 23, 2737–2750. [Google Scholar] [CrossRef]
- Nasir, N.F.M.; Shah, C.M.; Leech, P.W.; Reeves, G.K.; Pirogova, E.; Istivan, T.; Tanner, P.; Holland, A.S. Fabrication of 3c-silicon carbide membranes: Towards development of novel microdevices for biomedical applications. In Proceedings of the 2012 International Conference on Biomedical Engineering (ICoBE), Penang, Malaysia, 27–28 February 2012; pp. 589–593.
- Starr, P.; Agrawal, C.M.; Bailey, S. Biocompatibility of common polyimides with human endothelial cells for a cardiovascular microsensor. J. Biomed. Mater. Res. A 2016, 104, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Kalkandjiev, K.; Riegger, L.; Kosse, D.; Welsche, M.; Gutzweiler, L.; Zengerle, R.; Koltay, P. Microfluidics in silicon/polymer technology as a cost-efficient alternative to silicon/glass. J. Micromech. Microeng. 2011, 21. [Google Scholar] [CrossRef]
- Greene, J.A.; Podolsky, S.H. Reform, regulation, and pharmaceuticals--the kefauver-harris amendments at 50. N. Engl. J. Med. 2012, 367, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- FDA. Drug Approval Process. Available online: http://www.fda.gov/downloads/Drugs/ResourcesForYou/Consumers/UCM284393.pdf (accessed on 15 June 2016).
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations; FDA: Silver Spring, MD, USA, 2013.
- FDA. Combination Product Definition. Available online: http://www.fda.gov/CombinationProducts/AboutCombinationProducts/ucm118332.htm (accessed on 15 June 2016).
- FDA. Examples of Combination Product Approvals. Available online: http://www.fda.gov/CombinationProducts/AboutCombinationProducts/ucm101598.htm (accessed on 15 June 2016).
- Couto, D.S.; Perez-Breva, L.; Saraiva, P.; Cooney, C.L. Lessons from innovation in drug-device combination products. Adv. Drug Deliv. Rev. 2012, 64, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Staples, M.; Daniel, K.; Cima, M.J.; Langer, R. Application of micro- and nano-electromechanical devices to drug delivery. Pharm. Res. 2006, 23, 847–863. [Google Scholar] [CrossRef] [PubMed]
- FDA. Office of Combination Products; FDA: Silver Spring, MD, USA, 2016.
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, F.; Durando, P.; Icardi, G. Intradermal influenza vaccine and new devices: A promising chance for vaccine improvement. Expert Opin. Biol. Ther. 2011, 11, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Gilpin, E.; Farkas, A.J. Nicotine patch use in the general population: Results from the 1993 California tobacco survey. J. Natl. Cancer Inst. 1995, 87, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Abelin, T.; Buehler, A.; Muller, P.; Vesanen, K.; Imhof, P.R. Controlled trial of transdermal nicotine patch in tobacco withdrawal. Lancet 1989, 1, 7–10. [Google Scholar] [CrossRef]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Inoue, N.T.; Ashton-Barnett, J.; Barrow, D.A.; Allender, C.J. Evaluation of geometrical effects of microneedles on skin penetration by ct scan and finite element analysis. Eur. J. Pharm. Biopharm. 2016, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Hord, A.H.; Denson, D.D.; McAllister, D.V.; Smitra, S.; Allen, M.G.; Prausnitz, M.R. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001, 92, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Gardeniers, H.J.G.E.; Luttge, R.; Berenschot, E.J.W.; de Boer, M.J.; Yeshurun, S.Y.; Hefetz, M.; van’t Oever, R.; van den Berg, A. Silicon micromachined hollow microneedles for transdermal liquid transport. J. Microelectromech. Syst. 2003, 12, 855–862. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Majithiya, R.; Singh, T.R.; Morrow, D.I.; Garland, M.J.; Demir, Y.K.; Migalska, K.; Ryan, E.; Gillen, D.; Scott, C.J.; et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011, 28, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Bystrova, S.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectron. Eng. 2011, 88, 1681–1684. [Google Scholar] [CrossRef]
- Gower, M.C. Industrial applications of laser micromachining. Opt. Express 2000, 7, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, T.; Chujo, K.; Miyamoto, K.; Okida, M.; Nakamura, K.; Aoki, N.; Morita, R. Metal microneedle fabrication using twisted light with spin. Opt. Express 2010, 18, 17967–17973. [Google Scholar] [CrossRef] [PubMed]
- Yuzhakov, V.V. The adminpen™ microneedle device for painless & convenient drug delivery. Drug Deliv. Technol. 2010, 10, 32–36. [Google Scholar]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (dermaroller) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yoon, Y.K.; Choi, S.O.; Prausnitz, M.R.; Allen, M.G. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans. Biomed. Eng. 2007, 54, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, G.; Dong, P.; Gong, Z.; Li, G.; Zhang, K.; Wu, C. Investigation on fabrication process of dissolving microneedle arrays to improve effective needle drug distribution. Eur. J. Pharm. Sci. 2014, 66, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.C.; Lee, D.S.; Jung, H. Drawing lithography: Three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010, 22, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Chichkov, B.N.; Doraiswamy, A.; Narayan, R.J. Two-photon polymerization of microneedles for transdermal drug delivery. Expert Opin. Drug Deliv. 2010, 7, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, S.O.; Seo, S.; Choy, Y.B.; Prausnitz, M.R. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, C.Y.; Jung, H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials 2011, 32, 3134–3140. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.J.; McCrory, J.; Evans, S.L.; Allender, C.J. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, J.M.; Geiger, E.J.; Pisano, A.P. Polymer investment molding: Method for fabricating hollow, microscale parts. Sens. Actuators A Phys. 2007, 134, 2–10. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
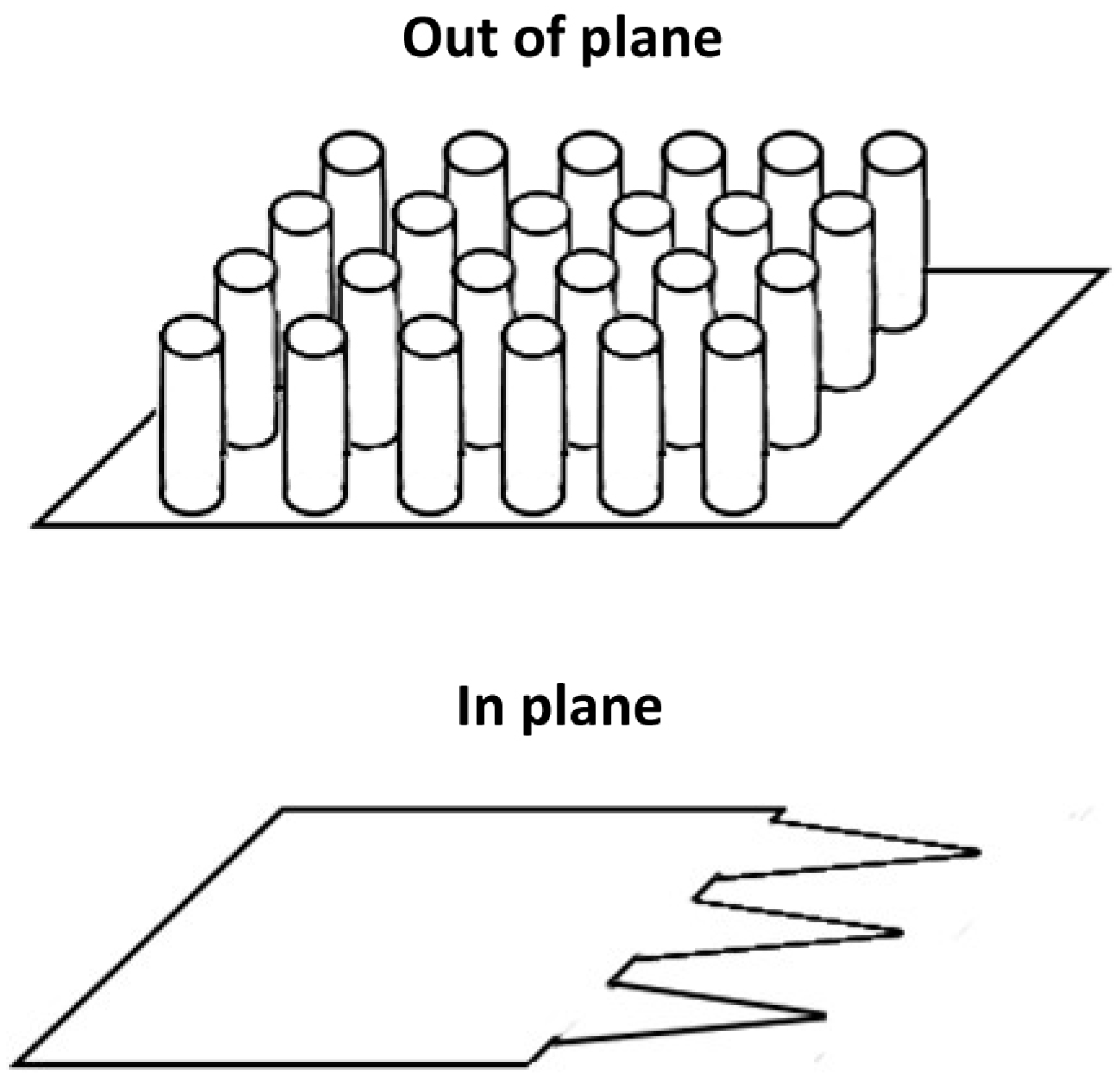
- Stoeber, B.; Liepmann, D. Arrays of hollow out-of-plane microneedles for drug delivery. J. Microelectromech. Syst. 2005, 14, 472–479. [Google Scholar] [CrossRef]
- Paik, S.J.; Byun, A.; Lim, J.M.; Park, Y.; Lee, A.; Chung, S.; Chang, J.K.; Chun, K.; Cho, D.D. In-plane single-crystal-silicon microneedles for minimally invasive microfluid systems. Sens. Actuators A Phys. 2004, 114, 276–284. [Google Scholar] [CrossRef]
- Baek, C.; Han, M.; Min, J.; Prausnitz, M.R.; Park, J.H.; Park, J.H. Local transdermal delivery of phenylephrine to the anal sphincter muscle using microneedles. J. Control. Release 2011, 154, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, L.; Roxhed, N.; Griss, P.; Stemme, G. Novel microneedle patches for active insulin delivery are efficient in maintaining glycaemic control: An initial comparison with subcutaneous administration. Pharm. Res. 2007, 24, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Huo, M.R.; Zhou, J.P.; Zhou, Y.Q.; Hao, B.H.; Liu, T.; Zhang, Y. Super-short solid silicon microneedles for transdermal drug delivery applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar] [PubMed]
- Zhang, Y.; Brown, K.; Siebenaler, K.; Determan, A.; Dohmeier, D.; Hansen, K. Development of lidocaine-coated microneedle product for rapid, safe, and prolonged local analgesic action. Pharm. Res. 2012, 29, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Siebenaler, K.; Brown, K.; Dohmeier, D.; Hansen, K. Adjuvants to prolong the local anesthetic effects of coated microneedle products. Int. J. Pharm. 2012, 439, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Denson, D.D.; Felner, E.I.; Prausnitz, M.R. Rapid local anesthesia in humans using minimally invasive microneedles. Clin. J. Pain 2012, 28, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Das, D.B. Potential of biodegradable microneedles as a transdermal delivery vehicle for lidocaine. Biotechnol. Lett. 2013, 35, 1351–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochhar, J.S.; Lim, W.X.; Zou, S.; Foo, W.Y.; Pan, J.; Kang, L. Microneedle integrated transdermal patch for fast onset and sustained delivery of lidocaine. Mol. Pharm. 2013, 10, 4272–4280. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, B.M.; Zarnitsyn, V.; Prausnitz, M.R.; Anstey, A.; Gateley, C.; Birchall, J.C.; Coulman, S.A. Pocketed microneedles for rapid delivery of a liquid-state botulinum toxin a formulation into human skin. J. Control. Release 2013, 165, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Pinninti, R.R.; Hammell, D.C.; Paudel, K.S.; Stinchcomb, A.L. Development of a codrug approach for sustained drug delivery across microneedle-treated skin. J. Pharm. Sci. 2013, 102, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.L.; Pinninti, R.R.; Gill, H.S.; Crooks, P.A.; Prausnitz, M.R.; Stinchcomb, A.L. Flux across microneedle-treated skin is increased by increasing charge of naltrexone and naltrexol in vitro. Pharm. Res. 2008, 25, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Murakami, A.; Maeda, T.; Sugioka, N.; Takada, K. Evaluation of self-dissolving needles containing low molecular weight heparin (lmwh) in rats. Int. J. Pharm. 2008, 349, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, H.; Zheng, J.; Mou, D.; Wan, J.; Zhang, J.; Shi, T.; Zhao, Y.; Xu, H.; Yang, X. Iontophoresis-driven penetration of nanovesicles through microneedle-induced skin microchannels for enhancing transdermal delivery of insulin. J. Control. Release 2009, 139, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Gao, Y.; Wu, Y.; Zhang, S.; Qiu, Y.; Li, F.; Xu, B. Simultaneous basal-bolus delivery of fast-acting insulin and its significance in diabetes management. Nanomedicine 2012, 8, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Yamazaki, T.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Pharmacokinetic and pharmacodynamic evaluation of insulin dissolving microneedles in dogs. Diabetes Technol. Ther. 2010, 12, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Felner, E.I.; Prausnitz, M.R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 2011, 13, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.C.; Gazelle, G.S.; Rocha, L.A.; Tavares, J.M. Medical device specificities: Opportunities for a dedicated product development methodology. Expert Rev. Med. Devices 2012, 9, 299–311. [Google Scholar] [CrossRef] [PubMed]
- 3M. 3M Solid Microstructured Transdermal System. Available online: http://multimedia.3m.com/mws/media/1004089O/solid-microstructured-transdermal-system-smts-sell-sheet.pdf (accessed on 15 June 2016).
- 3M. 3M™ Hollow Microstructured Transdermal System. Available online: http://www.3m.com/3M/en_US/drug-delivery-systems-us/technologies/microneedle/hollow/ (accessed on 15 June 2016).
- Dick, L.A. Innovative Drug Delivery Technology to Meet Evolving Need of Biologics & Small Moledules. Available online: http://multimedia.3m.com/mws/media/1080389O/ondrugdelivery-article-march-2015.pdf (accessed on 15 June 2016).
- MicropointTechnologies. Mpatch Mini. Available online: http://www.micropoint-tech.com/our-products/mpatch-mini/ (accessed on 15 June 2016).
- Zhou, C.P.; Liu, Y.L.; Wang, H.L.; Zhang, P.X.; Zhang, J.L. Transdermal delivery of insulin using microneedle rollers in vivo. Int. J. Pharm. 2010, 392, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, R.; Qin, Z.; Zhang, J.; Zhai, S.; Qiu, Y.; Gao, Y.; Xu, B.; Thomas, S.H. Microneedle pretreatment improves efficacy of cutaneous topical anesthesia. Am. J. Emerg. Med. 2010, 28, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Fernando, G.J.; Chen, X.; Primiero, C.A.; Yukiko, S.R.; Fairmaid, E.J.; Corbett, H.J.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Nanopatch targeted delivery of both antigen and adjuvant to skin synergistically drives enhanced antibody responses. J. Control. Release 2012, 159, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Li, P.Y.; Shih, J.; Lo, R.; Saati, S.; Agrawal, R.; Humayun, M.S.; Tai, Y.C.; Meng, E. An electrochemical intraocular drug delivery device. Sens. Actuators A Phys. 2008, 143, 41–48. [Google Scholar] [CrossRef]
- Meng, E.; Hoang, T. Mems-enabled implantable drug infusion pumps for laboratory animal research, preclinical, and clinical applications. Adv. Drug Deliv. Rev. 2012, 64, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Tng, D.J.H.; Hu, R.; Song, P.Y.; Roy, I.; Yong, K.T. Approaches and challenges of engineering implantable microelectromechanical systems (mems) drug delivery systems for in vitro and in vivo applications. Micromachines 2012, 3, 615–631. [Google Scholar] [CrossRef]
- Riahi, R.; Tamayol, A.; Shaegh, S.A.M.; Ghaemmaghami, A.M.; Dokmeci, M.R.; Khademhosseini, A. Microfluidics for advanced drug delivery systems. Curr. Opin. Chem. Eng. 2015, 7, 101–112. [Google Scholar] [CrossRef]
- LaVan, D.A.; McGuire, T.; Langer, R. Small-scale systems for in vivo drug delivery. Nat. Biotechnol. 2003, 21, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Hoang, T. Micro- and nano-fabricated implantable drug-delivery systems. Ther. Deliv. 2012, 3, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Graesslin, O.; Korver, T. The contraceptive efficacy of implanon: A review of clinical trials and marketing experience. Eur. J. Contracept. Reprod. Health Care 2008, 13 (Suppl. 1), 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mäkäräinen, L.; van Beek, A.; Tuomivaara, L.; Asplund, B.; Bennink, H.C. Ovarian function during the use of a single contraceptive implant: Implanon compared with norplant. Fertil. Steril. 1998, 69, 714–721. [Google Scholar] [CrossRef]
- Fuster, V.; Badimon, L.; Badimon, J.J.; Chesebro, J.H. The pathogenesis of coronary artery disease and the acute coronary syndromes. N. Engl. J. Med. 1992, 326, 242–250. [Google Scholar] [PubMed]
- Serruys, P.W.; Herrman, J.P.; Simon, R.; Rutsch, W.; Bode, C.; Laarman, G.J.; van Dijk, R.; van den Bos, A.A.; Umans, V.A.; Fox, K.A.; et al. A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. Helvetica investigators. N. Engl. J. Med. 1995, 333, 757–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, B.C.; Nascimento, G.A.; Rabelo, W.; Marino, M.A.; Marino, R.L.; Ribeiro, A.L. Clinical coronary in-stent restenosis follow-up after treatment and analyses of clinical outcomes. Arq. Bras. Cardiol. 2015, 104, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.X.; Yang, D.Z.; Wu, J.Z. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics 2014, 4, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Woods, T.C.; Marks, A.R. Drug-eluting stents. Annu. Rev. Med. 2004, 55, 169–178. [Google Scholar] [CrossRef] [PubMed]
- NanoPrecisionMedical. Nanoportal Technology. Available online: http://www.nanoprecisionmedical.com/ (accessed on 15 June 2016).
- Fischer, K.; Degenkolb, K.; Fischer, W.; Mendelsohn, A. Nanoscale Constrained Delivery: A Novel Technology for Subdermal Implants. Available online: http://www.nanoprecisionmedical.com/pdfs/publications/Nanoscale_Constrained_Delivery_A_Novel_Technology_for_Subdermal_Implants_2014.pdf (accessed on 15 June 2016).
- Wright, J.C.; Tao Leonard, S.; Stevenson, C.L.; Beck, J.C.; Chen, G.; Jao, R.M.; Johnson, P.A.; Leonard, J.; Skowronski, R.J. An in vivo/in vitro comparison with a leuprolide osmotic implant for the treatment of prostate cancer. J. Control. Release 2001, 75, 1–10. [Google Scholar] [CrossRef]
- Maillefer, D.; Lintel, H.V.; Rey-Mermet, G.; Hirschi, R. A high-performance silicon micropump for an implantable drug delivery system. In Proceedings of the Twelfth IEEE International Conference on Micro Electro Mechanical Systems (MEMS ’99), Orlando, FL, USA, 21 January 1999; pp. 541–546.
- Lo, R.; Li, P.Y.; Saati, S.; Agrawal, R.; Humayun, M.S.; Meng, E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip 2008, 8, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Elman, N.M.; Ho Duc, H.L.; Cima, M.J. An implantable mems drug delivery device for rapid delivery in ambulatory emergency care. Biomed. Microdevices 2009, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Leoni, L.; Desai, T.A. Nanoporous biocapsules for the encapsulation of insulinoma cells: Biotransport and biocompatibility considerations. IEEE Trans. Biomed. Eng. 2001, 48, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Zimerman, B.; Steffen, A.; Yavriants, K.; Azarov, D.; Reichel, A.; Vardi, P.; German, T.; Shabtay, N.; Rotem, A.; et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm. Metab. Res. 2010, 42, 918–922. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.; Reichel, A.; Steffen, A.; Zimerman, B.; Schally, A.V.; Block, N.L.; Colton, C.K.; Ludwig, S.; Kersting, S.; Bonifacio, E.; et al. Transplantation of human islets without immunosuppression. Proc. Natl. Acad. Sci. USA 2013, 110, 19054–19058. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, T.; Ludwig, B.; Barkai, U.; Weir, G.C.; Colton, C.K.; Evron, Y.; Balyura, M.; Yavriyants, K.; Zimermann, B.; Azarov, D.; et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.C.; Teng, Z.; Wang, X.A.; Wang, Q. Development of carboxymethyl chitosan hydrogel beads in alcohol-aqueous binary solvent for nutrient delivery applications. Food Hydrocoll. 2013, 31, 332–339. [Google Scholar] [CrossRef]
- Fucinos, C.; Fucinos, P.; Miguez, M.; Katime, I.; Pastrana, L.M.; Rua, M.L. Temperature- and PH-sensitive nanohydrogels of poly(n-isopropylacrylamide) for food packaging applications: Modelling the swelling-collapse behaviour. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in agriculture: A review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- Knipe, J.M.; Chen, F.; Peppas, N.A. Multiresponsive polyanionic microgels with inverse PH responsive behavior by encapsulation of polycationic nanogels. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Doring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels--structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, B. Smart delivery system for cosmetic ingredients using ph-sensitive polymer hydrogel particles. Korean J. Chem. Eng. 2011, 28, 1347–1350. [Google Scholar] [CrossRef]
- Klee, S.K.; Farwick, M.; Lersch, P. Triggered release of sensitive active ingredients upon response to the skin’s natural ph. Colloid Surface A 2009, 338, 162–166. [Google Scholar] [CrossRef]
- Pafiti, K.; Cui, Z.; Carney, L.; Freemont, A.J.; Saunders, B.R. Composite hydrogels of polyacrylamide and crosslinked ph-responsive micrometer-sized hollow particles. Soft Matter 2016, 12, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.A.; Alhnan, M.A.; Nilkumhang, S.; Murdan, S.; Basit, A.W. Fabrication and in vivo evaluation of highly ph-responsive acrylic microparticles for targeted gastrointestinal delivery. Eur. J. Pharm. Sci. 2009, 37, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Majumdar, D.K.; Panda, A.K. Insulin loaded eudragit l100 microspheres for oral delivery: Preliminary in vitro studies. J. Biomater. Appl. 2006, 21, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zhen, Z.; Hu, X.; Wu, S.; Xu, Z.; Chu, P.K. Hollow chitosan-silica nanospheres as ph-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials 2011, 32, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, H.; Thurecht, K.J.; Puttick, S.; Whittaker, A.K. PH-responsive star polymer nanoparticles: Potential 19f mri contrast agents for tumour-selective imaging. Polym. Chem. 2013, 4, 4480–4489. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal ph profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.P.; Ju, X.J.; Xie, R.; Liu, Y.M.; Wang, W.; Zhang, J.J.; Niu, C.H.; Chu, L.Y. Monodisperse core-shell chitosan microcapsules for ph-responsive burst release of hydrophobic drugs. Soft Matter 2011, 7, 4821–4827. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and ph-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Morishita, I.; Morishita, M.; Takayama, K.; Machida, Y.; Nagai, T. Enteral insulin delivery by microspheres in 3 different formulations using eudragit-l100 and eudragit-s100. Int. J. Pharm. 1993, 91, 29–37. [Google Scholar] [CrossRef]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printing. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Eldridge, J.H.; Hammond, C.J.; Meulbroek, J.A.; Staas, J.K.; Gilley, R.M.; Tice, T.R. Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the peyer’s patches. J. Control. Release 1990, 11, 205–214. [Google Scholar] [CrossRef]
- Iddan, G.; Meron, G.; Glukhovsky, A.; Swain, P. Wireless capsule endoscopy. Nature 2000, 405, 417. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, G.; Shah, S.K.; Riccioni, M.E.; Foschia, F.; Mutignani, M.; Perri, V.; Vecchioli, A.; Brizi, M.G.; Picciocchi, A.; Marano, P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology 2002, 123, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Preat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Bhardwaj, V.; Bala, I.; Sitterberg, J.; Bakowsky, U.; Ravi Kumar, M.N. Design of estradiol loaded plga nanoparticulate formulations: A potential oral delivery system for hormone therapy. Pharm. Res. 2006, 23, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Prego, C.; Fabre, M.; Torres, D.; Alonso, M.J. Efficacy and mechanism of action of chitosan nanocapsules for oral peptide delivery. Pharm. Res. 2006, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.M.; Bouwstra, J.A.; Kok, W.; Noach, A.B.; de Boer, A.G.; Junginger, H.E. Bioadhesion by means of specific binding of tomato lectin. Pharm. Res. 1992, 9, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Cuna, M.; Alonso-Sande, M.; Remunan-Lopez, C.; Alonso, M. Development of chitosan/glucomannan nanoparticles as carrier for oral protein administration. J. Nanosci. Nanotechnol. 2006, 6, 2887–2895. [Google Scholar] [PubMed]
- Clark, M.A.; Jepson, M.A.; Hirst, B.H. Exploiting m cells for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2001, 50, 81–106. [Google Scholar] [CrossRef]
- Jepson, M.A.; Clark, M.A.; Foster, N.; Mason, C.M.; Bennett, M.K.; Simmons, N.L.; Hirst, B.H. Targeting to intestinal m cells. J. Anat. 1996, 189 Pt 3, 507–516. [Google Scholar] [PubMed]
- Ruoslahti, E. Rgd and other recognition sequences for integrins. Annu. Rev. Cell. Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Fromell, K.; Gullberg, E.; Artursson, P.; Caldwell, K.D. Characterization of surface-modified nanoparticles for in vivo biointeraction. A sedimentation field flow fractionation study. Anal. Chem. 2005, 77, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Wang, Y.; Yang, X.; Zhao, N.; Zhang, X.; Xu, J. A bottom-up approach to fabricate patterned surfaces with asymmetrical TiO2 microparticles trapped in the holes of honeycomblike polymer film. J. Am. Chem. Soc. 2011, 133, 3736–3739. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Li, S.; Xu, J.; Luo, G. A one-step microfluidic approach for controllable preparation of nanoparticle-coated patchy microparticles. Microfluid. Nanofluid. 2012, 13, 491–498. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to ph-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Feng, S.S.; Chien, S. Chemotherapeutic engineering: Application and further development of chemical engineering principles for chemotherapy of cancer and other diseases. Chem. Eng. Sci. 2003, 58, 4087–4114. [Google Scholar] [CrossRef]
- Feng, S.-S.; Mu, L.; Win, K.; Huang, G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr. Med. Chem. 2004, 11, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Moingeon, P.; de Taisne, C.; Almond, J. Delivery technologies for human vaccines. Br. Med. Bull. 2002, 62, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Black, S.; Lambert, P.H. Vaccine discovery and translation of new vaccine technology. Lancet 2011, 378, 360–368. [Google Scholar] [CrossRef]
- Gupta, R.K. Aluminum compounds as vaccine adjuvants. Adv. Drug Deliv. Rev. 1998, 32, 155–172. [Google Scholar] [CrossRef]
- Willhite, C.C.; Karyakina, N.A.; Yokel, R.A.; Yenugadhati, N.; Wisniewski, T.M.; Arnold, I.M.; Momoli, F.; Krewski, D. Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Crit. Rev. Toxicol. 2014, 44 (Suppl. 4), 1–80. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D. Summary of Stability Data for Licensed Vaccines. Available online: http://www.path.org/vaccineresources/details.php?i=924 (accessed on 15 June 2016).
- Ding, Z.; Van Riet, E.; Romeijn, S.; Kersten, G.F.; Jiskoot, W.; Bouwstra, J.A. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in balb/c mice after microneedle array pretreatment. Pharm. Res. 2009, 26, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Verbaan, F.J.; Bivas-Benita, M.; Bungener, L.; Huckriede, A.; van den Berg, D.J.; Kersten, G.; Bouwstra, J.A. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in balb/c mice. J. Control. Release 2009, 136, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Clements, M.L.; Prince, S.J.; Murphy, B.R.; Mestecky, J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 1995, 13, 1006–1012. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Davitt, C.J.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef] [PubMed]
- FDA. Complete List of Vaccines Licensed for Immunization and Distribution in the US. Available online: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm (accessed on 15 June 2016).
- Lloyd, J. Technologies for Vaccine Delivery in the 21st Century; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Pearson, F.E.; McNeilly, C.L.; Crichton, M.L.; Primiero, C.A.; Yukiko, S.R.; Fernando, G.J.; Chen, X.; Gilbert, S.C.; Hill, A.V.; Kendall, M.A. Dry-coated live viral vector vaccines delivered by nanopatch microprojections retain long-term thermostability and induce transgene-specific t cell responses in mice. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.; Woolfson, A.D. Microneedle.-Mediated Transdermal and Intradermal Drug Delivery; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Kim, Y.C.; Jarrahian, C.; Zehrung, D.; Mitragotri, S.; Prausnitz, M.R. Delivery systems for intradermal vaccination. Curr. Top. Microbiol. Immunol. 2012, 351, 77–112. [Google Scholar] [PubMed]
- Taddio, A.; Ipp, M.; Thivakaran, S.; Jamal, A.; Parikh, C.; Smart, S.; Sovran, J.; Stephens, D.; Katz, J. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine 2012, 30, 4807–4812. [Google Scholar] [CrossRef] [PubMed]
- Kommareddy, S.; Baudner, B.C.; Bonificio, A.; Gallorini, S.; Palladino, G.; Determan, A.S.; Dohmeier, D.M.; Kroells, K.D.; Sternjohn, J.R.; Singh, M.; et al. Influenza subunit vaccine coated microneedle patches elicit comparable immune responses to intramuscular injection in guinea pigs. Vaccine 2013, 31, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Bondy, B.J.; Yoo, D.G.; Compans, R.W.; Kang, S.M.; Prausnitz, M.R. Stability of whole inactivated influenza virus vaccine during coating onto metal microneedles. J. Control. Release 2013, 166, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fernando, G.J.; Raphael, A.P.; Yukiko, S.R.; Fairmaid, E.J.; Primiero, C.A.; Frazer, I.H.; Brown, L.E.; Kendall, M.A. Rapid kinetics to peak serum antibodies is achieved following influenza vaccination by dry-coated densely packed microprojections to skin. J. Control. Release 2012, 158, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Arya, J.M.; McClain, M.A.; Frew, P.M.; Meltzer, M.I.; Prausnitz, M.R. Microneedle patches: Usability and acceptability for self-vaccination against influenza. Vaccine 2014, 32, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Bartsch, S.M.; Mvundura, M.; Jarrahian, C.; Zapf, K.M.; Marinan, K.; Wateska, A.R.; Snyder, B.; Swaminathan, S.; Jacoby, E.; et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine 2015, 33, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Wang, Y.; Edens, C.; Gentsch, J.R.; Prausnitz, M.R.; Jiang, B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine 2013, 31, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Dybdahl-Sissoko, N.C.; Weldon, W.C.; Oberste, M.S.; Prausnitz, M.R. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine 2015, 33, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Collins, M.L.; Ayers, J.; Rota, P.A.; Prausnitz, M.R. Measles vaccination using a microneedle patch. Vaccine 2013, 31, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Edens, C.; Collins, M.L.; Goodson, J.L.; Rota, P.A.; Prausnitz, M.R. A microneedle patch containing measles vaccine is immunogenic in non-human primates. Vaccine 2015, 33, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Hirao, L.A.; Wu, L.; Khan, A.S.; Satishchandran, A.; Draghia-Akli, R.; Weiner, D.B. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 2008, 26, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Pearton, M.; Kang, S.M.; Song, J.M.; Kim, Y.C.; Quan, F.S.; Anstey, A.; Ivory, M.; Prausnitz, M.R.; Compans, R.W.; Birchall, J.C. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on langerhans cells in human skin. Vaccine 2010, 28, 6104–6113. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.N.V.R. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Attwood, D. Surfactant Systems: Their Chemistry, Pharmacy and Biology; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Ravi, S.; Peh, K.K.; Darwis, Y.; Murthy, B.K.; Singh, T.R.; Mallikarjun, C. Development and characterization of polymeric microspheres for controlled release protein loaded drug delivery system. Indian J. Pharm. Sci. 2008, 70, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Blix, H.S.; Viktil, K.K.; Moger, T.A.; Reikvam, A. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm. Pract. 2010, 8, 50–55. [Google Scholar] [CrossRef]
- Levy, G. What are narrow therapeutic index drugs? Clin. Pharmacol. Ther. 1998, 63, 501–505. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, B.; Rubino, I.; Quan, F.-S.; Yoo, B.; Choi, H.-J. Microfabrication for Drug Delivery. Materials 2016, 9, 646. https://doi.org/10.3390/ma9080646
Koch B, Rubino I, Quan F-S, Yoo B, Choi H-J. Microfabrication for Drug Delivery. Materials. 2016; 9(8):646. https://doi.org/10.3390/ma9080646
Chicago/Turabian StyleKoch, Brendan, Ilaria Rubino, Fu-Shi Quan, Bongyoung Yoo, and Hyo-Jick Choi. 2016. "Microfabrication for Drug Delivery" Materials 9, no. 8: 646. https://doi.org/10.3390/ma9080646