1. Introduction
Semiconductor solar cells incorporating thin-film absorbers are an attractive option for replacing classical silicon-based solar cells. In recent years, several thin-film absorbers such as III–V compound material (GaInP/GaAs) [
1,
2,
3], I–III–VI compound material (Cu(In, Ga)Se
2, CIGS) [
4,
5,
6], and I–II–VI compound material (Cu
2ZnSnS
4, CZTS) [
7,
8,
9] have been investigated for semiconductor thin-film solar cells. Among these studied materials, direct band gap chalcopyrite-structured CIGS and kesterite-structured CZTS are potential absorber materials for the next generation of thin-film solar cells because of their large optical absorption coefficients over the visual light range. A record conversion efficiency of 20.3% was reported for a CIGS thin-film solar cell [
4]. However, use of toxic Se and scarce or expensive elements, such as gallium (Ga) and indium (In), may limit the production development of CIGS thin-film solar cells. Researchers strongly desire to identify a nontoxic inexpensive thin-film absorber with high conversion efficiency to replace CIGS. CZTS has been considered as an alternative to CIGS because of its majority carrier type (p-type), direct band gap of 1.4–1.5 eV, and large optical absorption coefficient (>10
4 cm
−1) over the visible light range [
10]. In addition, CZTS thin films are composed of elements that are nontoxic, abundant, and inexpensive, namely copper, zinc, tin, and sulfur.
CZTS thin films are generally fabricated using vacuum-based techniques such as thermal evaporation [
11], sputtering [
12], and pulsed laser deposition [
13]. These methods can deposit high-quality CZTS thin films on molybdenum (Mo)-coated glass substrates. However, these techniques require complicated equipment to maintain a vacuum and high process temperatures. In addition to high conversion efficiency, film content and composition as well as large-scale rather than small-area cell structural uniformity are challenges in developing CZTS thin-film solar cells for industrial use. Many nonvacuum methods of reducing production costs and satisfying the large-area requirements have been studied, including electrodeposition [
14], spray pyrolysis techniques [
15], sol-gel processing [
16], spin-coating methods [
17], and chemical bath methods [
18]. Among these, the spin-coating method has the advantages of simple construction, low cost, ecological safety, and the large-scale deposition of different semiconductor thin films. However, few studies have reported the preparation of spin-coated CZTS thin films without toxic precursors such as hydrazine [
19] or ethanolamine (MEA) [
17]. Researchers do not completely and clearly understand the relationships of the deposition parameters to the atomic contents of Cu, Zn, Sn, and S, surface morphology, and electrical properties of spin-coated CZTS thin films.
In this study, we developed spin-coating Cu-poor and Zn-rich CZTS thin films with optimal atomic contents of Cu, Zn, Sn, and S onto glass and silicon (Si) substrates. The atomic contents of Cu, Zn, Sn, and S, crystalline morphology, and surface morphology of these CZTS thin films were characterized using energy-dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) patterns, field-emission scanning electron microscopy (FE-SEM) images, and Raman measurements. The optical band gap and electrical properties were measured using absorption spectra and Hall measurements. Finally, an optimal CZTS thin was spun on an n-type Si substrate to form a Mo/p-CZTS/n-Si/Al solar cell.
2. Results and Discussion
The crystallinity, morphology, electrical properties, and band gap energy of CZTS thin films show a strong dependence on the stoichiometry of Cu, Zn, Sn, and S in those CZTS thin films [
9,
16,
20,
21,
22]. Well-controlled atomic contents of Cu, Zn, Sn, and S in CZTS thin films has become a crucial issue for fabricating high-performance kesterite CZTS solar cells. To determine the optimal atomic contents of Cu, Zn, Sn, and S in CZTS thin films, the chemical concentrations of CuCl
2, ZnCl
2, SnCl
2, and thiourea aqueous precursor solution should be analyzed.
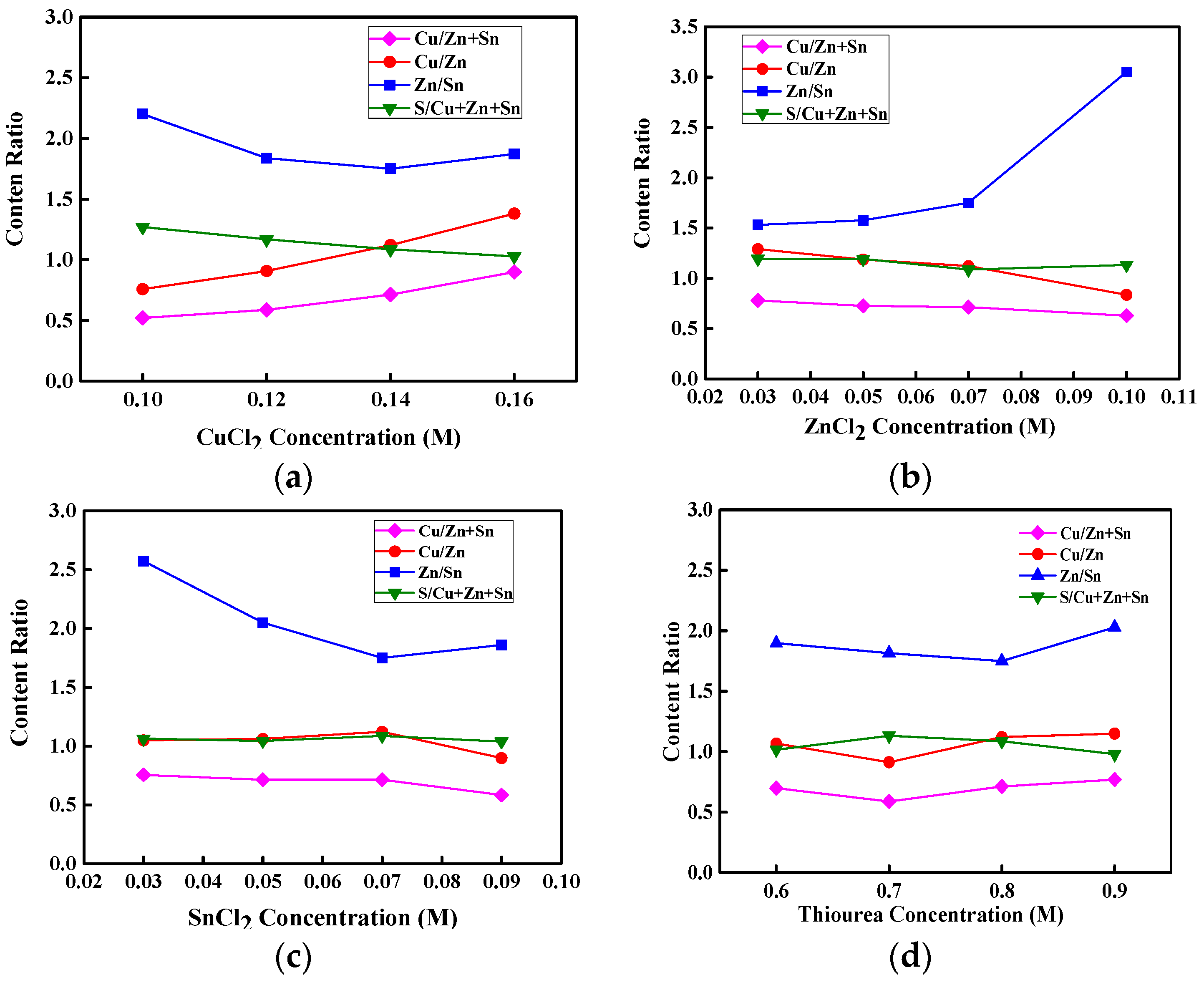
Figure 1a–d show the atomic content ratios of Cu/(Zn + Sn), Cu/Zn, Zn/Sn, and S/(Cu + Zn + Sn) for CZTS thin films as functions of the concentrations of CuCl
2, ZnCl
2, SnCl
2, and thiourea aqueous precursor solution, respectively, for an annealing temperature of 350 °C. As shown in
Figure 1a–c, the atomic contents of Cu, Zn, and Sn increase with raising the concentration of CuCl
2, ZnCl
2, and SnCl
2 aqueous precursor solution, respectively, implying that the atomic contents of Cu, Zn, Sn, and S in CZTS thin films can be modulated by changing the concentrations of aqueous precursor solution. Cu-rich CZTS thin films can facilitate the formation of passivated defect clusters such as Cu
Zn + Sn
Zn and 2Cu
Zn + Sn
Zn. The former produces a deep donor level in the band gap of CZTS, whereas the latter can significantly reduce the band gap of CZTS [
20]. However, a high Zn/Sn ratio or high Zn atomic content is necessary to form the Zn
Sn acceptors and reduce the Sn
Zn donors to increase the conductivity of the CZTS thin film [
23]. However, high Zn atomic content, which results in a low Cu/Zn ratio, can bring about a p/n conduction type [
22]. To avoid defect clusters and increase the conductivity of p-type CZTS thin films, the Cu/(Zn + Sn) atomic content ratio should be less than 1, the Zn/Sn atomic content ratio should be more than 1, and the Cu/Zn atomic content ratio should not be too low. In
Figure 1a, the atomic content ratio of Cu/(Zn + Sn) is lower than 1 for the CuCl
2 concentration below 0.14 M and approaches 1 at the CuCl
2 concentration of 0.16 M. When the ZnCl
2, SnCl
2, and thiourea concentrations increase with a CuCl
2 concentration of 0.14 M, the Cu/(Zn + Sn) atomic content ratio remains below 1, as seen in
Figure 1b–d. These results indicate that Cu-poor and Zn-rich CZTS thin films can be produced from properly controlled concentrations of aqueous precursor solutions. The atomic content ratio of S/Cu + Zn + Sn in CZTS thin films can approach 1 for the concentrations of CuCl
2, ZnCl
2, and SnCl
2, as shown in
Figure 1a–c, respectively. In addition, in
Figure 1d, the atomic content of S increases slightly as the thiourea concentration rises from 0.6 to 0.8 M, and saturates at a thiourea concentration above 0.8 M, possibly because of the solubility of S in the CZT thin film.
Figure 2a–c represent the XRD patterns for films with various concentrations of CuCl
2, ZnCl
2, and SnCl
2 aqueous precursor solutions that were annealed at a temperature of 350 °C. The XRD peaks were indexed using JADE software (Jade 5.0, Christchurch, New Zealand) as the CZTS kesterite structure; and they closely matched the standard data (PDF#26-5075). All of the spin-coated CZTS thin films exhibited a polycrystalline kesterite crystal structure with six broad peaks along the (101), (112), (200), (220), (312) and (224) planes as shown in
Figure 2a–c. The secondary phases including Cu
2SnS
3, CuS, SnS and ZnS would form during growth of CZTS thin films. They will degrade the performance of CZTS solar cells. It is important to avoid the formation of secondary phases in CZTS thin films. Among these secondary phases, tetragonal-structured Cu
2SnS
3 (PDF#33-0501) and cubic-structured ZnS (PDF#05-0566) show the similar XRD patterns to kesterite-structured CZTS between diffraction angles of 20 and 80 degrees because of the same lattice constants [
15]. As a result, the measured XRD patterns of CZTS thin film might be related to either CZTS or Cu
2SnS
3 or ZnS pahse. The weak peak at 18.2° attributed to (101) plane indicates a kesterite-structured CZTS thin film because it is not found in the XRD patterns of tetragonal-structured Cu
2SnS
3 and cubic-structured ZnS. Consequently, a CZTS thin film without secondary phase, which leads to a degraded performance of CZTS solar cells, can be obtained using properly controlled concentrations of aqueous precursor solutions and a proper annealing process. To further confirm the XRD characteristics of this spin-coated CZTS thin film, which differ from similar XRD patterns such as those of ZnS and Cu
2SnS
3, Raman spectroscopy was performed.
Figure 2e shows the Raman spectrum for a spin-coated CZTS thin film prepared from 0.14 M CuCl
2, 0.07 M ZnCl
2, 0.07 M SnCl
2, and 0.8 M thiourea aqueous precursor solutions and annealed at 350 °C. The characteristic CZTS peak in
Figure 2e is at 336.2 cm
−1, which is different from the Raman peaks of Cu
2–xS at 475 cm
−1, ZnS at 273 and 351 cm
−1, Cu
2SnS
3 at 290 and 352 cm
−1, and of Sn
2S
3 at 234 and 307 cm
−1, indicating a spin-coated CZTS thin film deposited on glass substrate [
24,
25].
The mean crystallite grain size (D) of CZTS thin film can be estimated by the Scherrer formula [
26]:
where β, λ, and θ
B are the line width at full width at half the maximum of the film diffraction peak at 2θ, X-ray wavelength (0.15406 nm), and Bragg diffraction angle, respectively.
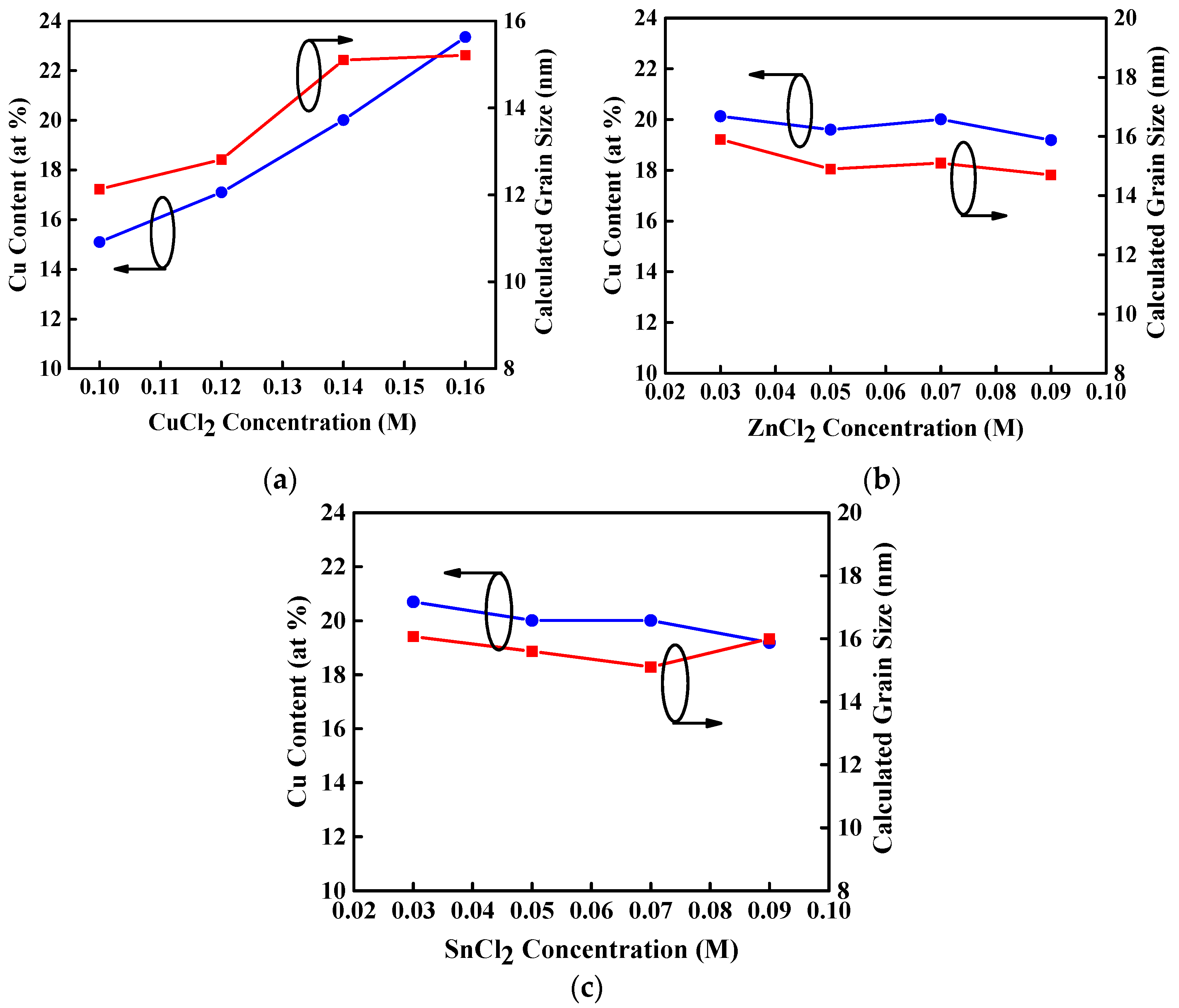
Figure 3a–c show the full width at half maximum (FWHM) of (112) peak and calculated mean crystallite grain size of CZTS thin films as a function of the varied concentration of CuCl
2, ZnCl
2, and SnCl
2 aqueous precursor solutions that were annealed at a temperature of 350 °C. The grain size enlarges with increasing CuCl
2 concentration, as shown in
Figure 3a because of the increase of Cu atomic content in the CZTS thin films [
27,
28]. This increase in the grain size might be due to agglomeration of grains. In addition, the grain size of CZTS thin films represents a slight change as ZnCl
2, and SnCl
2 concentrations increase or decrease while CuCl
2 concentration is maintained at 0.14 M, as shown in
Figure 3b,c. This is attributed to the fairly constant Cu atomic content in these CZTS thin films. Consequently, the grain size of CZTS thin films with the same annealing temperature is determined by the Cu atomic content.
The conversion efficiency of a CZTS thin-film solar cell is strongly associated with the grain size because an absorber layer with a large grain size maximizes the minority carrier diffusion length and the inherent potential of a polycrystalline solar cell [
16,
24].
Figure 4 shows the FE–SEM images of the CZTS thin film.
Figure 4a–c show plane views of CZTS thin films with CuCl
2 concentrations of 0.1, 0.14, and 0.16 M with concentrations of ZnCl
2, SnCl
2, and thiourea at 0.07, 0.07, and 0.8 M, respectively. The thickness of all the CZTS thin films was approximately 2.4 μm, and
Figure 4d represents the FE-SEM cross section image of CZTS thin film with concentrations of CuCl
2, ZnCl
2, SnCl
2, and thiourea at 0.14, 0.07, 0.07, and 0.8 M. As shown in
Figure 4a–c, large numbers of voids were observed in the Cu-rich CZTS thin films. Voids of Cu-rich CZTS absorbers in thin-film solar cells cause low conversion efficiencies for photovoltaic applications [
16,
29]. Well-controlled Cu atomic content is important to obtain a large grain size and less number of voids in CZTS thin films.
Figure 4e shows the plane view of a CZTS thin film with a high ZnCl
2 concentration of 0.1 M and CuCl
2, SnCl
2, and thiourea concentrations of 0.14, 0.07, and 0.8 M. The surface of Zn-rich CZTS thin film is smoother than that of Zn-poor CZTS thin films because of the large columnar grains at the bottom [
30].
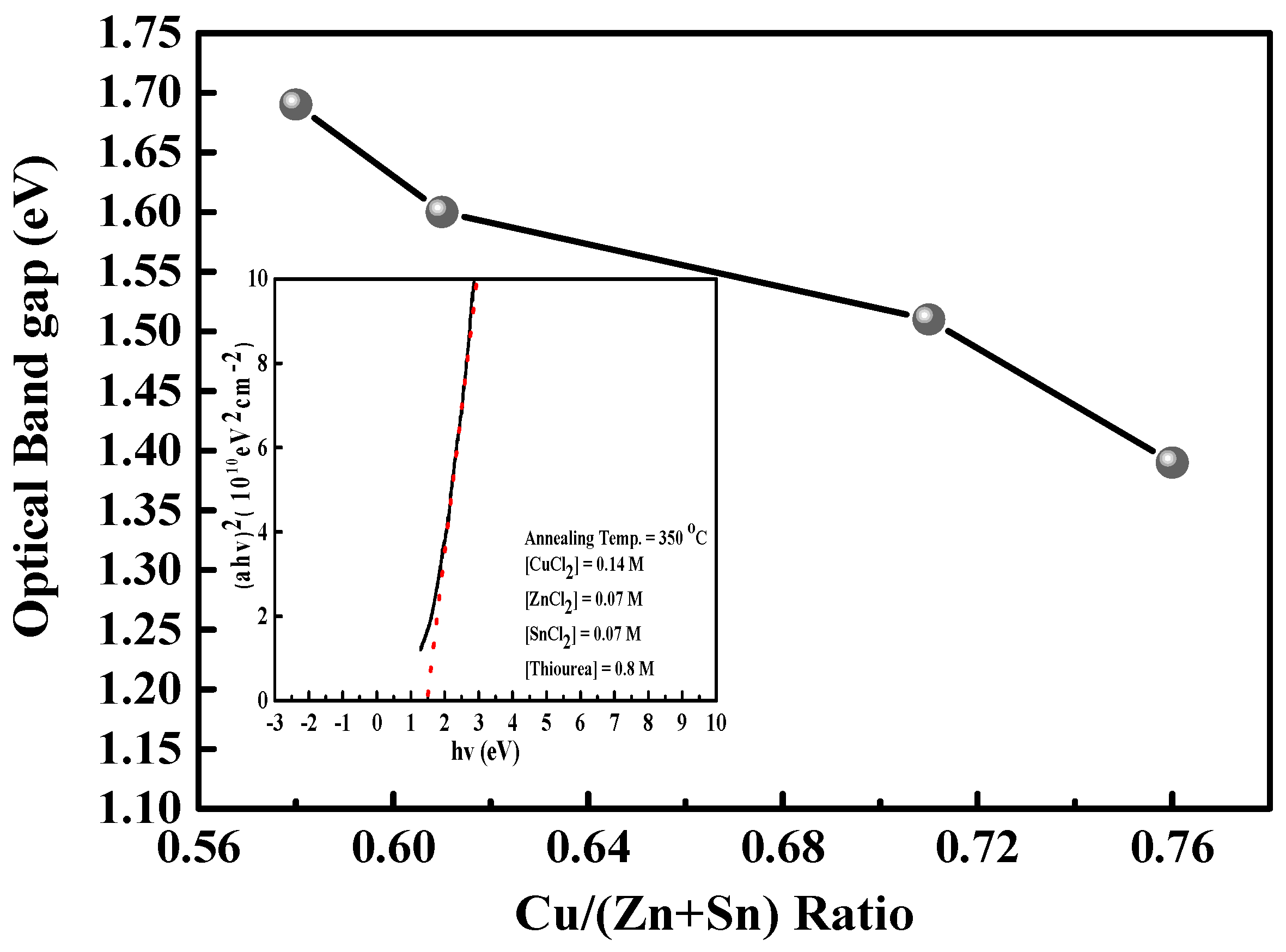
Figure 5 shows the graph of optical band gap energy as a function of the Cu/(Zn + Sn) atomic content ratio. The optical band gaps of these CZTS thin films range from 1.39 to 1.69, whereas the Cu/(Zn + Sn) atomic content ratios range from 0.58 to 0.76. This shift is attributed to the change in the p-d hybridization between Cu d-levels and S p-levels [
16]. In addition, the relation of the extended optical band gap to the decrease in grain size is attributed to the fact that the band gap energy of a material is dependent on the particle size of that material according to the quantum size effect [
15]. As shown in
Figure 3a–c, the grain size of a Cu-poor CZTS thin film is smaller than that of a Cu-rich CZTS thin film, resulting in a wide optical band gap. The inset of
Figure 5 shows a plot of (αhν)
2 against the photon energy of a Cu-poor and Zn-rich (Cu/Zn = 1.12) CZTS thin film where α is the absorption coefficient and hν is the photon energy. The optical band gap energy is estimated by extrapolating the linear region in the (αhν)
2 plot and determining the intercept with the photon energy axis. The optical band gap for an optimal CZTS absorber is approximately 1.5 eV.
Figure 6a,b show the carrier concentration and resistivity of CZTS thin films as a function of CuCl
2 and ZnCl
2 concentration, respectively. The carrier concentration shown in
Figure 6a increases with CuCl
2 concentration. The formation of a Cu
Zn + Sn
Zn deep level depends on the Cu/(Zn + Sn) and Zn/Sn atomic content ratio, and an optimal condition occurs at Cu/(Zn + Sn) ≈ 0.8 and Zn/Sn ≈ 1.2 [
20]. As the CuCl
2 concentration increases from 0.1 to 0.14 M, the Cu/(Zn + Sn) atomic content ratio approaches the optimal value because of the suppression of the formation of a Cu
Zn + Sn
Zn deep donor level in the CZTS band gap, leading to a significant increase in hole concentration. Nevertheless, the Cu/(Zn + Sn) atomic content ratio increases with CuCl
2 concentration to 1.6 M, causing the generation of a Cu
Zn + Sn
Zn deep donor defect and a reduction of the Cu
Zn antisite acceptor defects, resulting in a decrease of carrier concentration. Under the optimal Cu/(Zn + Sn) atomic content ratio of 0.714, the CZTS thin film has a high carrier concentration of 1.07 × 10
19 cm
−3 and a resistivity of 1.19 Ωcm. The atomic content ratio of Zn/Sn is also a crucial factor determining the electrical properties of a CZTS thin film. The Zn
Sn antisite defect with an inherent p-type conduction type in a CZTS thin film can easily form under a high Zn/Sn atomic content ratio. However, further increases in Zn/Sn atomic content ratio would generate a large population of intrinsic defects, causing a detrimental influence relative to a CZTS thin film with a atomic content ratio of Cu/(Zn + Sn) = 1 and Zn/Sn = 1. In
Figure 6b, the hole concentration decreases as the ZnCl
2 concentration rises from 0.7 to 0.9 M because of the population of donor defect clusters [
20]. Further increases in ZnCl
2 concentration would move the Cu/(Zn + Sn) atomic content ratio away from 0.8 (see
Figure 1b), leading to a translation of conduction types.
The sulfer vacancy (V
S) is also a dominant donor-like defect in a CZTS thin film because of its low formation energy. The conduction type would change from p-type to n-type and the hole concentration might decrease in an S-poor CZTS thin film due to a high concentration of V
S. High S content or S-rich in CZTS thin film is crucial to reduce the concentration of V
S. Kosyak et al. [
31] have calculated the concentration of Zn
Sn antisite and V
S in CZTS thin films with different Cu, Zn, and Sn content, and represented that the concentration of acceptor-like Zn
Sn antisite is higher than that of donor-like V
S in a Zn-rich and Sn-poor CZTS thin film. Based on this result, we measure the electrical properties of CZTS thin films with various thiourea concentration under optimal Cu/(Zn + Sn) atomic content ratio of 0.714.
Table 1 lists the measured results of S/(Cu + Zn + Sn) atomic content ratio and electrical characteristics of CZTS thin films depending on various thiourea concentration. The S/(Cu + Zn + Sn) atomic content ratio increases with rising thiourea concentration, implying that the a high S content or S-rich CZTS thin film can be obtained by changing the concentration thiourea aqueous solution. As thiourea concentration rises from 0.6 to 0.8 M, the hole concentration and conductivity of the p-type CZTS thin film increase because of reduction of V
S concentration. However, further increase in thiourea concentration would reduce the hole concentration and conductivity possible due to the formation of ZnS [
32].
Table 2 shows the relation between annealing temperature and conduction type, carrier concentration, mobility, and resistivity of CZTS thin films with concentrations of CuCl
2, ZnCl
2, SnCl
2, and thiourea aqueous precursor solution at 0.14, 0.07, 0.07, and 0.8 M, respectively. The hole concentration for annealing temperatures of 300 °C, 350 °C, 400 °C, and 450 °C are 2.02 × 10
14, 1.07 × 10
19, 2.80 × 10
15, and 1.24 × 10
16 cm
−3. The varied carrier concentrations of CZTS thin films with increasing annealing temperatures is attributed to the changes of Cu/(Zn + Sn) and Zn/Sn content ratios, which cause the formation of Cu
Zn + Sn
Zn deep donor defects by reducing the Cu
Zn antisite acceptor defects [
20,
25]. The mobility of CZTS thin films shows a counterclockwise dependence on the carrier concentration because of the impurity scattering. In conclusion, with an annealing temperature of 350 °C, CZTS thin films have a stable p-type conduction type with a high hole concentration of 1.07 × 10
19 cm
−3 and a low resistivity of 1.19 Ωcm. To realize the optimal CZTS absorber for photovoltaic solar cell applications, a CZTS thin film was spin-coated onto an Si substrate to fabricate a Mo/p-CZTS/n-Si/Al heterostructured solar cell.
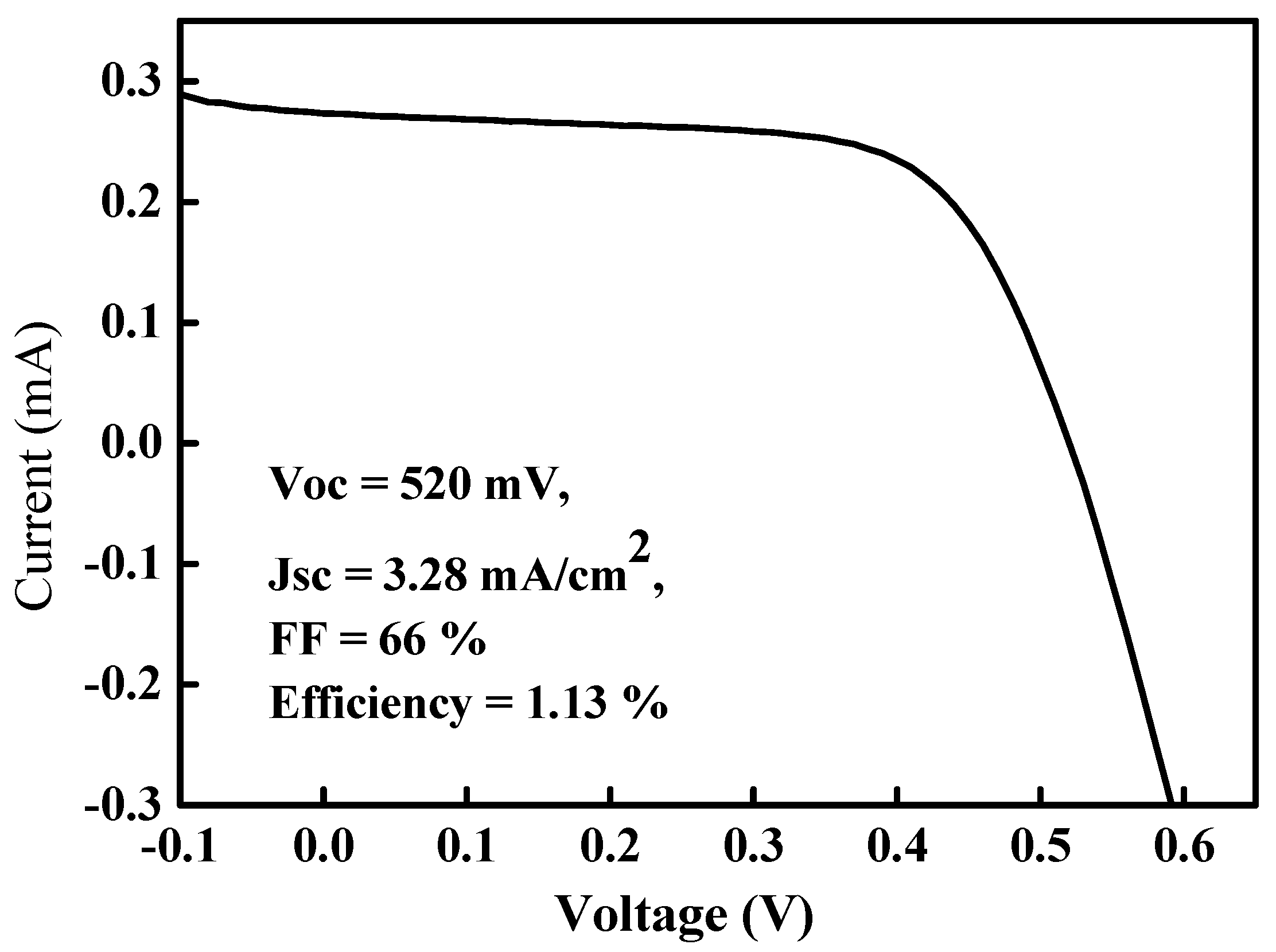
Figure 7 shows the (current-voltage)
I–
V characteristics of this Mo/CZTS/Si/Al solar cell under (air mass) AM 1.5 G. The open circuit voltage, short current density, fill factor, and efficiency were 520 mV, 3.28 mA/cm
2, 66%, and 1.13%, respectively. The Mo/CZTS/Si/Al heterostructured solar cell prepared in this work was simple and the characteristics of the CZTS thin film used as the absorber in this solar cell should be investigated as soon as possible. Compared with the techniques of preparing CZTS thin films reported by other authors, our CZTS thin film preparation method of using spin-coating exhibits superior performance for photovoltaic solar cell applications.