Crystal Structures and Mechanical Properties of Ca2C at High Pressure
Abstract
:1. Introduction
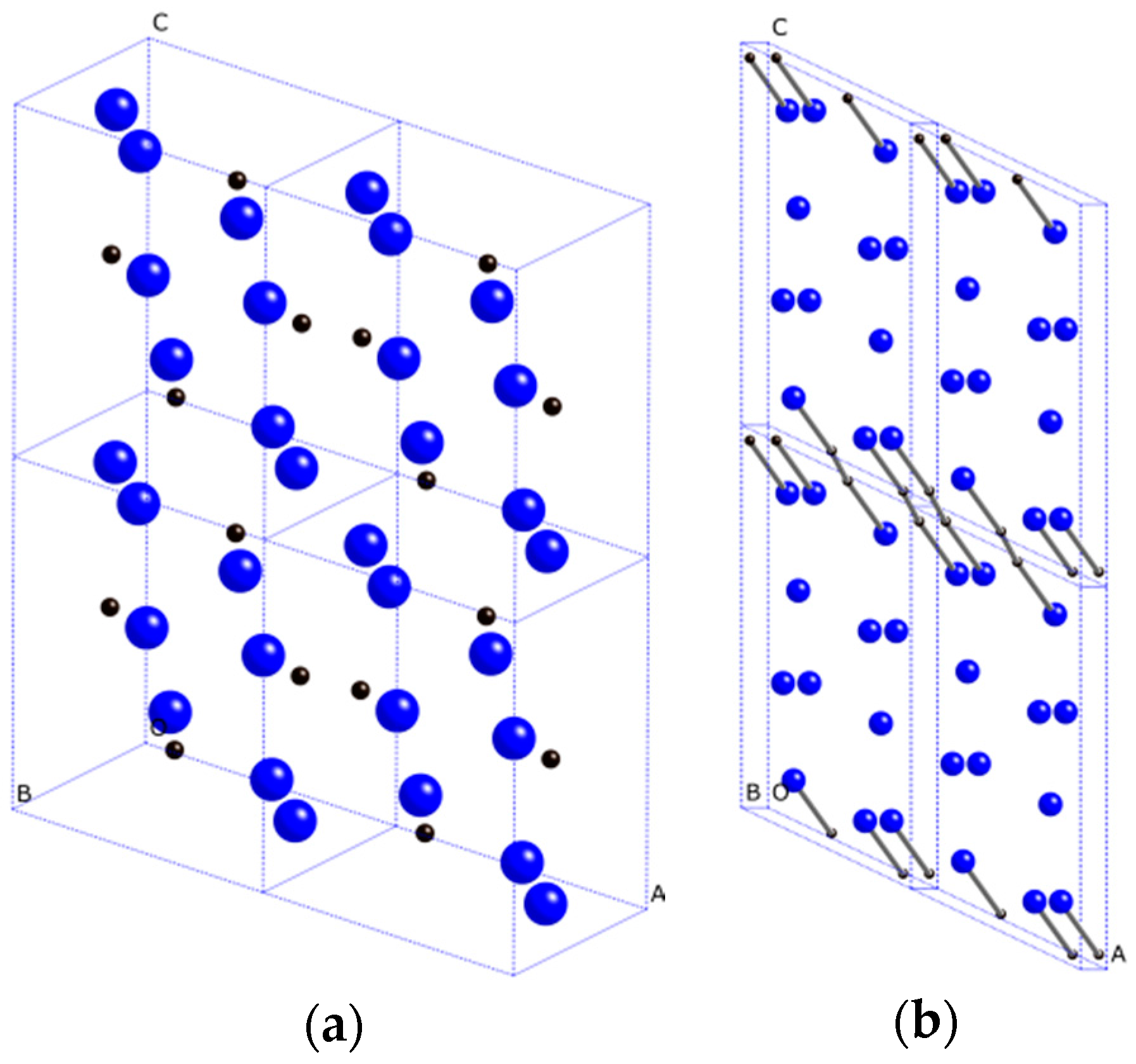
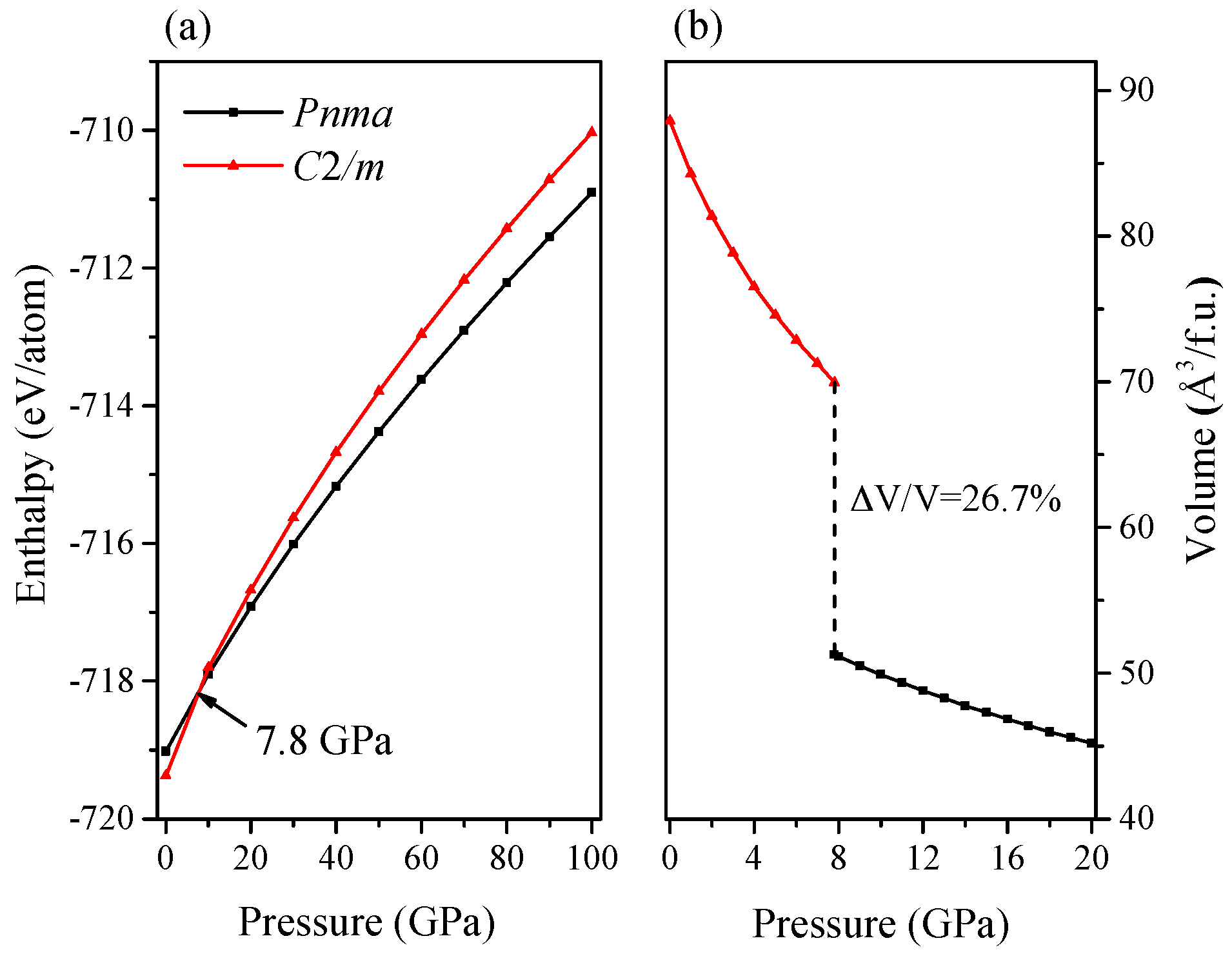
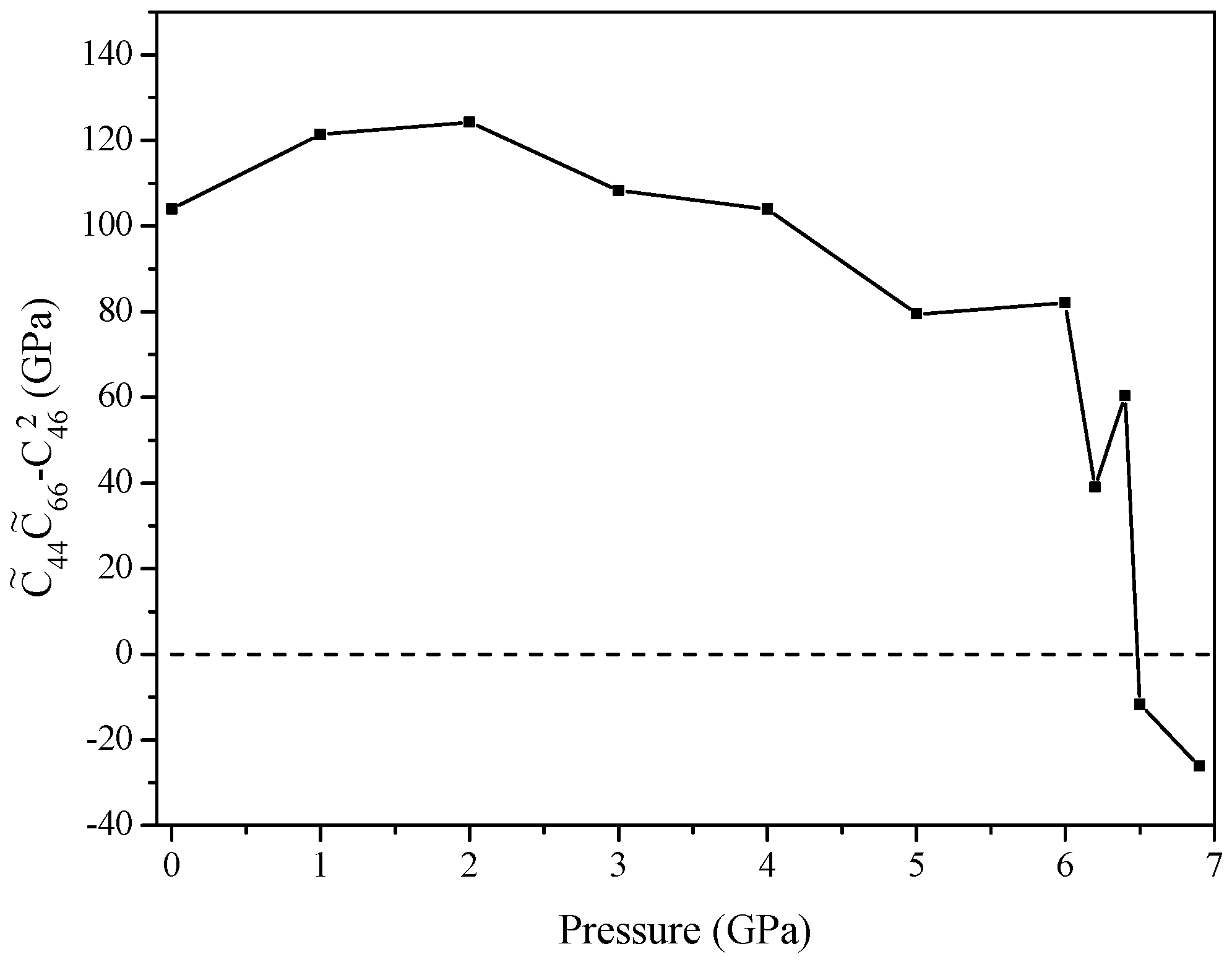
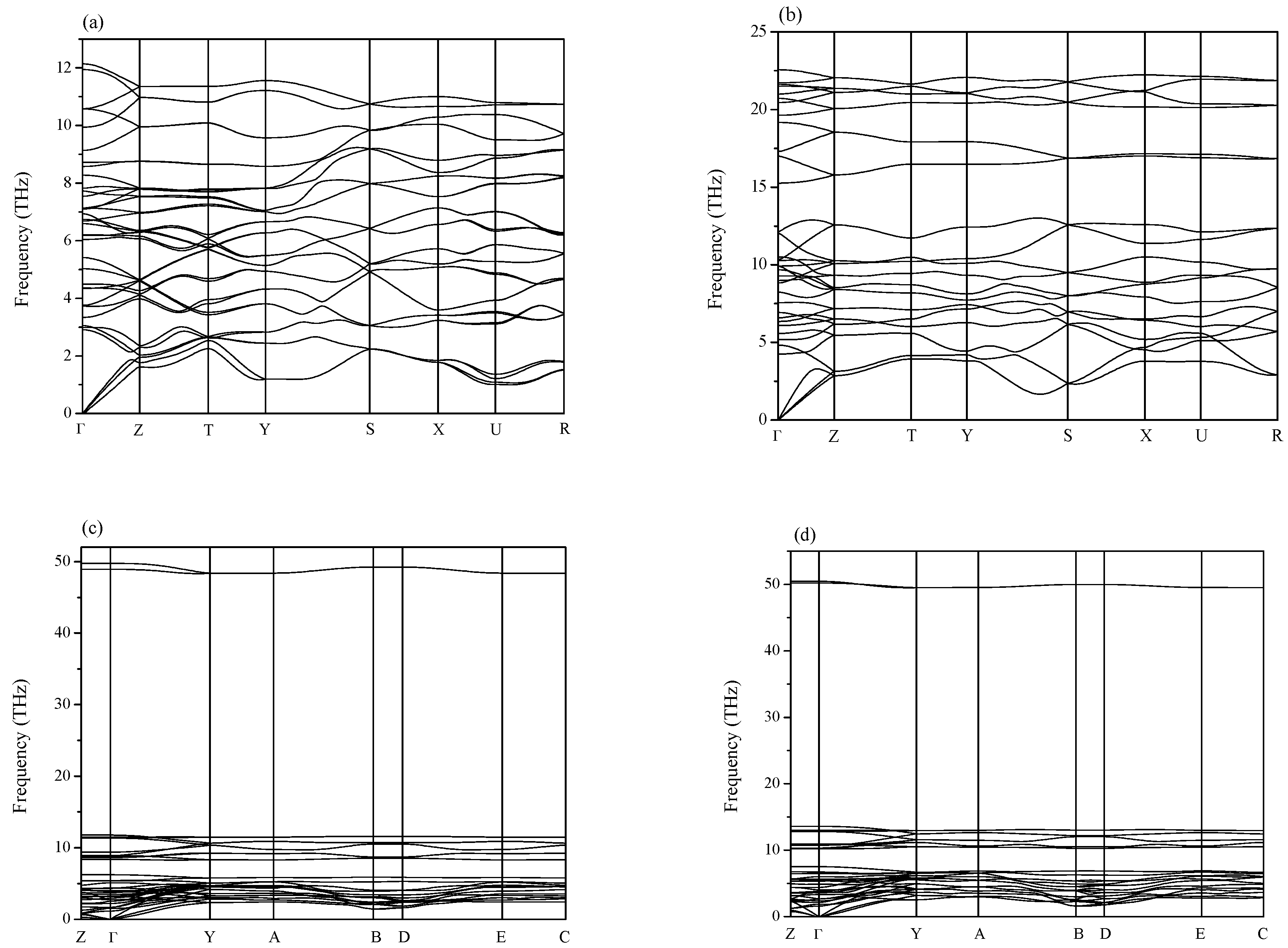
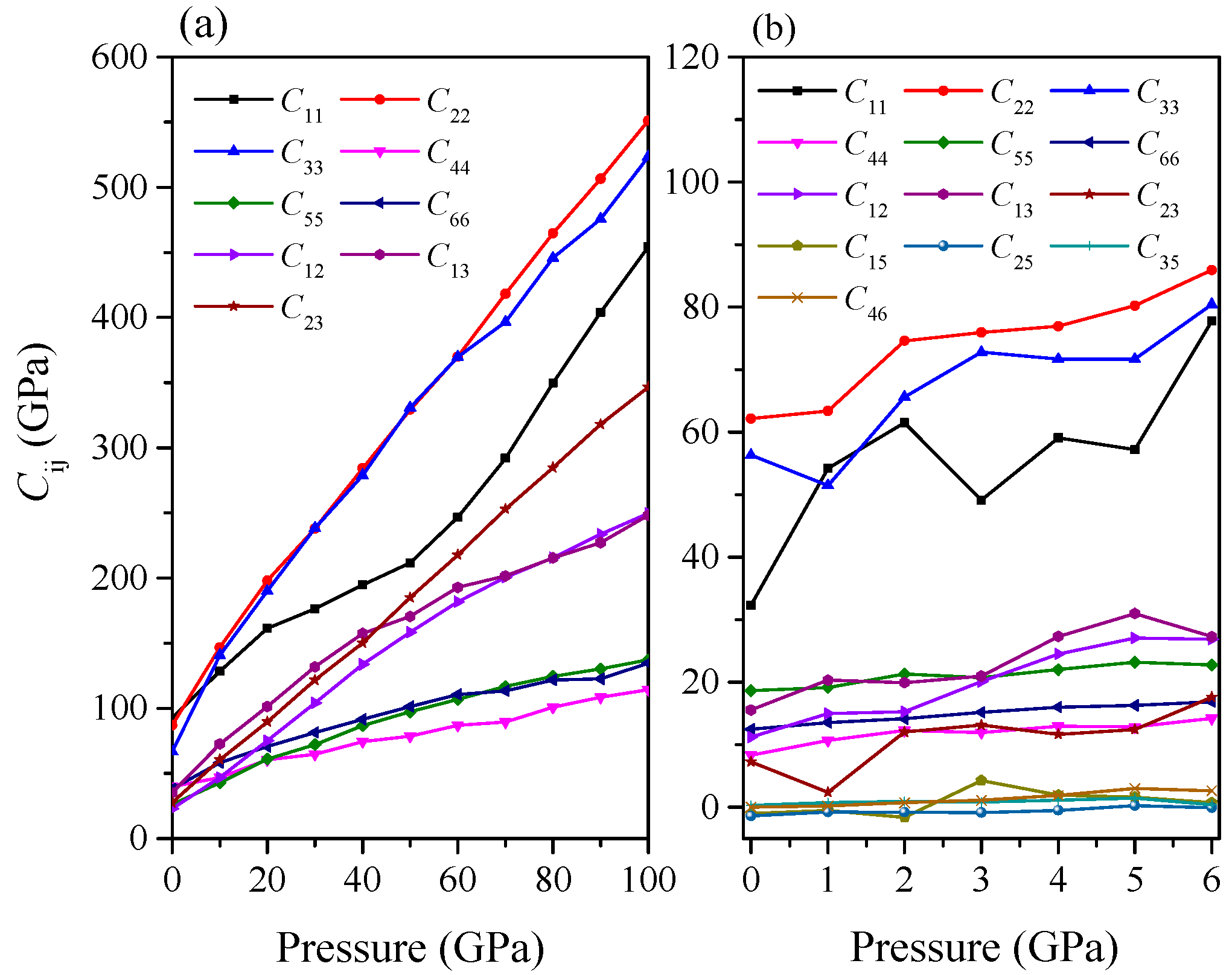
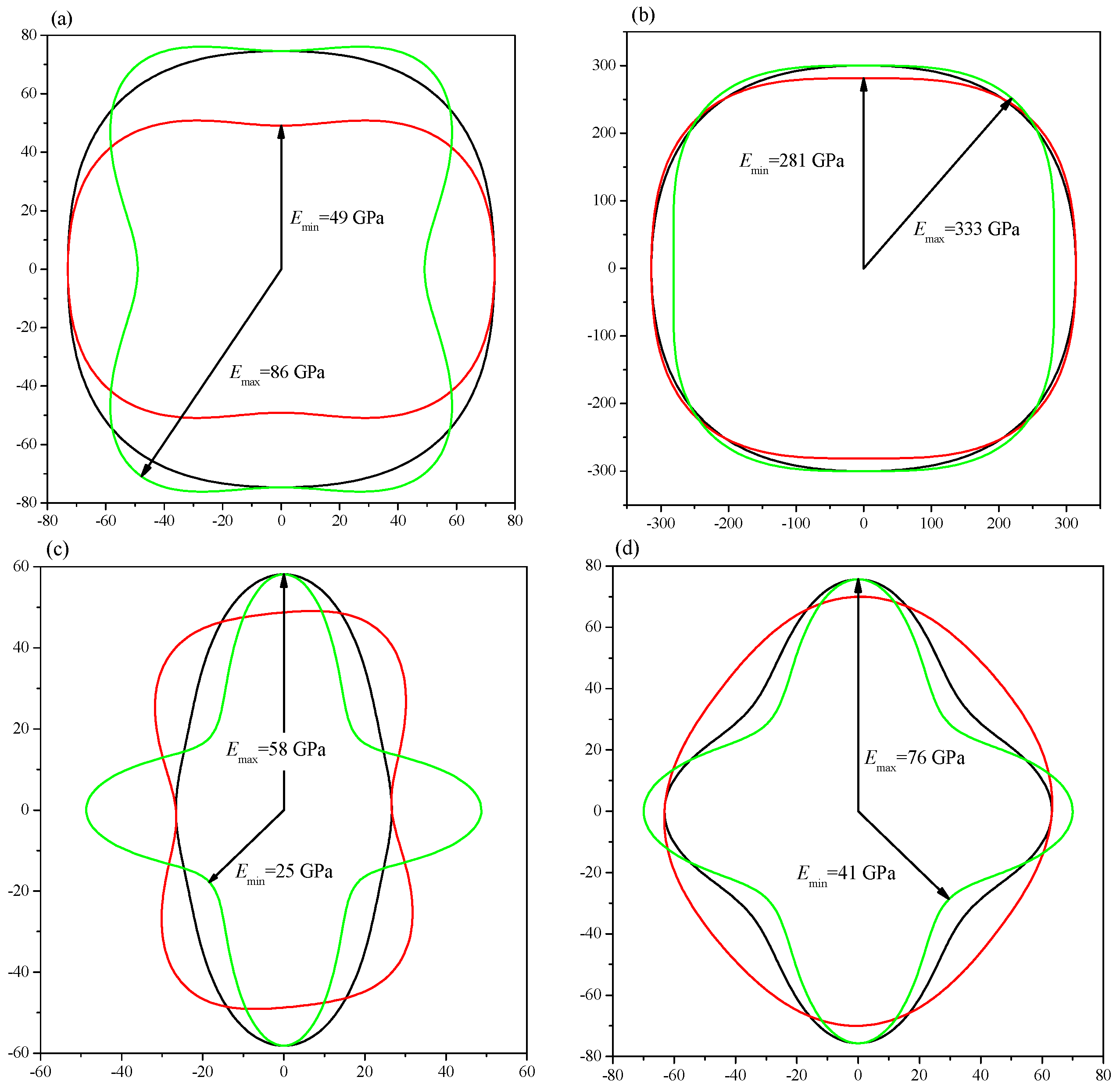
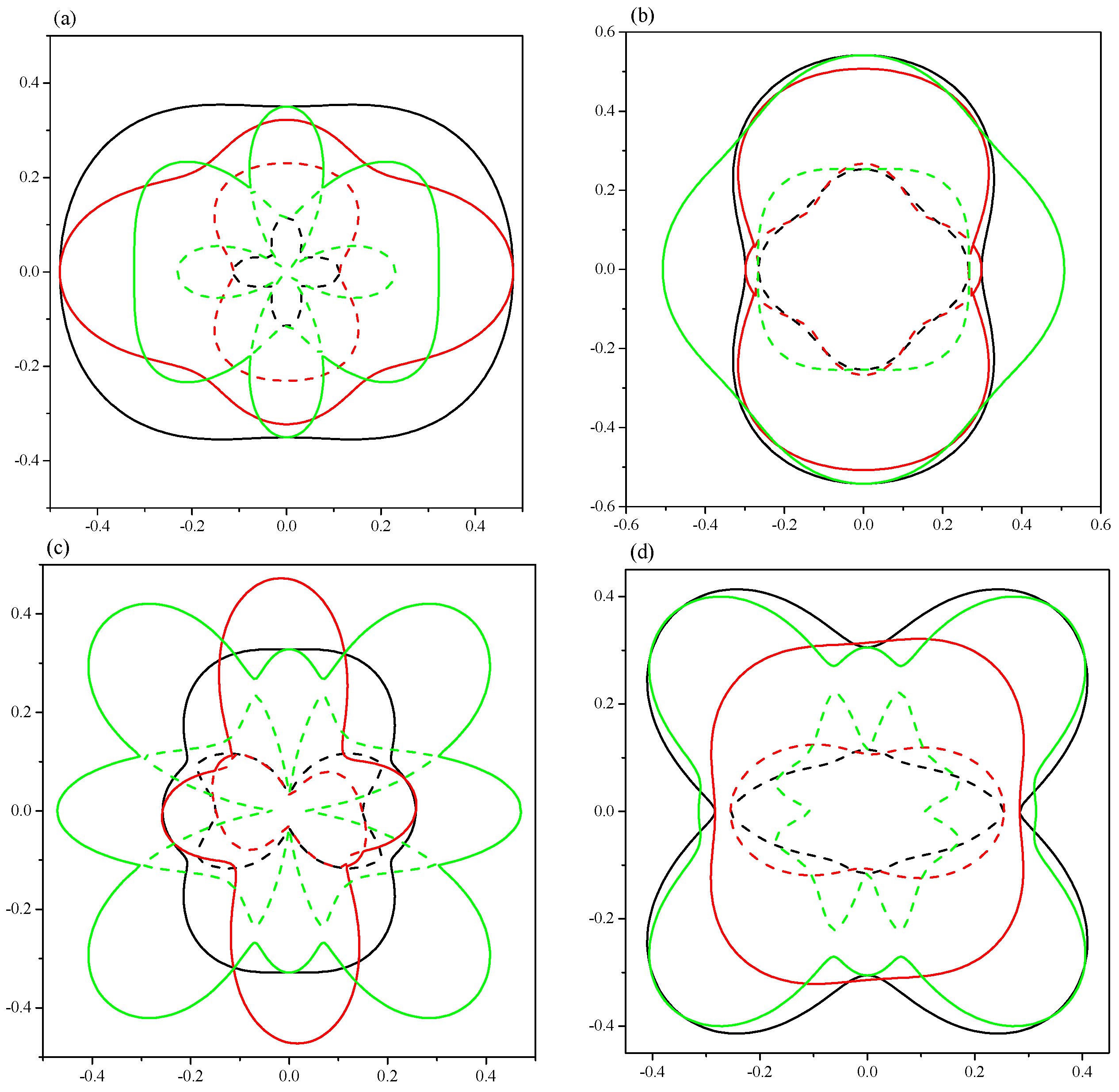
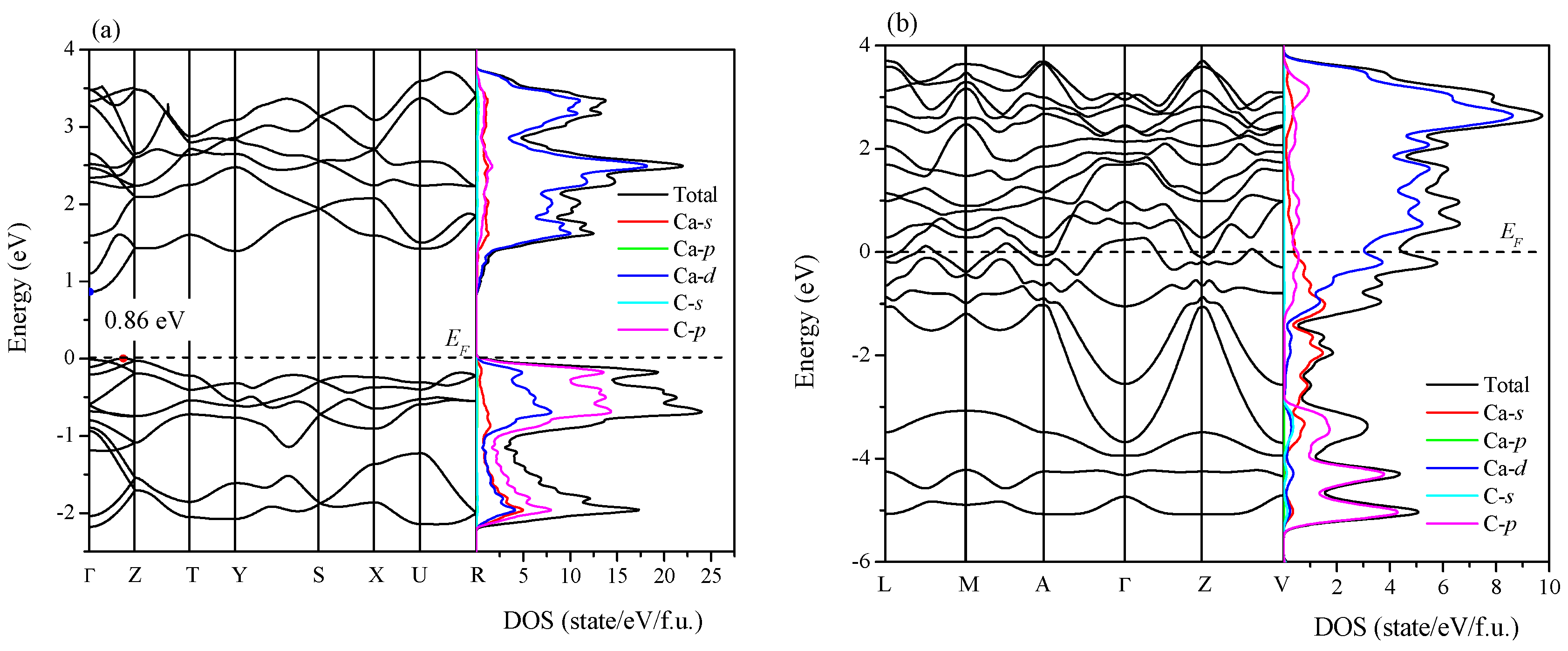
2. Results and Discussion
3. Computational Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, W.; Oganov, A.R.; Goncharov, A.F.; Zhu, Q.; Boulfelfel, S.E.; Lyakhov, A.O.; Stavrou, E.; Somayazulu, M.; Prakapenka, V.B.; Konôpková, Z. Unexpected stable stoichiometries of Sodium Chlorides. Science 2013, 342, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Kurakevych, O.O.; Strobel, T.A.; Kim, D.Y.; Cody, G.D. Synthesis of Mg2C: A Magnesium Methanide. Angew. Chem. Int. Ed. 2013, 52, 8930–8933. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Chai, C.; Wei, Q.; Yang, Y.; Yang, Q.; Chen, P.; Xing, M.; Zhang, J.; Yao, R. Prediction of novel phase of silicon and Si-Ge alloys. J. Solid State Chem. 2016, 233, 471–483. [Google Scholar] [CrossRef]
- Wei, Q.; Yan, H.; Zhu, X.; Lin, Z.; Yao, R. Theoretical investigations on the elastic and thermodynamic properties of Rhenium Phosphide. Z. Naturforsch. A 2016, 71, 1–8. [Google Scholar] [CrossRef]
- Gauzzi, A.; Takashima, S.; Takeshita, N.; Terakura, C.; Takagi, H.; Emery, N.; Hérold, C.; Lagrange, P.; Loupias, G. Enhancement of superconductivity and evidence of structural instability in intercalated Graphite CaC6 under high pressure. Phys. Rev. Lett. 2007, 98, 067002. [Google Scholar] [CrossRef] [PubMed]
- Nylén, J.; Konar, S.; Lazor, P.; Benson, D.; Häussermann, U. Structural behavior of the acetylide carbides Li2C2 and CaC2 at high pressure. J. Chem. Phys. 2012, 137, 224507. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Luo, W.; Zeng, Z.; Lin, H.Q.; Mao, H.K.; Ahuja, R. Pressure-induced superconductivity in CaC2. Proc. Natl. Acad. Sci. USA 2013, 110, 9289–9294. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, Z.; Hashemifar, S.J.; Akbarzadeh, H. First-principles insights into p magnetism in CaC and CaN ionic compounds. J. Magn. Magn. Mater. 2013, 341, 56–59. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, S.N.; Oganov, A.R.; Gou, H.; Smith, J.S.; Strobel, T.A. Investigation of exotic stable calcium carbides using theory and experiment. Nat. Commun. 2015, 6, 6974. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ding, H.; Zhou, B.; Duan, C.; Wu, P.; Zhao, H. First-principles and Monte Carlo studies on the magnetic stability of half-metallic zinc-blende CaC and similar compounds. J. Magn. Magn. Mater. 2015, 378, 469–477. [Google Scholar] [CrossRef]
- Debessai, M.; Hamlin, J.J.; Schilling, J.S.; Rosenmann, D.; Hinks, D.G.; Claus, H. Superconductivity for CaC6 to 32 GPa hydrostatic pressure. Phys. Rev. B 2010, 82, 132502. [Google Scholar] [CrossRef]
- Mazin, I.I.; Boeri, L.; Dolgov, O.V.; Golubov, A.A.; Bachelet, G.B.; Giantomassi, M.; Andersen, O.K. Unresolved problems in superconductivity of CaC6. Physics C 2007, 460–462, 116–120. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Cui, T.; Li, Y.; He, Z.; Ma, Y.; Zou, G. Pressure-induced enhancement of electron-phonon coupling in superconducting CaC6 from first principles. Phys. Rev. B 2006, 74, 184519. [Google Scholar] [CrossRef]
- Moradi, M.; Mohammadi, A.; Afshari, M.; Soltani, Z. The half-metallicity of zinc-blende CaC/GaAs (001) heterojunction: A density functional theory study. J. Magn. Magn. Mater. 2013, 332, 81–84. [Google Scholar] [CrossRef]
- Nourbakhsh, Z.; Hashemifar, S.J.; Akbarzadeh, H. Ab initio study of different structures of CaC: Magnetism, bonding, and lattice dynamics. J. Alloys Compd. 2013, 579, 360–364. [Google Scholar] [CrossRef]
- Gao, G.Y.; Yao, K. Surface sp half-metallicity of zinc-blende calcium monocarbide. J. Appl. Phys. 2009, 106, 053703. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Zhao, E.-J.; Xiang, H.-P.; Hao, X.-F.; Liu, X.-J.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Fan, Q.Y.; Wei, Q.; Yan, H.Y.; Zhang, M.G.; Zhang, D.Y.; Zhang, J.Q. A New Potential Superhard Phase of OsN2. Acta Phys. Pol. A 2014, 126, 740–746. [Google Scholar] [CrossRef]
- Sin’Ko, G.V.; Smirnov, N.A. Ab initio calculations of elastic constants and thermodynamic properties of bcc, fcc, and hcp Al crystals under pressure. J. Phys. Condens. Matter 2002, 14, 6989–7005. [Google Scholar]
- Voigt, W. Lehrburch der Kristallphysik; Teubner: Leipzig, Germany, 1928. [Google Scholar]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Phys. Soc. Lond. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Pugh, S.F. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W.; Clarke, D.R. Anisotropic elastic and thermal properties of the double perovskite slab-rock salt layer Ln2SrAl2O7 (Ln = La, Nd, Sm, Eu, Gd or Dy) natural superlattice structure. Acta Mater. 2012, 60, 3380–3392. [Google Scholar] [CrossRef]
- Ravindran, P.; Fast, L.; Korzhavyi, P.A.; Johansson, B.; Wills, J.; Eriksson, O. Density functional theory for calculation of elastic properties of orthorhombic crystals: Application to TiSi2. J. Appl. Phys. 1998, 84, 4891–4904. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, M.; Yan, H.; Lin, Z.; Zhu, X. Structural, electronic and mechanical properties of Imma-carbon. Europhys. Lett. 2014, 107, 27007. [Google Scholar] [CrossRef]
- Marmiera, A.; Lethbridge, Z.A.D.; Walton, R.I.; Smith, C.W.; Parker, S.C.; Evans, K.E. ElAM: A computer program for the analysis and representation of anisotropic elastic properties. Comput. Phys. Commun. 2010, 181, 2102–2115. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of Crystals with the Quasi-Newton Method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]


| Phase | Ionic Radius (Å) | Charge Transfers (e) | |
|---|---|---|---|
| C | Ca | Ca → C | |
| C2/m | 1.534 | 1.871 | 0.928 |
| Pnma | 1.788 | 1.485 | 2.348 |
| Phase | Pressure (GPa) | a (Å) | b (Å) | c (Å) | β (deg) | V (Å3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pnma | 0 | 6.677 | 6.689 a | 4.384 | 4.389 a | 7.979 | 7.981 a | 233.58 | 234.32 a | ||
| 10 | 6.404 | 6.415 a | 4.150 | 4.154 a | 7.513 | 7.518 a | 199.68 | 200.35 a | |||
| 6.449 b | 4.157 b | 7.523 b | 201.7 b | ||||||||
| 30 | 5.919 | 5.929 a | 3.931 | 3.933 a | 7.195 | 7.204 a | 167.41 | 168.04 a | |||
| C2/m | 0 | 7.166 | 3.775 | 15.490 | 122.9 | 351.59 | |||||
| 5 | 6.743 | 6.701 a | 3.594 | 3.587 a | 14.65 | 14.68 a | 122.8 | 122 a | 298.32 | ||
| 6.4 | 6.674 | 3.554 | 14.45 | 122.6 | 288.87 | ||||||
| Pressure (GPa) | Pnma-Ca2C | C2/m-Ca2C | ||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 0 | 6 | 6.4 | |
| C11 | 92 | 212 | 454 | 32 | 78 | 71 |
| C22 | 87 | 329 | 551 | 62 | 86 | 84 |
| C33 | 67 | 331 | 523 | 56 | 80 | 86 |
| C44 | 40 | 79 | 114 | 8 | 13 | 14 |
| C55 | 26 | 97 | 137 | 19 | 23 | 20 |
| C66 | 38 | 101 | 135 | 13 | 17 | 15 |
| C12 | 23 | 158 | 250 | 11 | 26 | 28 |
| C13 | 35 | 170 | 248 | 16 | 27 | 29 |
| C23 | 27 | 185 | 347 | 7 | 18 | 23 |
| C15 | −1 | 0.75 | 4 | |||
| C25 | −1.4 | −0.06 | 3 | |||
| C35 | 0.3 | 0.44 | −1.31 | |||
| C46 | 0.04 | 2.65 | 2.67 | |||
| B | 46 | 203 | 353 | 24 | 43 | 45 |
| G | 30 | 75 | 122 | 15 | 21 | 19 |
| E | 74 | 200 | 328 | 37 | 54 | 50 |
| υ | 0.23 | 0.34 | 0.35 | 0.24 | 0.29 | 0.31 |
| B/G | 1.53 | 2.71 | 2.89 | 1.60 | 2.05 | 2.37 |
| Phase | Pressure (GPa) | AU | A1 | A2 | A3 | AB | AG |
|---|---|---|---|---|---|---|---|
| Pnma | 0 | 0.37 | 1.79 | 1.06 | 1.14 | 0.6 | 3.5 |
| 50 | 0.62 | 1.56 | 1.34 | 1.81 | 3.8 | 5.1 | |
| 100 | 0.15 | 0.95 | 1.44 | 1.06 | 1.3 | 1.2 | |
| C2/m | 0 | 0.95 | 0.58 | 0.72 | 0.69 | 2.7 | 8.2 |
| 6 | 0.54 | 0.53 | 0.70 | 0.62 | 0.06 | 5.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Zhang, Q.; Zhang, M. Crystal Structures and Mechanical Properties of Ca2C at High Pressure. Materials 2016, 9, 570. https://doi.org/10.3390/ma9070570
Wei Q, Zhang Q, Zhang M. Crystal Structures and Mechanical Properties of Ca2C at High Pressure. Materials. 2016; 9(7):570. https://doi.org/10.3390/ma9070570
Chicago/Turabian StyleWei, Qun, Quan Zhang, and Meiguang Zhang. 2016. "Crystal Structures and Mechanical Properties of Ca2C at High Pressure" Materials 9, no. 7: 570. https://doi.org/10.3390/ma9070570






