Pulsed Laser Porosification of Silicon Thin Films
Abstract
:1. Introduction
2. Experimental Section
2.1. Laser Porosification Process
2.2. Thin Film Silicon on Stainless Steel Foil Substrates
2.2.1. Electron Beam Evaporated Films
2.2.2. Sputtered Films
2.3. PECVD Films
3. Results and Discussion
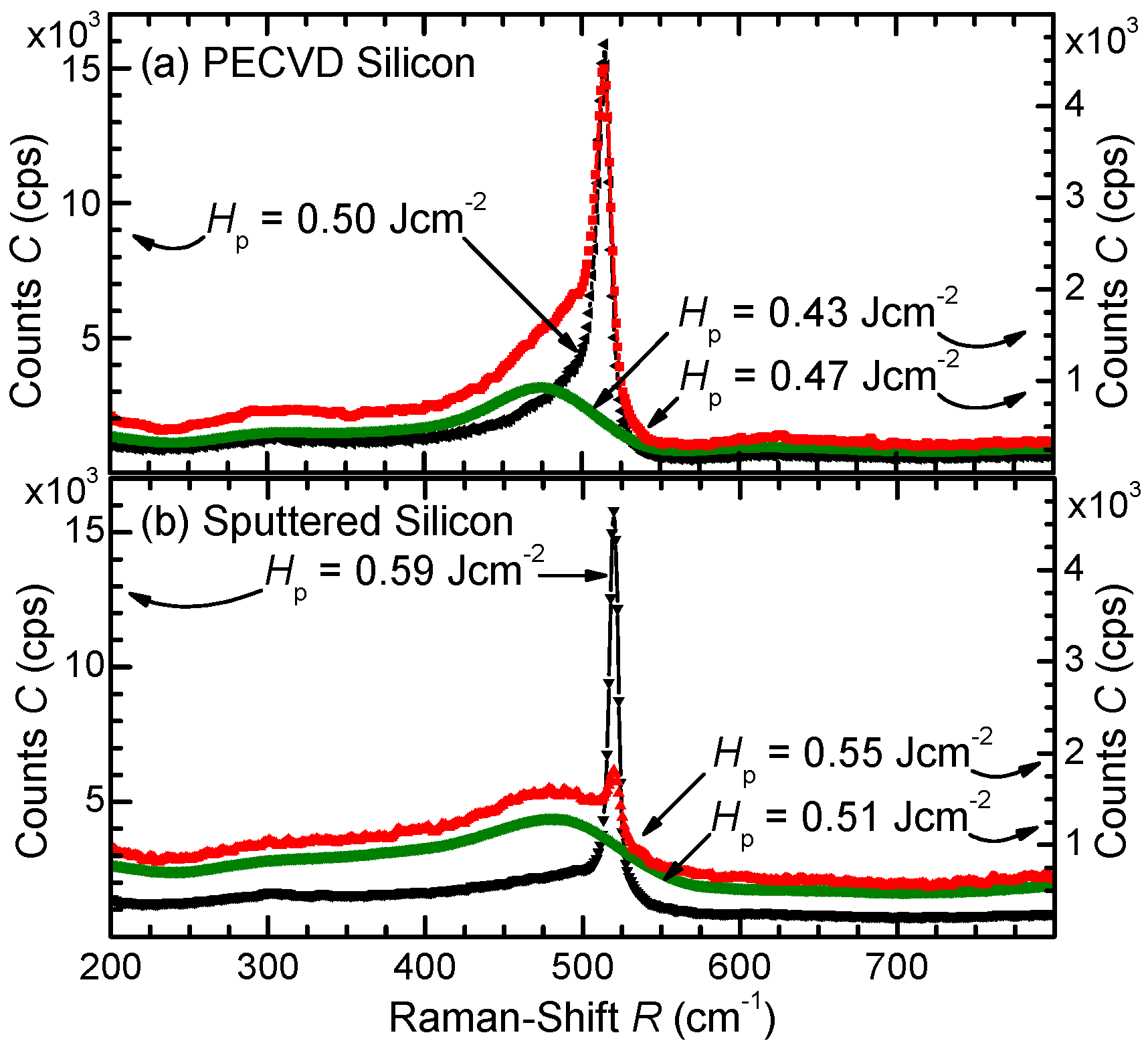
3.1. Raman Spectroscopy
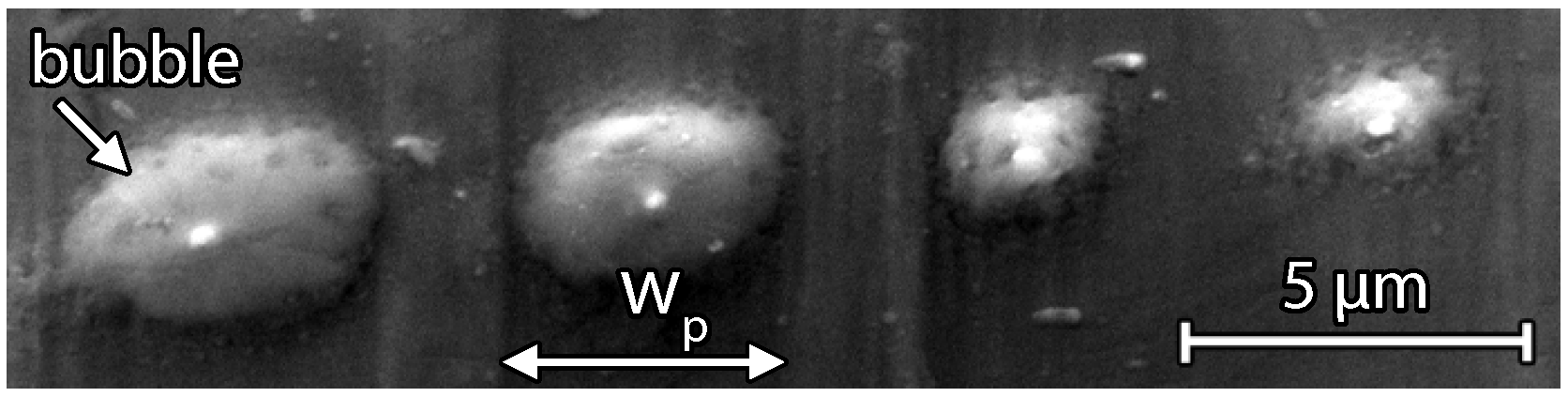
3.2. Scanning Electron Microscopy
3.2.1. Evaporated Silicon
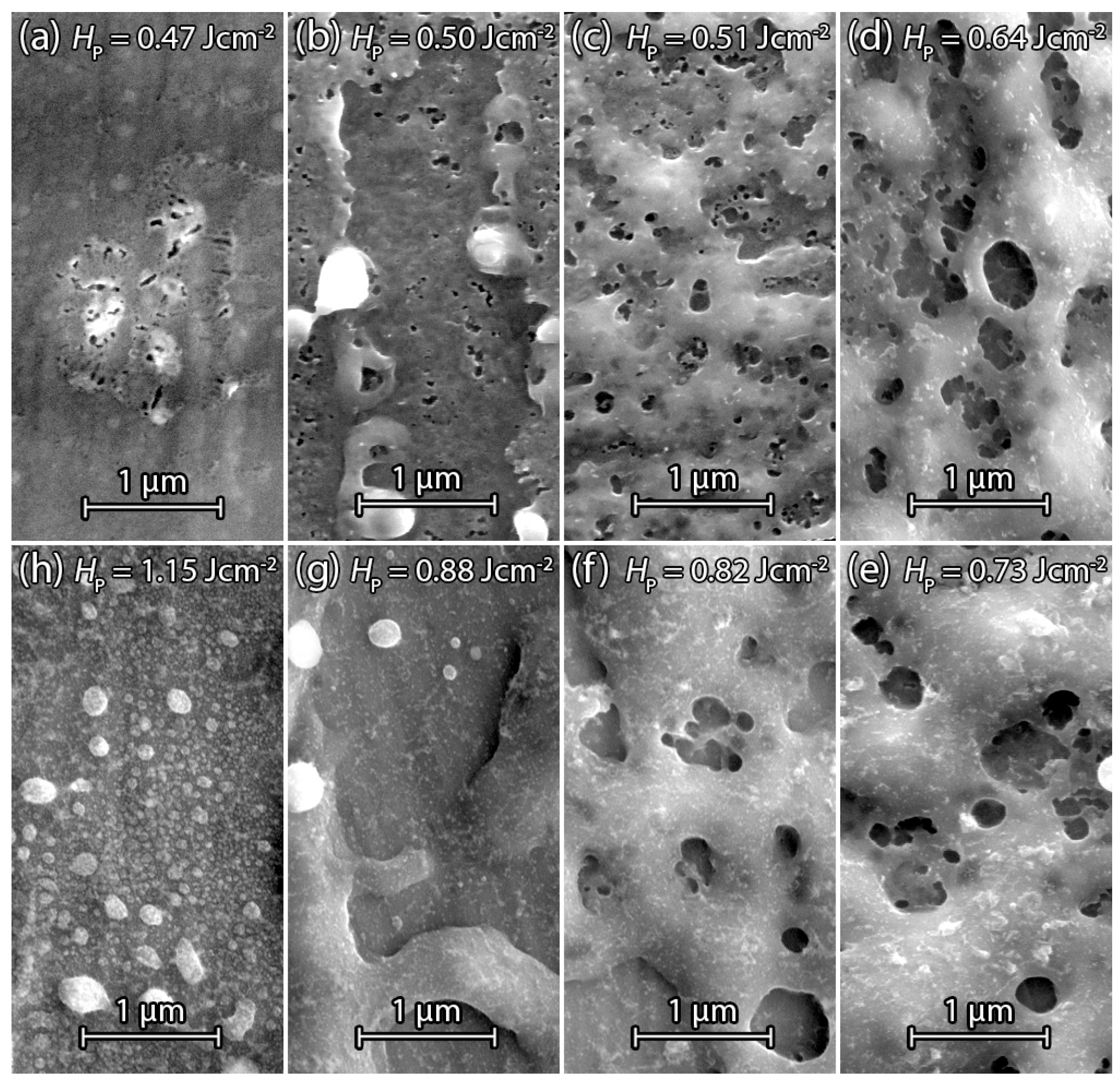
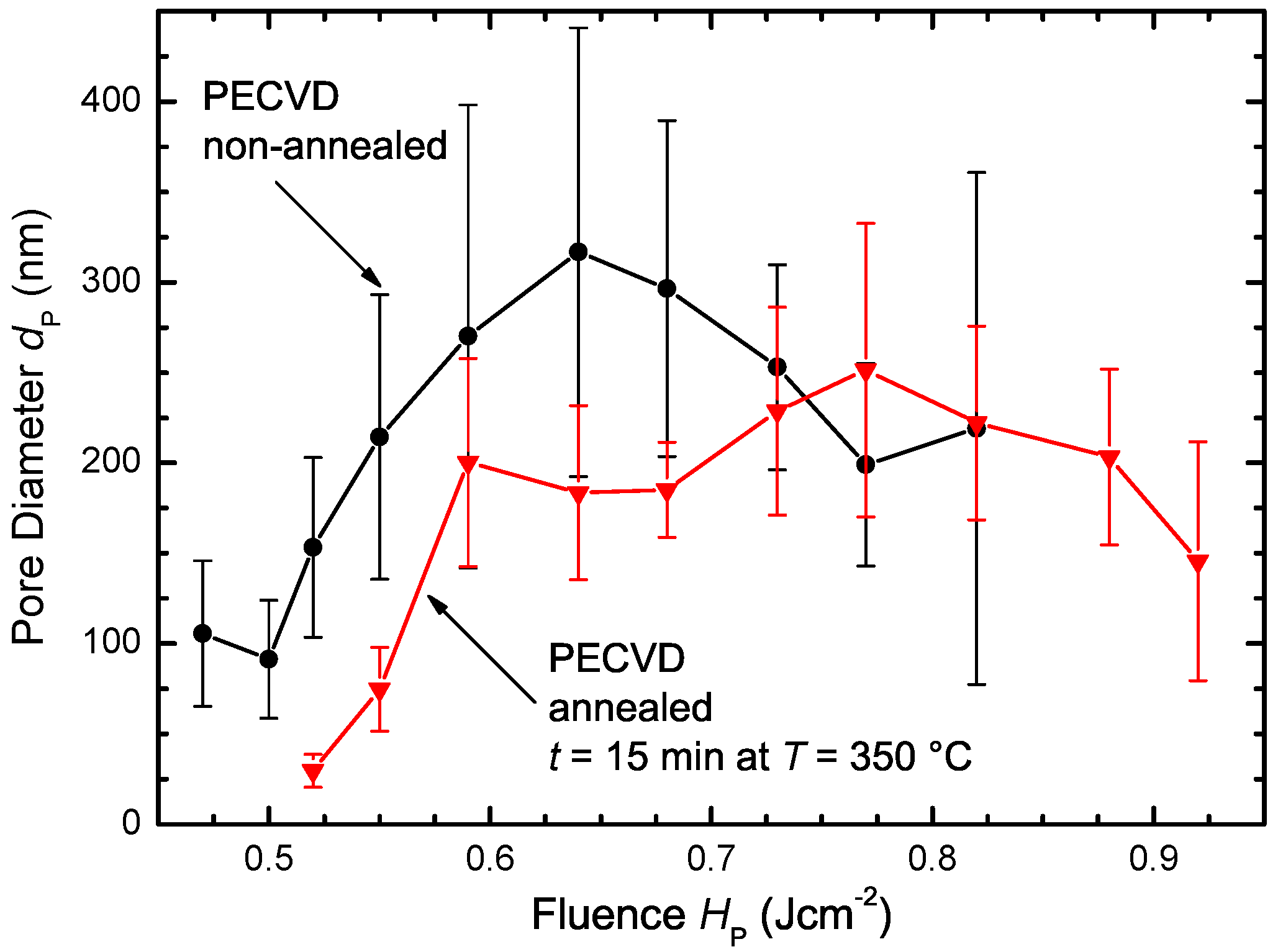
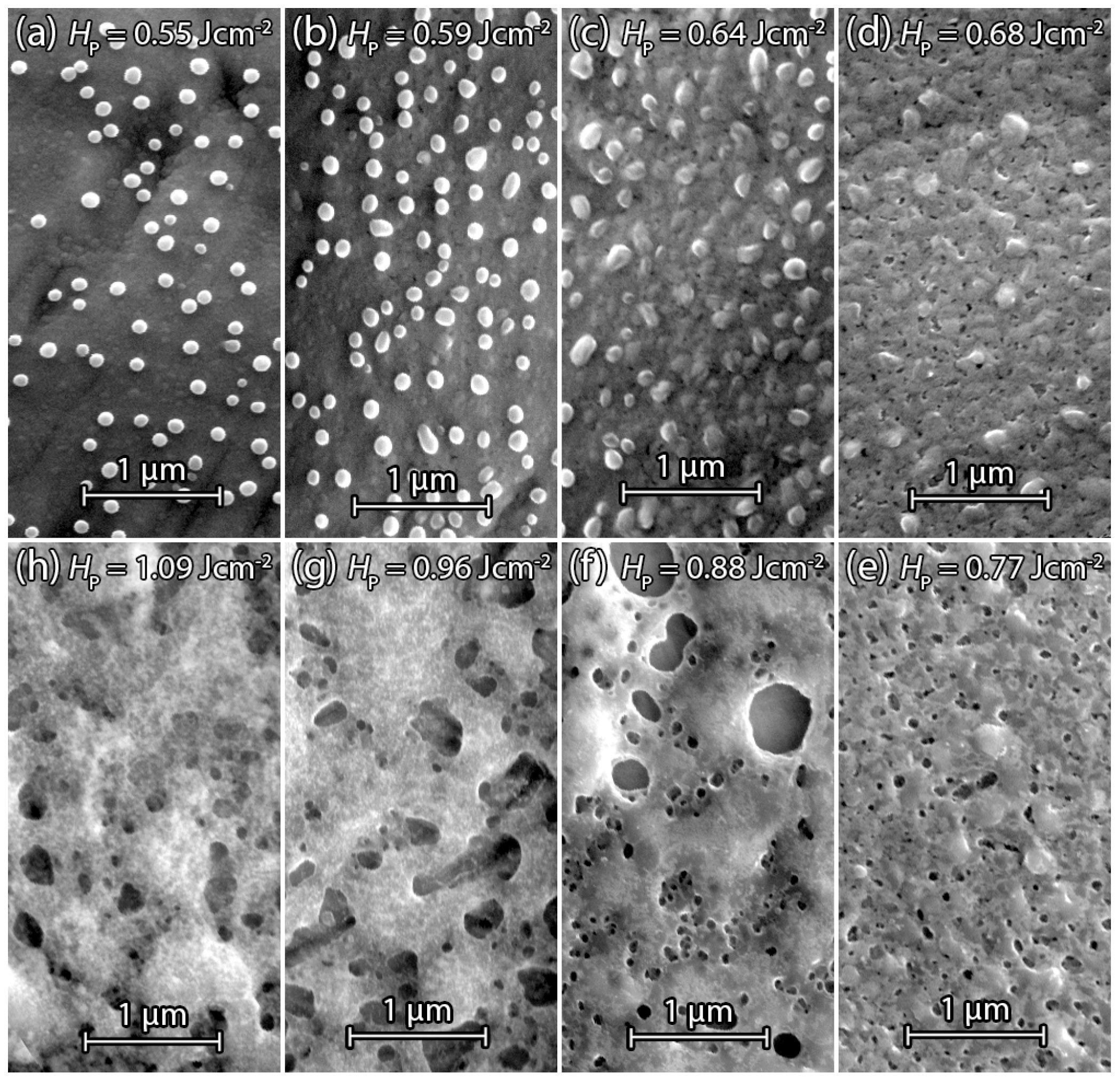
3.2.2. PECVD Silicon
3.2.3. Annealed PECVD Silicon
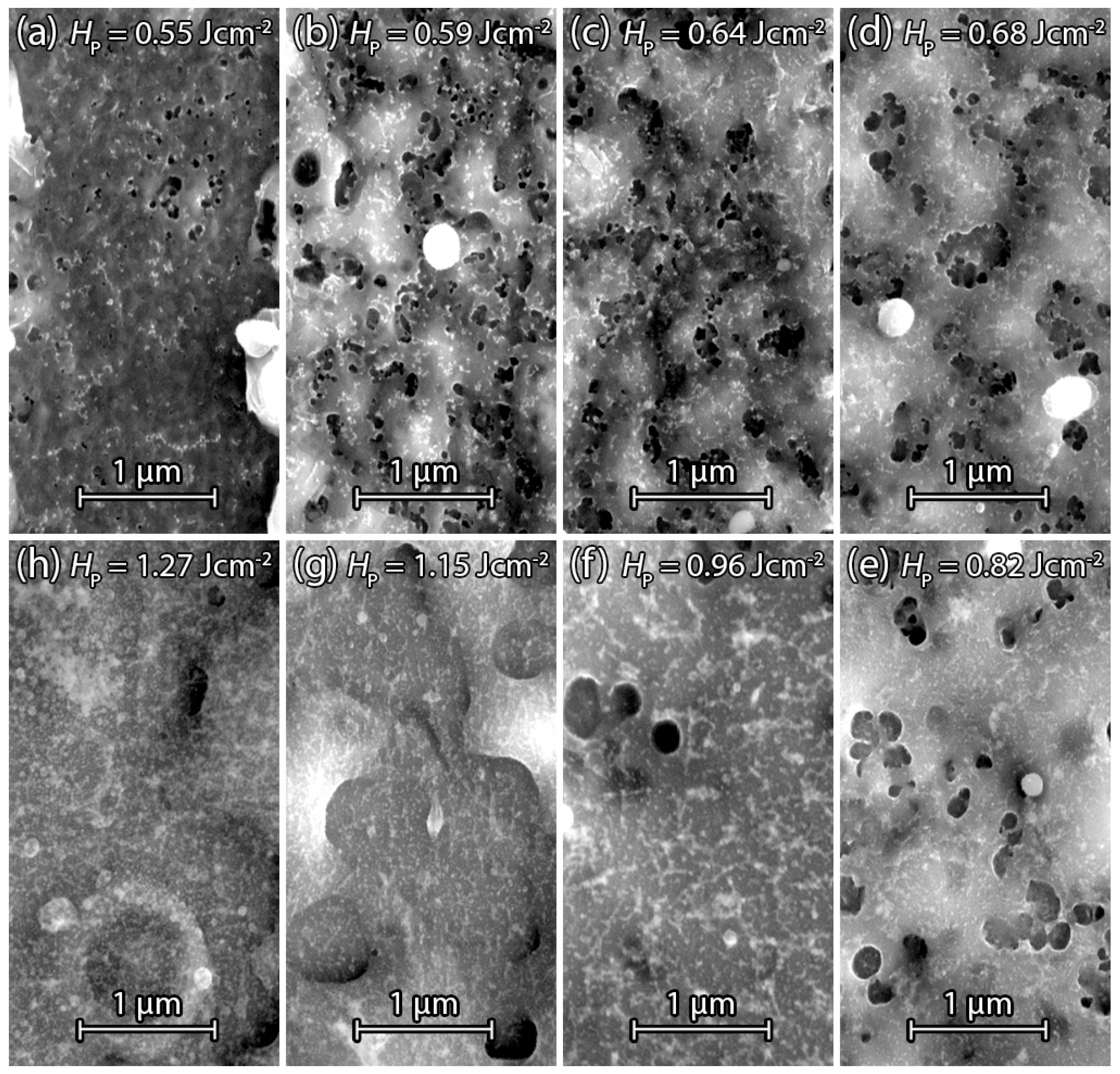
3.2.4. Sputtered Silicon
3.2.5. Annealed Sputtered Silicon
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Föll, H.; Christophersen, M.; Carstensen, J.; Hasse, G. Formation and application of porous silicon. Mat. Sci. Eng. R. 2002, 39, 93–141. [Google Scholar] [CrossRef]
- Schechter, I.; Ben-Chorin, M.; Kux, A. Gas sensing properties of porous silicon. Anal. Chem. 1995, 67, 3727–3732. [Google Scholar] [CrossRef]
- Tang, G.H.; Bi, C.; Fu, B. Thermal conduction in nano-porous silicon thin film. J. Appl. Phys. 2013, 114, 184302. [Google Scholar] [CrossRef]
- McDowell, M.T.; Lee, S.W.; Nix, W.D.; Cui, Y. 25th Anniversary Article: Understanding the Lithiation of Silicon and Other Alloying Anodes for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 4966–4985. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Collins, S.D. Porous silicon formation mechanisms. J. Appl. Phys. 1992, 71, R1–R22. [Google Scholar] [CrossRef]
- Lehmann, V.; Gösele, U. Porous silicon formation: A quantum wire effect. Appl. Phys. Lett. 1991, 58, 856–858. [Google Scholar] [CrossRef]
- Li, X.; Bohn, P.W. Metal-assisted chemical etching in HF/H2O2 produces porous silicon. Appl. Phys. Lett. 2000, 77, 2572. [Google Scholar] [CrossRef]
- Beale, M.; Benjamin, J.; Uren, M.; Chew, N.; Cullis, A. The formation of porous silicon by chemical stain etches. J. Cryst. Growth 1986, 75, 408–414. [Google Scholar] [CrossRef]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fan, Z.; Dhanabalan, A.; Chen, C.; Wang, C. Mesoporous silicon anodes prepared by magnesiothermic reduction for lithium ion batteries. J. Electrochem. Soc. 2011, 158, A1055–A1059. [Google Scholar] [CrossRef]
- Winters, H.F.; Kay, E. Gas incorporation into sputtered films. J. Appl. Phys. 1967, 38, 3928–3934. [Google Scholar] [CrossRef]
- Pawlewicz, W.T. Influence of deposition conditions on sputter-deposited amorphous silicon. J. Appl. Phys. 1978, 49, 5595–5601. [Google Scholar] [CrossRef]
- Chapman, B. Glow Discharge Processes: Sputtering and Plasma Etching; Wiley-Interscience: New York, NY, USA, 1980; pp. 206–208. [Google Scholar]
- Knights, J.C. (Invited) Characterization of Plasma-Deposited Amorphous Si: H Thin Films. Jpn. J. Appl. Phys. 1979, 18, 101. [Google Scholar] [CrossRef]
- Brennen, C.E. Cavitation and Bubble Dynamics; Number 44 in Oxford Engineering Science Series; Oxford University Press: New York, NY, USA, 1995; pp. 21–34. [Google Scholar]
- Thompson, M.O.; Galvin, G.J.; Mayer, J.W.; Peercy, P.S.; Poate, J.M.; Jacobson, D.C.; Cullis, A.G.; Chew, N.G. Melting temperature and explosive crystallization of amorphous silicon during pulsed laser irradiation. Phys. Rev. Lett. 1984, 52, 2360. [Google Scholar] [CrossRef]
- Im, J.S.; Kim, H.J.; Thompson, M.O. Phase transformation mechanisms involved in excimer laser crystallization of amorphous silicon films. Appl. Phys. Lett. 1993, 63, 1969. [Google Scholar] [CrossRef]
- Zellama, K.; Chahed, L.; Sládek, P.; Thèye, M.L.; von Bardeleben, J.H.; Roca i Cabarrocas, P. Hydrogen-effusion-induced structural changes and defects in a-Si:H films: Dependence upon the film microstructure. Phys. Rev. B 1996, 53, 3804–3812. [Google Scholar] [CrossRef]
- Sinke, W.C.; Polman, A.; Roorda, S.; Stolk, P.A. Explosive crystallization of amorphous silicon: Triggering and propagation. Appl. Surf. Sci. 1989, 43, 128–135. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sämann, C.; Köhler, J.R.; Dahlinger, M.; Schubert, M.B.; Werner, J.H. Pulsed Laser Porosification of Silicon Thin Films. Materials 2016, 9, 509. https://doi.org/10.3390/ma9070509
Sämann C, Köhler JR, Dahlinger M, Schubert MB, Werner JH. Pulsed Laser Porosification of Silicon Thin Films. Materials. 2016; 9(7):509. https://doi.org/10.3390/ma9070509
Chicago/Turabian StyleSämann, Christian, Jürgen R. Köhler, Morris Dahlinger, Markus B. Schubert, and Jürgen H. Werner. 2016. "Pulsed Laser Porosification of Silicon Thin Films" Materials 9, no. 7: 509. https://doi.org/10.3390/ma9070509





