Microstructure and Electrical Properties of AZO/Graphene Nanosheets Fabricated by Spark Plasma Sintering
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
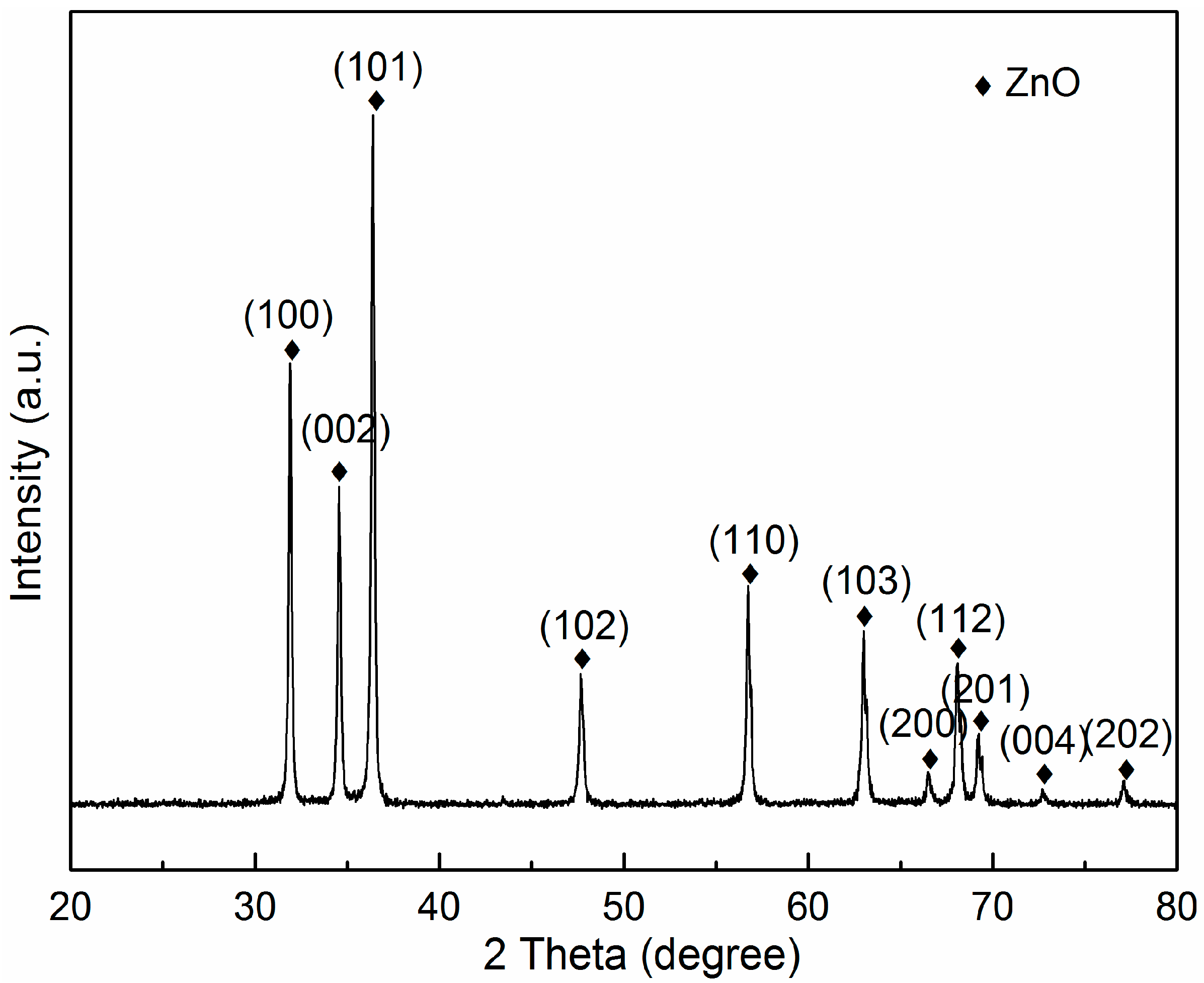
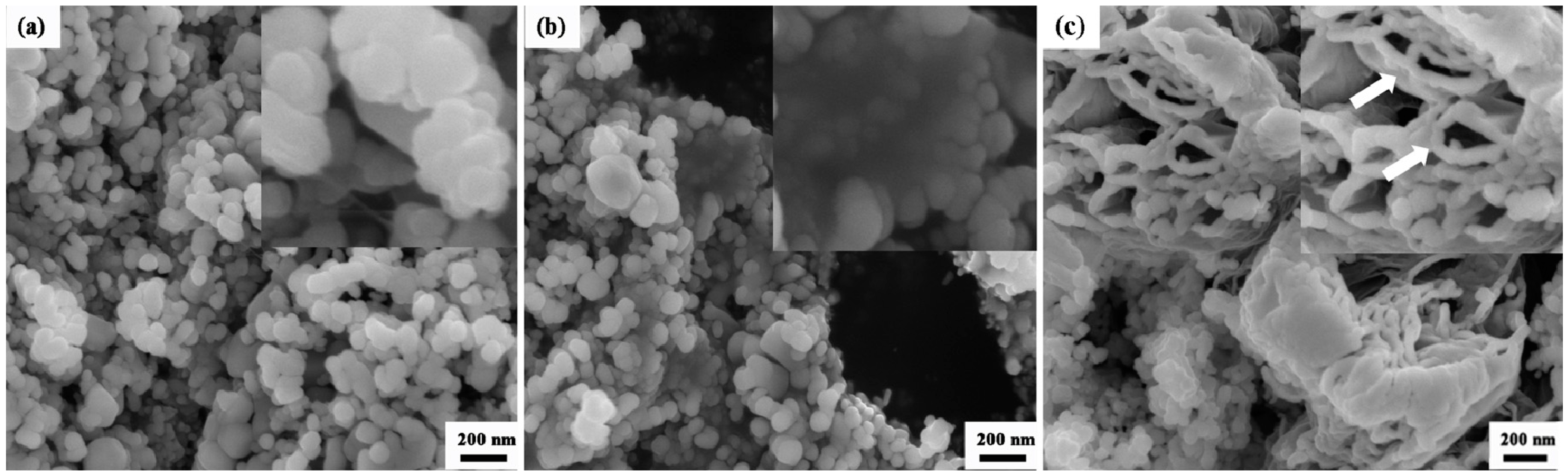
3.1. Phase Composition and Microstructure of AZO-G Composite Nanoparticles
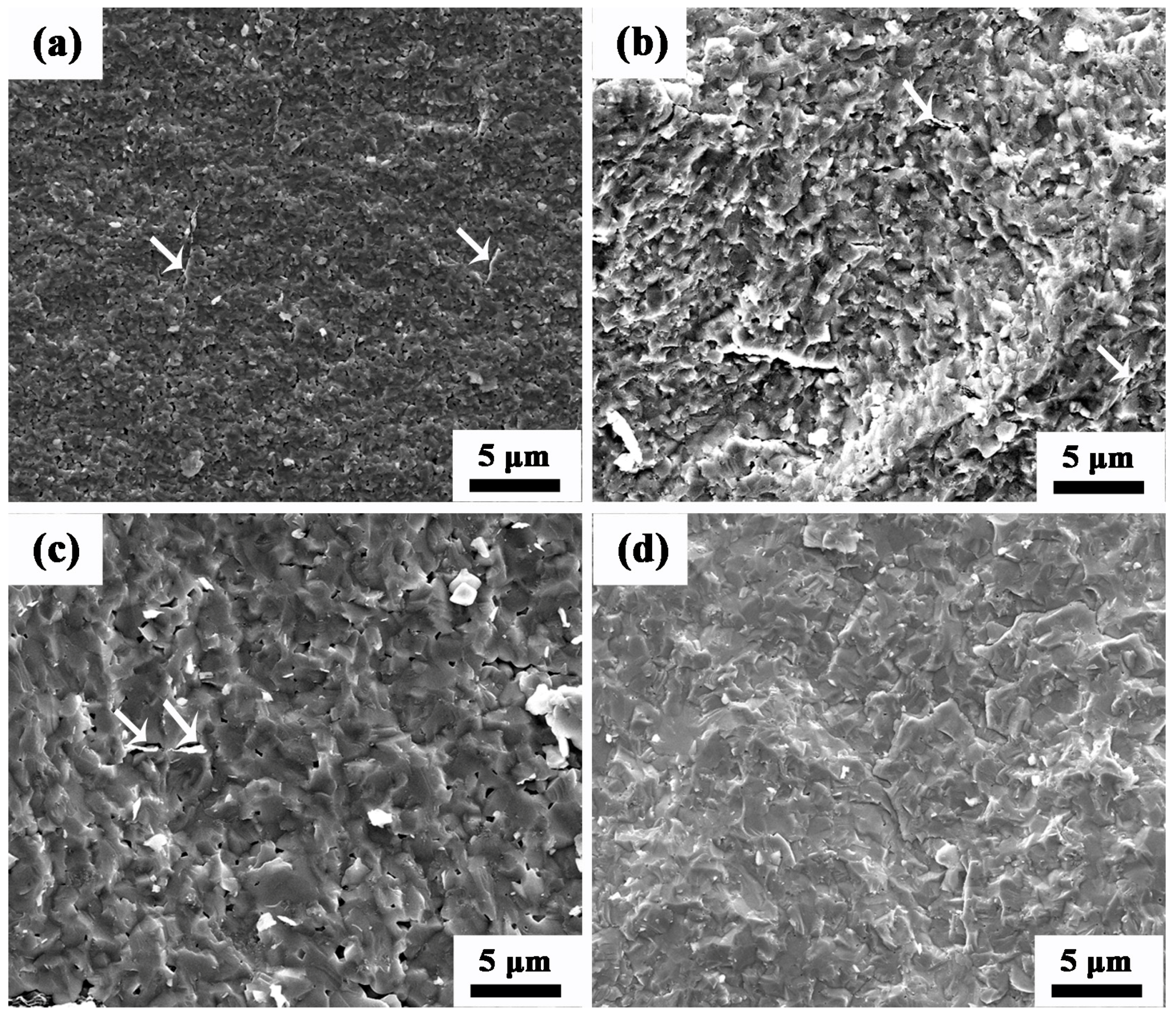
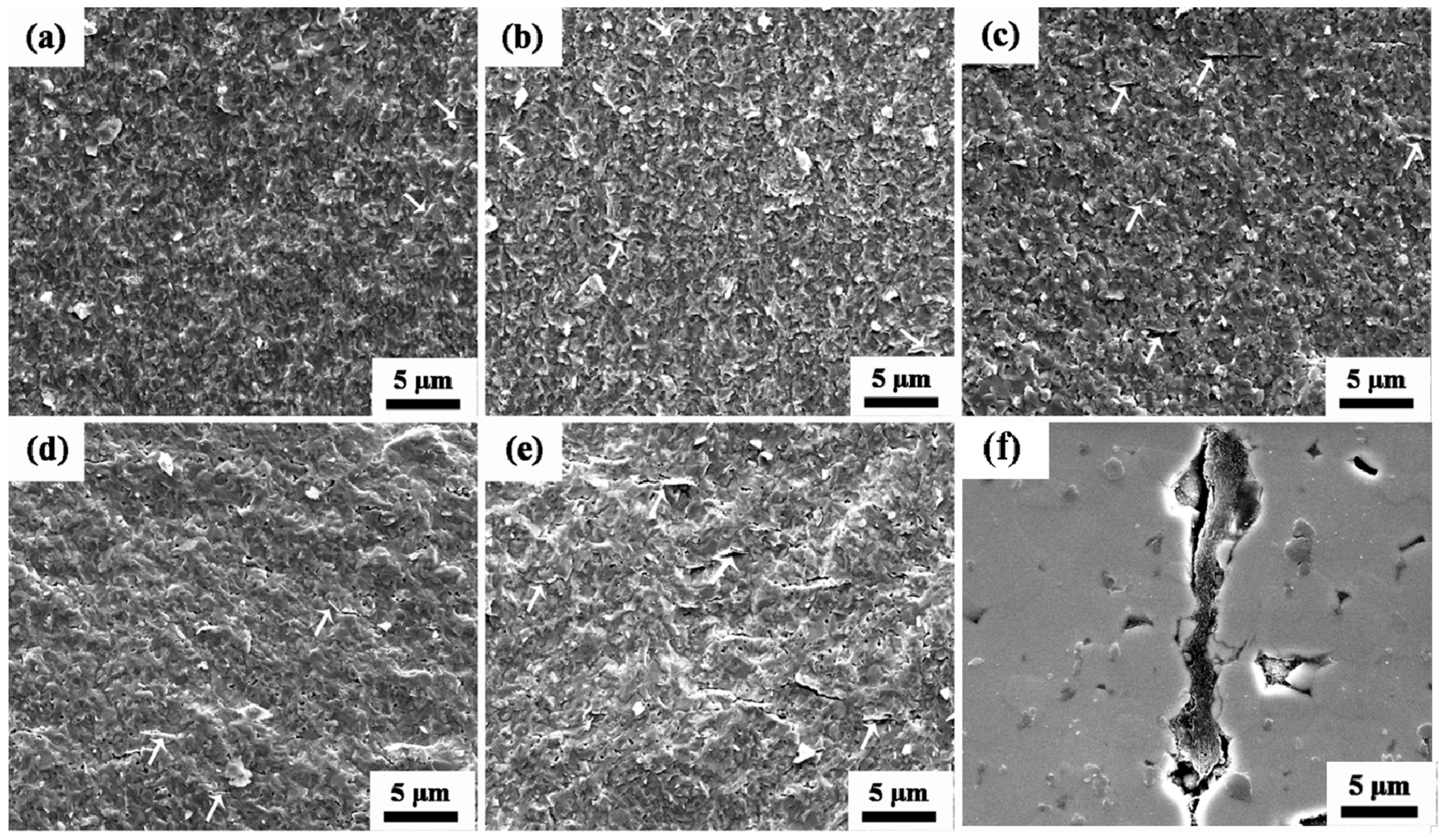
3.2. Relative Density and Grain Size of Composite Ceramics
3.3. Microstructure of AZO-G Composite Ceramics
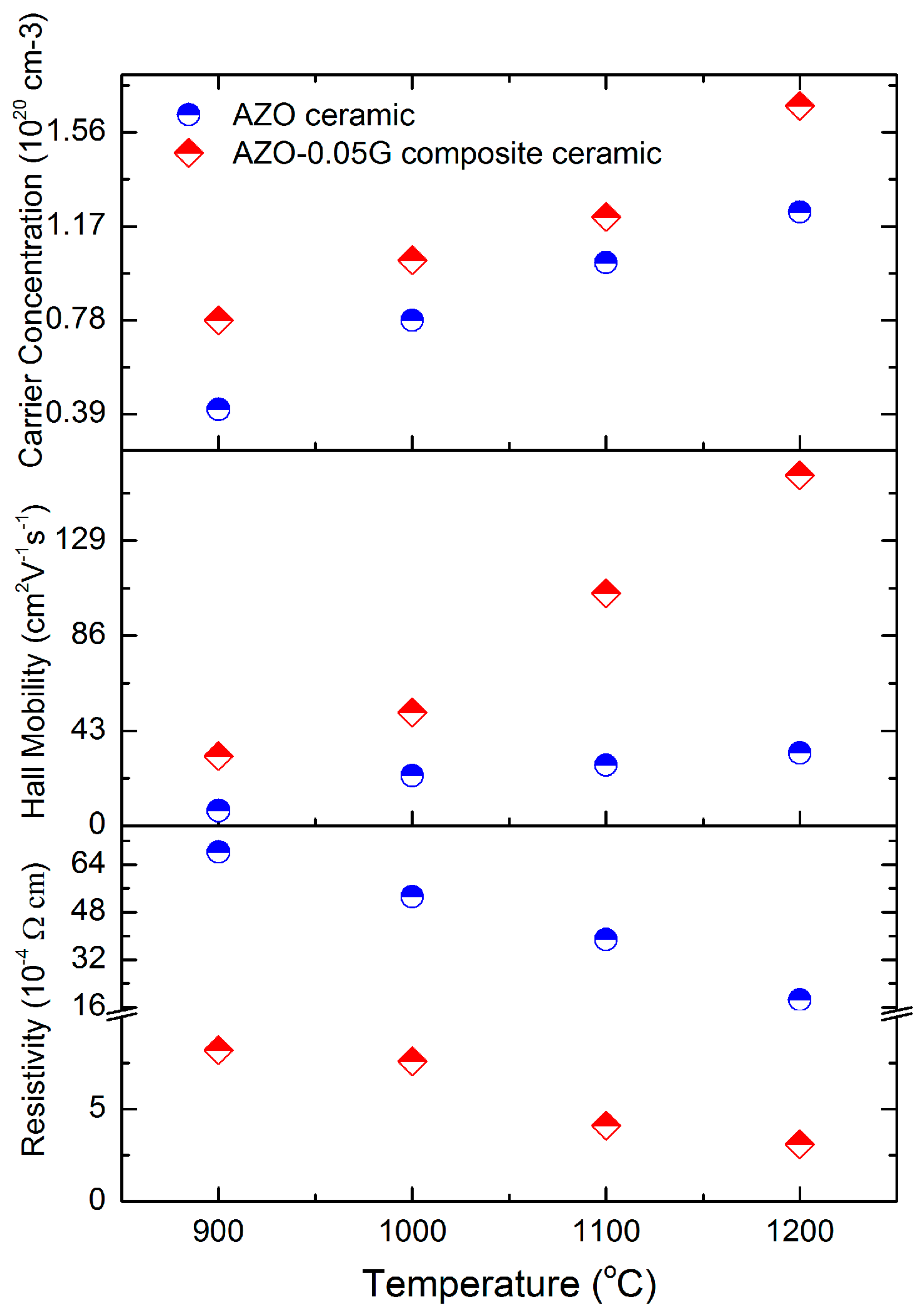
3.4. Electrical Properties of AZO-G Composite Ceramics
4. Discussion
4.1. Influence of GNSs on Densification of AZO-GNSs during SPS
4.2. Influence of GNSs on Electrical Properties of AZO-G
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seung, W.B.; Jae, M.L.; Tae, Y.K.; Min, S.S.; Youngsin, P. Garnet related lithium ion conductor processed by spark plasma sintering for all solid state batteries. J. Power Sources 2014, 249, 197–206. [Google Scholar]
- Lee, J.H.; Ko, K.H.; Park, B.O. Electrical and optical properties of ZnO transparent conducting films by the sol-gel method. J. Cryst. Growth 2003, 247, 119–125. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Tu, J.P.; Wu, H.M.; Yang, Y.Z.; Shi, D.Q.; Zhao, X.B. Electrochemical performance and morphology evolution of nanosized ZnO as anode material of Ni-Zn batteries. Electrochim. Acta 2006, 51, 3632–3636. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, X.; Wu, X.; Wu, H.; Liu, X.; Yue, G.; Yang, Y.; Peng, D.L. Yolk-shell ZnO-C microspheres with enhanced electrochemical performance as anode material for lithium ion batteries. Electrochim. Acta 2014, 125, 659–665. [Google Scholar] [CrossRef]
- Guler, M.O.; Cetinkaya, T.; Tocoglu, U.; Akbulut, H. Electrochemical performance of MWCNT reinforced ZnO anodes for Li-ion batteries. Microelectron. Eng. 2014, 118, 54–60. [Google Scholar] [CrossRef]
- Subramanian, V.; Karki, A.; Gnanasekar, K.I.; Fannie, P.E.; Rambabu, B. Nanocrystalline TiO2 for Li-ion batteries. J. Power Sources 2006, 159, 186–192. [Google Scholar] [CrossRef]
- Kavan, L.; Kalbác, M.; Zukalová, M.; Exnar, I.; Lorenzen, V.; Nesper, R.; Graetzel, M. Lithium storage in nanostructured TiO2 made by hydrothermal growth. Chem. Mater. 2004, 16, 477–485. [Google Scholar] [CrossRef]
- Li, F.; Yang, L.; Xu, G. Hydrothermal self-assembly of hierarchical flower-like ZnO nanospheres with nanosheets and their application in Li-ion batteries. J. Alloy. Compd. 2013, 577, 663–668. [Google Scholar] [CrossRef]
- Bresser, D.; Mueller, F.; Fiedler, M. Transition-metal-doped zinc oxide nanoparticles as a new lithium-ion anode material. Chem. Mater. 2013, 25, 4977–4985. [Google Scholar] [CrossRef]
- Chae, O.B.; Park, S.; Ryu, J.H.; Oh, S.M. Flower-like ZnO–NiO–C films with high reversible capacity and rate capability for lithium-ion batteries. Electrochem. Soc. 2013, 160, 11–14. [Google Scholar] [CrossRef]
- Park, S.K.; Jin, A.; Yu, S.H.; Ha, J.; Jang, B.; Bong, S.; Woo, S.; Sung, Y.E.; Piao, Y. In Situ Hydrothermal Synthesis of Mn3O4 Nanoparticles on Nitrogen-doped Graphene as High-Performance Anode materials for Lithium Ion Batteries. Electrochim. Acta 2014, 120, 452–459. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Zhang, J.; Su, Q.M.; Shi, J.J.; Liu, Y.; Du, G.H. Nanoleaf-on-sheet CuO/graphene composites: Microwave-assisted assemble and excellent electrochemical performances for lithium ion batteries. Electrochim. Acta 2014, 125, 615–621. [Google Scholar] [CrossRef]
- Yue, W.B.; Yang, S.; Ren, Y.; Yang, X.J. In situ growth of Sn, SnO on graphene nanosheets and their application as anode materials for lithium-ion batteries. Electrochim. Acta 2013, 92, 412–420. [Google Scholar] [CrossRef]
- Yue, W.B.; Jiang, S.H.; Huang, W.J.; Gao, Z.Q.; Li, J.; Ren, Y.; Zhao, X.H.; Yang, X.J.J. Sandwich-structural graphene-based metal oxides as anode materials for lithium-ion batteries. Mater. Chem. A 2013, 1, 6928–6933. [Google Scholar] [CrossRef]
- Yue, W.B.; Lin, Z.Z.; Jiang, S.H.; Yang, X. Preparation of graphene-encapsulated mesoporous metal oxides and their application as anode materials for lithium-ion batteries. Mater. Chem. 2012, 22, 16318–16323. [Google Scholar] [CrossRef]
- Zhao, L.; Yue, W.B.; Ren, Y. Synthesis of graphene-encapsulated mesoporous In2O3 with different particle size for high-performance lithium storage. Electrochim. Acta 2014, 116, 31–38. [Google Scholar] [CrossRef]
- Liu, J.P.; Li, Y.Y.; Ding, R.M.; Jiang, J.; Hu, Y.Y.; Ji, X.X.; Chi, Q.B.; Zhu, Z.H.; Huang, X.T. Carbon/ZnO nanorod array electrode with significantly improved lithium storage capability. J. Phys. Chem. C 2009, 113, 5336–5339. [Google Scholar] [CrossRef]
- Shen, X.Y.; Mu, D.B.; Chen, S.; Wu, B.R.; Wu, F. Enhanced Electrochemical Performance of ZnO-Loaded/Porous Carbon Composite as Anode Materials for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 3118–3125. [Google Scholar] [CrossRef] [PubMed]
- Köse, H.; Karaal, Ş.; Aydın, A.O. A facile synthesis of zinc oxide/multiwalled carbon nanotube nanocomposite lithium ion battery anodes by sol–gel method. J. Power Sources 2015, 295, 235–245. [Google Scholar] [CrossRef]
- Yang, S.; Yue, W.B.; Zhu, J.; Ren, Y.; Yang, X.J. Graphene-based mesoporous SnO2 with enhanced electrochemical performance for Lithium-Ion batteries. Adv. Funct. Mater. 2013, 23, 3570–3576. [Google Scholar] [CrossRef]
- Neves, N.; Lagoa, A.; Calado, J. Al-doped ZnO nanostructured powders by emulsion detonation synthesis—Improving materials for high quality sputtering targets manufacturing. J. Eur. Ceram. Soc. 2014, 34, 2325–2338. [Google Scholar] [CrossRef]
- Mendelson, M.I. Average grain size in polycrystalline ceramics. J. Am. Ceram. Soc. 1969, 52, 443–446. [Google Scholar] [CrossRef]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in graphene ceramic composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Huang, L.; Han, Y.H. Sintering behavior of spark plasma sintered alumina with graphene nanoplatelet reinforcement. Ceram. Int. 2015, 41, 5926–5936. [Google Scholar] [CrossRef]
- Andy, N.; Debrupa, L.; Arvind, A. Graphene NanoPlatelets reinforced tantalum carbide consolidated by spark plasma sintering. Mater. Sci. Eng. A 2013, 582, 338–346. [Google Scholar]
- Bérardan, D.; Byl, C.; Dragoe, N. Influence of the preparation conditions on the thermoelectric properties of Al-doped ZnO. J. Am. Ceram. Soc. 2010, 93, 2352–2358. [Google Scholar] [CrossRef]
- Govindaraajan, B.; Sriharsha, K.; Arif, R.; Raman, P.S.; Kalkan, A.K.; Harimkar, S.P. Spark plasma sintering of graphene reinforced zirconium diboride ultra-high temperature ceramic composites. Ceram. Int. 2013, 39, 6637–6646. [Google Scholar]
- Ma, N.; Li, J.F.; Zhang, B.P. Microstructure and thermoelectric properties of Zn1–xAlxO ceramics fabricated by spark plasma sintering. J. Phys. Chem. Solids 2010, 71, 1344–1349. [Google Scholar] [CrossRef]
- Zhang, D.H.; Ma, H.L. Scattering mechanisms of charge carriers in transparent conducting oxide films. Appl. Phys. A 1996, 62, 487. [Google Scholar] [CrossRef]
- Ellmer, K.; Mientus, R. Carrier transport in polycrystalline ITO and ZnO: Al II: the influence of grain barriers and boundaries. Thin Solid Films 2008, 516, 5829–5835. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, F.; Shen, Q.; Zhang, L.M. Microstructure and electrical property of aluminum doped zinc oxide ceramics by isolating current under spark plasma sintering. J. Eur. Ceram. Soc. 2016, 36, 1953–1959. [Google Scholar] [CrossRef]


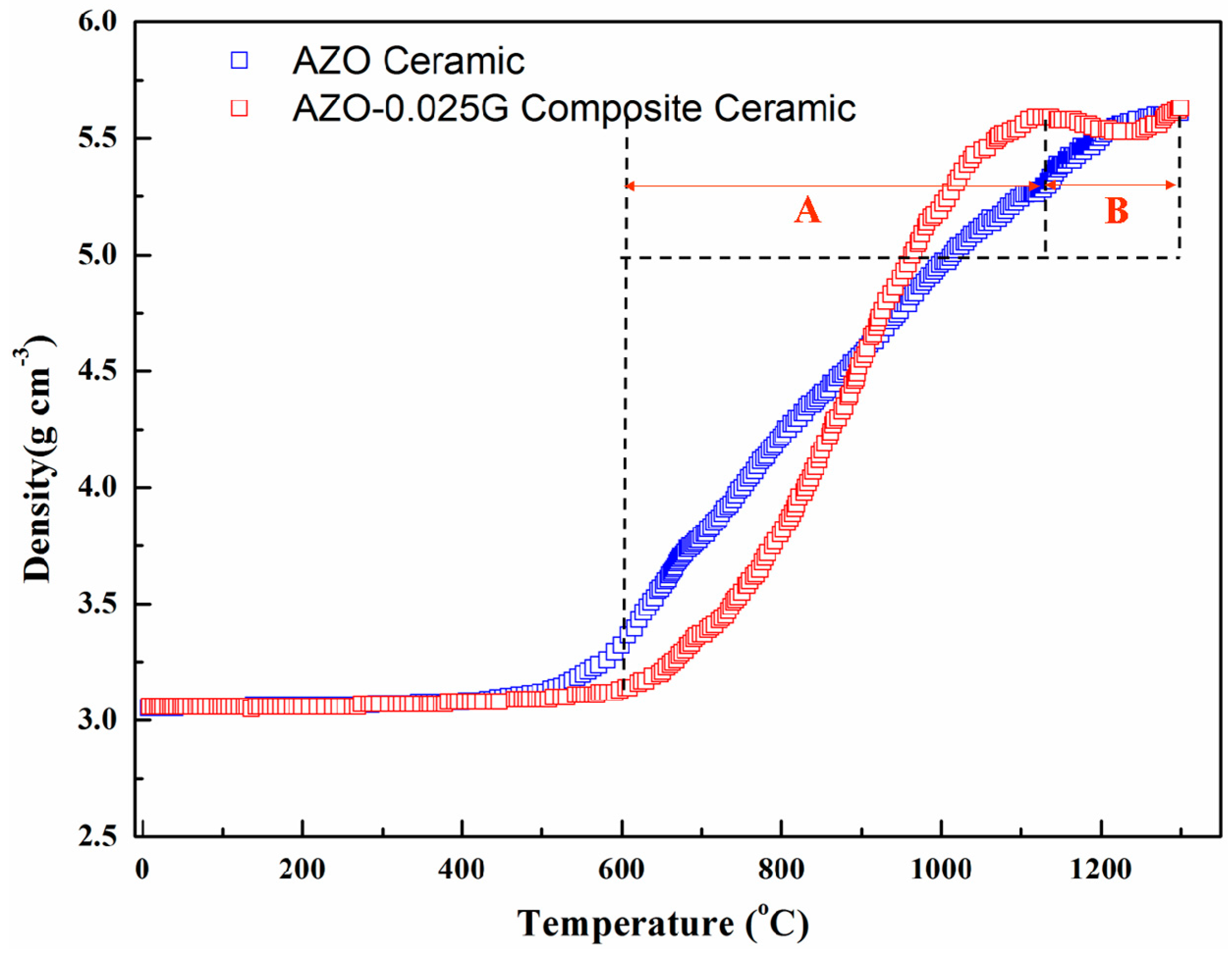
| GNSs (wt. %) | Temperature (°C) | Relative Density (%) | Average Grain Size (μm) |
|---|---|---|---|
| 0 | 1000 | 97.8 | 2.1 |
| 0 | 1100 | 98.4 | 3.3 |
| 0 | 1200 | 99.3 | 5.3 |
| 0.025 | 1000 | 98.3 | 1.8 |
| 0.025 | 1100 | 99.2 | 2.8 |
| 0.025 | 1200 | 97.2 | 4.3 |
| 0.05 | 1100 | 98.3 | 1.5 |
| 0.1 | 1100 | 97.2 | 0.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chen, F.; Shen, Q.; Lavernia, E.J.; Zhang, L. Microstructure and Electrical Properties of AZO/Graphene Nanosheets Fabricated by Spark Plasma Sintering. Materials 2016, 9, 638. https://doi.org/10.3390/ma9080638
Yang S, Chen F, Shen Q, Lavernia EJ, Zhang L. Microstructure and Electrical Properties of AZO/Graphene Nanosheets Fabricated by Spark Plasma Sintering. Materials. 2016; 9(8):638. https://doi.org/10.3390/ma9080638
Chicago/Turabian StyleYang, Shuang, Fei Chen, Qiang Shen, Enrique J. Lavernia, and Lianmeng Zhang. 2016. "Microstructure and Electrical Properties of AZO/Graphene Nanosheets Fabricated by Spark Plasma Sintering" Materials 9, no. 8: 638. https://doi.org/10.3390/ma9080638






