Rotation Disk Process to Assess the Influence of Metals and Voltage on the Growth of Biofilm
Abstract
:1. Introduction
2. Results and Discussion
2.1. SEM Data
2.2. Rotation Disk Reactor Compared to Others
3. Materials and Methods
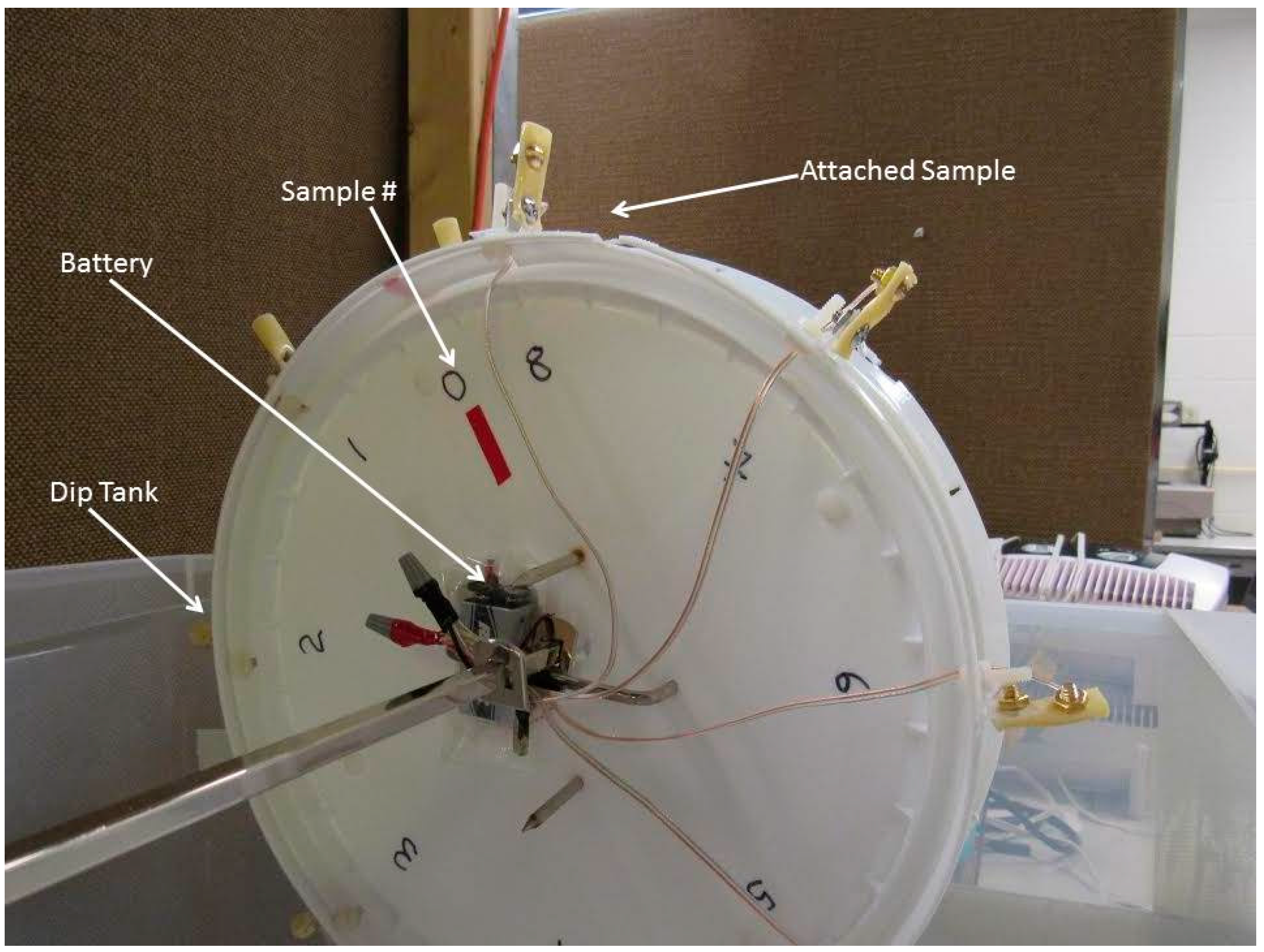
3.1. Test Samples
| Sample Number | Electrode Details |
| 0 | control: nylon mounted |
| 1 | single silver |
| 2 | single stainless steel |
| 3 | single brass |
| 4 | stainless steel—brass |
| 5 | stainless steel—stainless steel with voltage applied |
| 6 | brass—brass with voltage applied |
| 7 | brass positive—stainless steel negative with voltage applied |
| 8 | brass negative—stainless steel positive with voltage applied |
3.2. Observations
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Costerton, J.W. Introduction to Biofilm. Int. J. Antimicrob. Agents 1999, 11, 217–221. [Google Scholar] [CrossRef]
- Schmitt, J.; Flemming, H.-C. Water Binding Biofilms. Water Sci. Technol. 1999, 39, 77–82. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo Models of Bacterial Biofilm-Related Infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial Adhesion and Biofilms on Surfaces. Prog. Natl. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Ig. 2013, 25, 31–42. [Google Scholar] [PubMed]
- Barry, D.M.; McGrath, P.B. Rotation disk process for accelerated assessment of biofilm formation on medical materials. Mater. Technol. Adv. Biomater. 2015, 30, 33–37. [Google Scholar]
- Negri, M.; Silva, S.; Henriques, M.; Azeredo, J.; Svidzinski, T.; Oliveira, R. Candida tropicalis biofilms: Artificial urine, urinary catheters and flow model. Med. Mycol. 2011, 49, 739–747. [Google Scholar] [PubMed]
- Drury, W.J.; Characklis, W.G.; Stewart, P.S. Interactions of 1-um Latex Particles with Pseudomonas aeruginosa Biofilms. Wat. Res. 1993, 27, 1119–1126. [Google Scholar] [CrossRef]
- Drury, W.J.; Stewart, P.S.; Characklis, W.G. Transport of 1-um latex particles in Pseudomonas aeruginosa biofilms. Biotechnol. Bioeng. 1993, 42, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, L.C.; Charone, S.; Maia, L.C.; Soares, R.M.; Portela, M.B. Biofilm formation by Candida species on silicone surfaces and latex pacifier nipples: An in vitro study. J. Clin. Pediatr. Dent. 2009, 33, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, A.; Makisalo, H.; Talja, M.; Siitonen, A.; Vuopio-Varkila, J.; Ruuru, M.; Hockerstedt, K. Bacterial adherence and biofilm formation on latex and silicone T-tubes in relation to bacterial contamination of bile. Scand. J. Gastroenterol. 1996, 4, 398–403. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Das Ghatak, P.; Roy, S.; Khanna, S.; Hemann, C.; Deng, B.; Das, A.; Zweier, J.L.; Wozniak, D.; Sen, C.K. Siver-Zinc Redox-Coupled Electroceutical Wound Dressing Disrupts Bacterial Biofilm. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Perez-Roa, R.E.; Tompkins, D.T.; Paulose, M.; Grimes, C.A.; Anderson, M.A.; Noguera, D.R. Effects of localized, low-voltage pulseds electric fields on the development and inhibition of Pseudomonas aeruginosa biofilms. Biofouling 2006, 22, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Subramanian, S.; Gerasopoulos, K.; Ben-Yoav, H.; Wu, H.-C.; Quan, D.; Carter, K.; Meyer, M.T.; Bentley, W.E.; Ghodss, R. Effect of Electrical Energy on the Efficacy of Biofilm Treatment Using the Bioelectric Effect. Nature 2015. [Google Scholar] [CrossRef]
- Sandvik, E.L.; McLeod, B.R.; Parker, A.E.; Stewart, P.S. Direct Electric Current Treatment under Physiologic Saline Conditions Kills Staphylococcus epidermidis Biofilms via Electrolytic Generation of Hypochlorous Acid. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Newsletter of the Rubber Foundation Information Center for Natural Rubber; Natural Rubber-17; Natural Rubber: Beerise, Belgium, 2000.
- Li, G.Y.; Koenig, J.L. A review of rubber oxidation. Rubber Chem. Technol. 2005, 78, 355–390. [Google Scholar] [CrossRef]
- Goh, S.H. Thermo analytical studies of rubber oxidation. J. Elastomers Plast. 1977, 9, 186–192. [Google Scholar]
- Gould, S.W.; Fielder, M.D.; Kelly, A.F.; Morgan, M.; Kenny, J.; Naughton, D.P. The antimicrobial properties of copper surfaces against a range of important nosocomial pathogens. Ann. Microbiol. 2009, 59, 151–156. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Vine, G.J.; Beezer, A.E.; Bishop, A.H.; Hadgraft, J.; Labetoulle, C.; Bowler, P.G. Antimicrobial properties of silver-containing wound dressings: A microcalorimetric study. Int. J. Pharm. 2003, 263, 61–68. [Google Scholar] [CrossRef]
- Kanematsu, H.; Ikigai, H.; Yoshitake, M. Evaluation of various metallic coatings on steel to mitigate biofilm formation. Int. J. Mol. Sci. 2009, 10, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Mc Carthy, T.J.; Zeelie, J.J.; Krause, D.J. The antimicrobial action of zinc ion/antioxidant combinations. J. Clin. Pharm. Ther. 1992, 17, 51–54. [Google Scholar] [CrossRef]
- Yuvaraj, A.L.; Santhanaraj, D. A systematic study on electrolytic production of hydrogen gas by using graphite as electrode. Mat. Res. 2014, 17. [Google Scholar] [CrossRef]
- Ramanarayanan, T.A.; Smith, S.N. Corrosion of iron in gaseous environments and in gas-saturated aqueous environments. Corrosion 1990, 46, 66–74. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and Recent Advanced Strategies for Combating Biofilms. Compr. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric effect and bacterial biofilms: A Systematic review. Int. J. Artif. Organs 2008, 31, 786–795. [Google Scholar] [PubMed]
- Bierre, E.; Fullerton, R. Hydrogen sulphide removal from geothermal power station cooling water using a biofilm reactor. In Proceedings of the World Geothermal Congress 2015, Melbourne, Australia, 19–25 April 2015.
- Santek, B.; Ivancic, M.; Horvat, P.; Novak, S.; Maric, V. Horizontal tubular bioreactors in biotechnology. Chem. Biochem. Eng. Q. 2006, 20, 389–399. [Google Scholar]
- Denac, M.A.; Dunn, I.J. Packed-and fluidized-bed biofilm reactor performance for anaerobic wastewater treatment. Biotechnol. Bioeng. 1988, 32, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prabhu, A.A.; Dasu, V. Venkata, and pakshirajan, kannan, l-asparaginase from Pectobacterium carotovorum MTCC 1428. Appl. Biochem. Biotechnol. 2011, 163, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production-A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Brindle, K.; Stephenson, T. The application of membrane biological reactors for the treatment of wastewaters. Biotechnol. Bioeng. 1996, 49, 601–610. [Google Scholar] [CrossRef]
- Rosenberger, S.; Kruger, U.; Witzig, R.; Manz, W.; Szewzyk, U.; Kraume, M. Perfomance of a bioreactor with submerged membranes for aerobic treatment of a municipal waste water. Water Res. 2002, 36, 413–420. [Google Scholar] [CrossRef]
- Chan, Y.J.; Chong, M.F.; Law, C.L.; Hassell, D.G. A review on anaerobic-aerobic treatment of industrial and municipal wastewater. J. Chem. Eng. 2009, 155, 1–18. [Google Scholar] [CrossRef]
- Kanematsu, H.; Hirai, N.; Miura, Y.; Tanaka, M.; Kogo, T.; Itho, H. Various metals from water by biofilm from ambient germs in a reaction container. In Proceedings of the 2013 Conference & Exhibition Materials Science & Technology (MS & T), Montreal, QC, Canada, 27–31 October 2013; pp. 2154–2161.
- Barry, D.M.; Kanematsu, H.; McGrath, P.B. Artificial Biofilm on the Laboratory Scale. In Biofilm and Materials Science; Kanematsu, H., Barry, D.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 181–185. [Google Scholar]
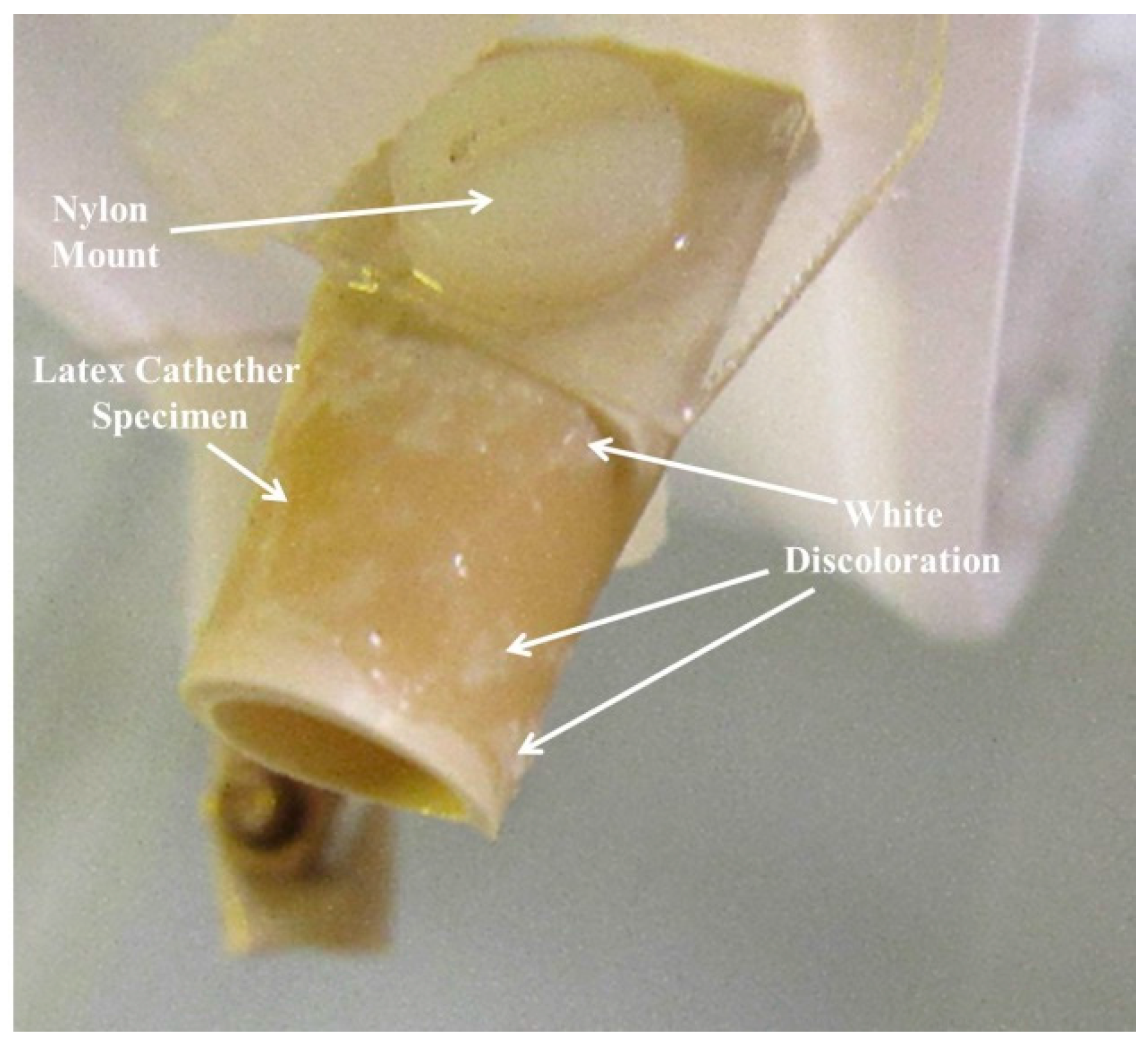
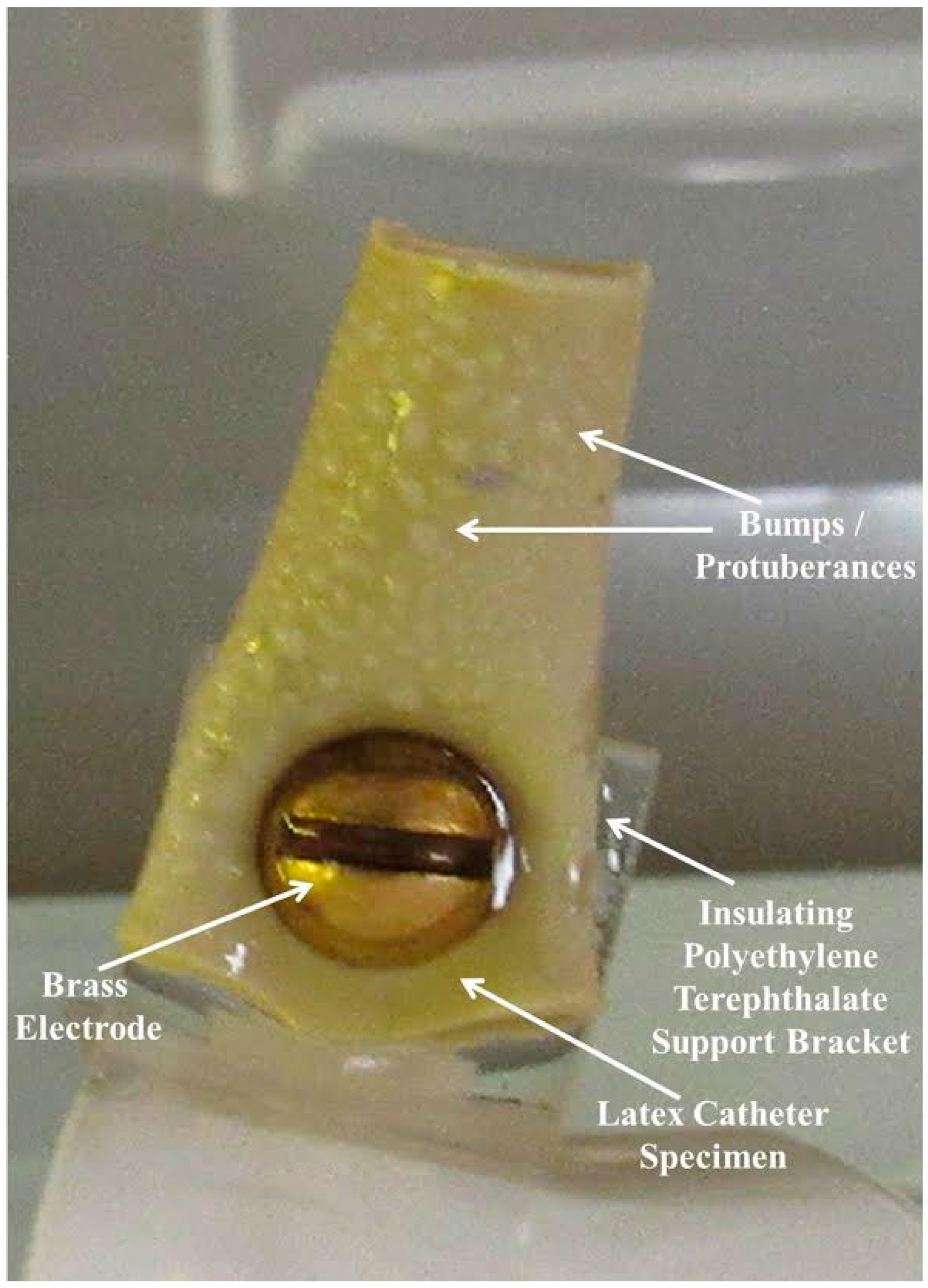

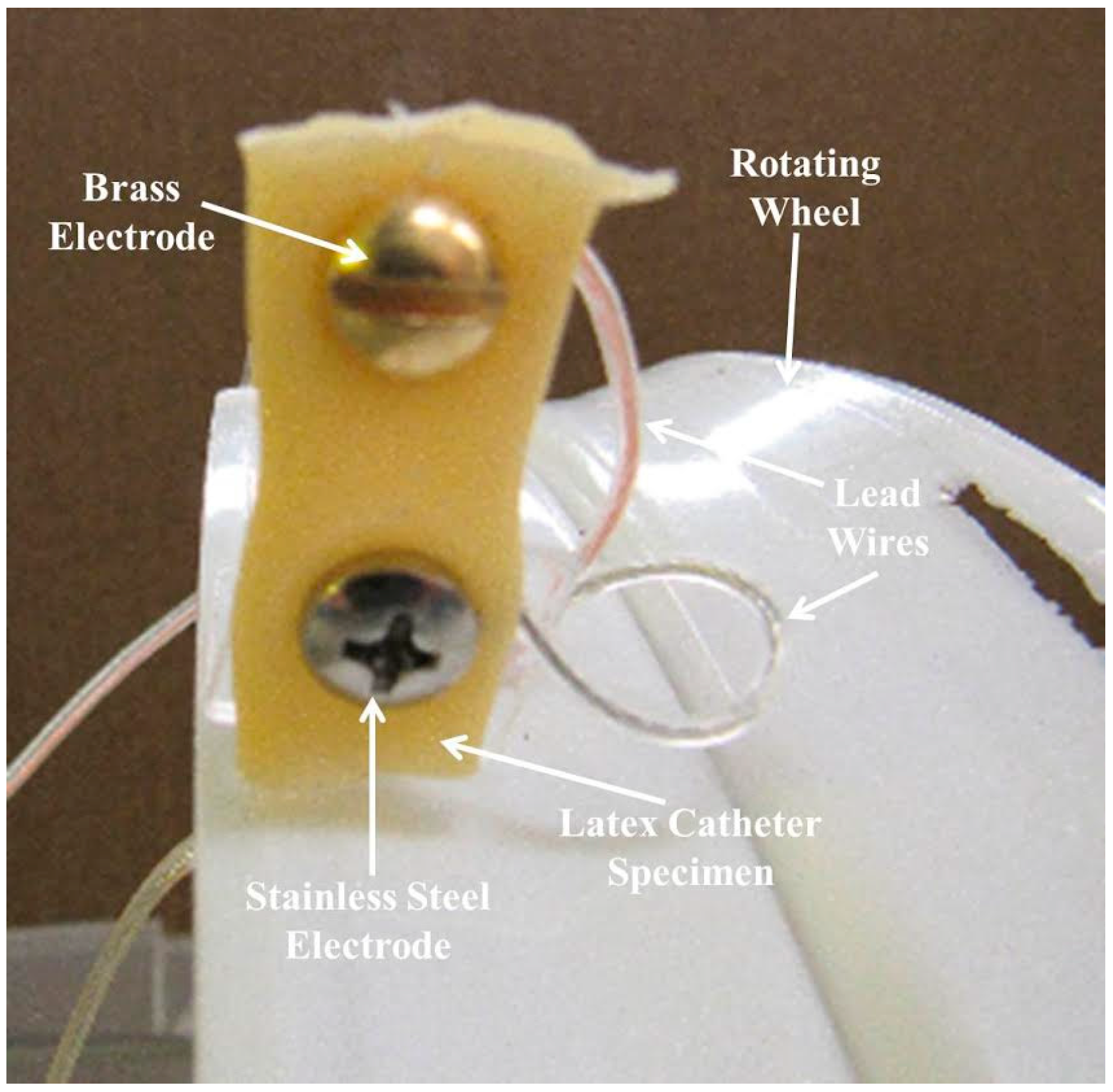


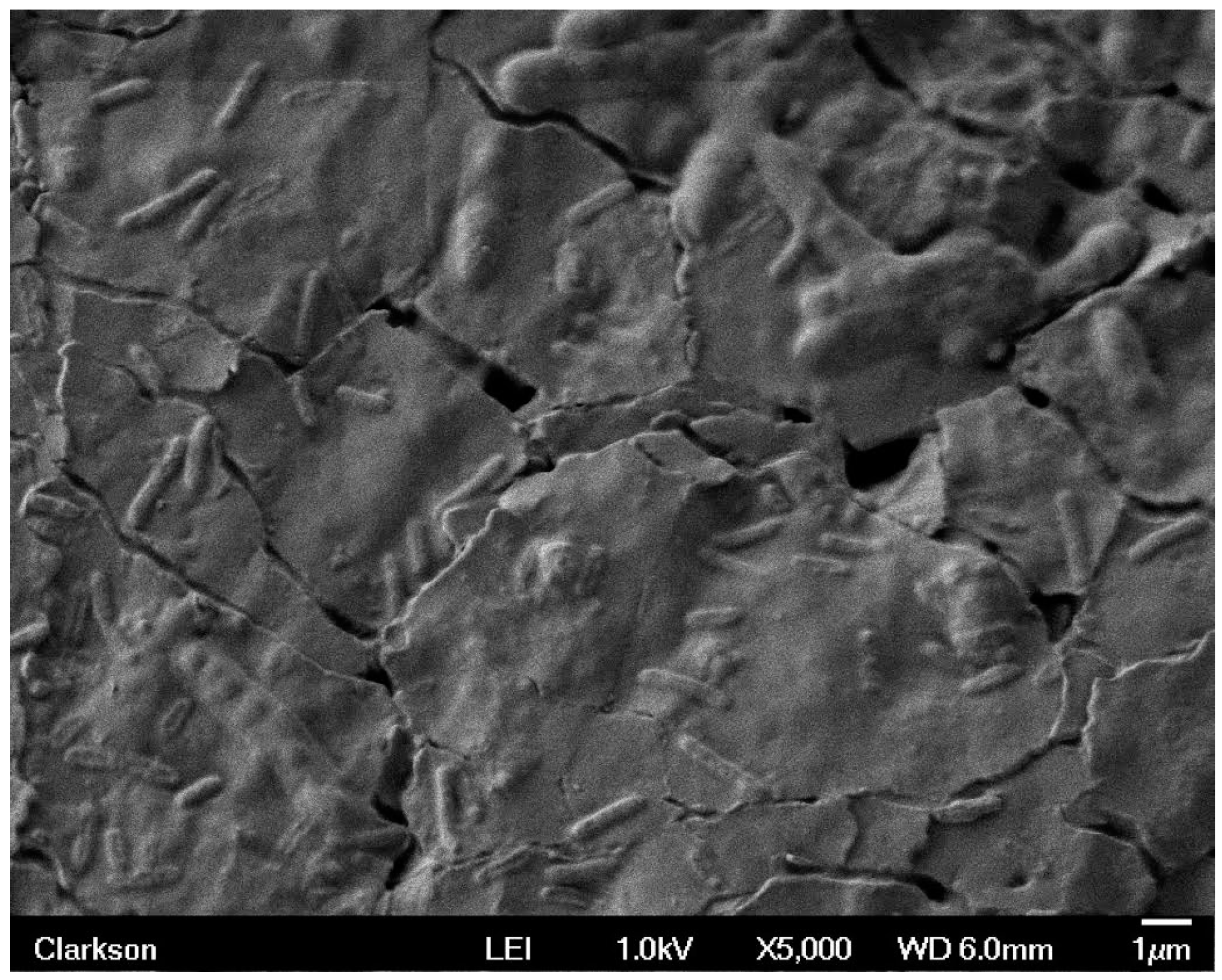
| Sample | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| 0 Control: Latex with nylon Mounting | Hydrophobic, Whitish-yellow discoloration near nylon bolt. | 90% discolored and wetted on front and 25% on back with small protuberances. | 100% wetted. 30% Coverage of small protuberances on front & some on back. | Similar to Week 3. |
| 1 Latex with silver | Hydrophobic, No discoloration near bolt head. | 50% discoloration on front with a few protuberances and 60% discoloration on back. | Hydrophilic, 60% Medium-sized bumps on front & 30% on back. | 80% medium sized protuberances (bumps) on front and 80% on back. |
| 2 Latex with stainless steel | Hydrophobic, discoloration near bolt head. | 30% discoloration near metal on front and 15% discoloration on back. | Front hydrophilic. 80% large bumps on front & 20% small on back. | Similar to Week 3. White–yellow and grey discoloration on back. |
| 3 Latex with brass | Hydrophobic with no discoloration near bolt head. | 50% discolor on front near electrode & 50% discoloration on back. | Hydrophilic, 90% large bumps on front & 30% small ones on back. | Similar to Week 3. White–yellow and grey discoloration on back. |
| 4 Latex with stainless steel–brass electrodes | Hydrophobic, discolor near stainless steel electrode & 50% discoloration on back. | 60% discoloration on front near electrodes. 75% discoloration on back. | Hydrophilic with 50% small protuberances on front. | Similar to Week 3. White–yellow and grey discoloration on back and tip. |
| 5 Latex with stainless steel–stainless steel electrodes and voltage applied | Hydrophobic, clear between electrodes, & 80% discolor on back. | 50% discoloration on front, mostly around electrodes and 95% discoloration on back. | Hydrophilic with 80% small protuberances on front. | Similar to Week 3. White–yellow and grey discoloration on back. |
| 6 Latex with brass–brass electrodes and voltage applied | Hydrophobic, clear between electrodes, & 40% discoloration on back. | 40% discoloration on front and 80% discoloration on back. | Hydrophilic with 30% large protuberances on front and 10% small protuberances on back. | Similar to Week 3. Some additional discoloration on front. |
| 7 Latex with brass positive electrode and stainless steel negative electrode | Hydrophobic. Discolor between electrodes & near brass electrode & 50% discoloration on back. | 80% discoloration on front, particularly around brass electrode. 85% discoloration on back. | Hydrophilic with a few small protuberances on back. | Similar to week 3. Some discoloration on front. |
| 8 Latex with brass negative electrode and stainless steel positive electrode | Hydrophobic, equal discolor near & between electrodes. 70% discoloration on back and near the brass. | 85% discoloration on front and 95% discoloration on back. | Hydrophilic with a few protuberances. | 20% small protuberances on front with some discoloration on front and back between electrodes. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barry, D.M.; McGrath, P.B. Rotation Disk Process to Assess the Influence of Metals and Voltage on the Growth of Biofilm. Materials 2016, 9, 568. https://doi.org/10.3390/ma9070568
Barry DM, McGrath PB. Rotation Disk Process to Assess the Influence of Metals and Voltage on the Growth of Biofilm. Materials. 2016; 9(7):568. https://doi.org/10.3390/ma9070568
Chicago/Turabian StyleBarry, Dana M., and Paul B. McGrath. 2016. "Rotation Disk Process to Assess the Influence of Metals and Voltage on the Growth of Biofilm" Materials 9, no. 7: 568. https://doi.org/10.3390/ma9070568





