1. Introduction
The European Community has recently issued a series of regulations to limit the energy consumption and the exploitation of natural resources, besides promoting the use of secondary raw materials and minimizing the amount of wastes disposed in landfills.
Working in this direction, the present study is focused on the development of dense and porous alkali-activated materials for the building sector, mainly realized by waste products.
In fact, the EU Community purpose of cutting greenhouse emission by 80%–95% by 2050 [
1] has a serious implication for the construction industry, being one of the greatest consumers of energy, resources and raw materials. In Europe, buildings through their life cycle, including construction, operation and demolition consume approximately 50% of the total energy demand and contribute almost 50% to the CO
2 emissions released in the atmosphere [
2]. In this frame, the EU has recently put forward a package to support the transition to a circular economy [
3], in which the value of products and materials is maintained for the longest time as possible. In this economic scheme, wastes and resources are minimized, whereas the use of secondary raw materials is encouraged to create further value. To this aim, the aspect of “sustainable use of natural resources” has been recently introduced in the European Construction Product Regulation (ECPR), which is laying down harmonized conditions for the marketing of construction products. To fulfil this request, the use of environmentally-compatible raw and secondary materials is becoming a fundamental need [
4].
In the last few years, the research has focused on the development of green alternatives to Ordinary Portland Cement (OPC), in terms of the limitation of the CO
2 footprint and conscious use of natural resources. On the ground of the good results achieved, growing attention has been paid to the family of alkali-activated binders and in particular to the geopolymers. These materials have shown to offer an environmentally-friendly, technically-competitive alternative for both mechanical [
5] and durability properties [
6,
7,
8] to OPC and are beginning to be used in several applications [
9,
10,
11].
The geopolymerization process starts with the dissolution of Al and Si (from the Al-Si-rich materials) in alkali solutions as hydrated reaction products with NaOH or KOH, hence forming a [M
x(AlO
2)
y(SiO
2)
z∙nMOH∙mH
2O] gel (M: Na or K) [
12]. Several silico-aluminates powders, such as kaolinitic clays [
13], metakaolin, fly ash [
14,
15], blast furnace slag and mixtures of them, could be used as precursors of geopolymerization [
12,
16]. The most important parameters that affect the mechanical properties of the final product are the reactive silica content, the Si/Al ratio, the amorphous phase content and the calcium content [
17,
18,
19].
Metakaolin-based geopolymers can be manufactured consistently with predictable properties due to the common features of the powder, which is commercially available worldwide [
16]. Curing is normally carried out at low temperature (20–120 °C) for 3–10 h, depending on the raw material and the composition used. The complete development of mechanical properties generally takes place after five days and is strongly related to the Si/Al ratio, as previously mentioned [
12,
17,
18,
19,
20,
21,
22]. The lack of production standards resulted in a wide range of data related to the mechanical properties, since the compressive strength ranges from 6–60 MPa [
16].
As an alternative to metakaolin, fly ash (FA) can be used as well. FA is an industrial waste with pozzolanic properties, obtained from thermal power plants. Differently from the metakaolin, FA does not derive from a well-defined starting material. Even if the predominant network of the final material is composed of silicon and aluminum, the presence of impurities (CaO, iron, blast furnace slag) plays an important role in modifying setting time, slump, strength and shrinkage [
16]. Due to the high levels of amorphous silica and alumina, FA are often used to produce geopolymers with interesting properties [
16,
17]. In addition, the curing conditions play an essential role in the development of FA-based geopolymers properties, as shown in the work of Kovalchuk
et al. [
23], in which an excellent mechanical strength (
Rck up to 102 MPa) was reached for samples cured in covered molds at 95 °C for 2 h.
Concerning the environmental impact of geopolymers, the available literature data are highly dispersed [
9,
16,
24,
25] depending on the raw materials and alkali activator used. FA is particularly interesting from the environmental point of view, since it is an industrial waste and does not require any energy input apart from transportation; on the opposite side, when clay is used as the raw material, it requires a calcination process at high temperature (≈650–750 °C) to dehydrate the powder and improve its reactivity in the geopolymerization process.
Concerning the alkaline activator, sodium silicate solution, which is frequently modified by adding extra amorphous silica nanopowder [
26] and sodium or potassium hydroxide, is commonly used to prepare geopolymers. However, sodium silicate is manufactured through an energy-intensive process, involving the calcination of sodium carbonate and quartz sand at temperatures ranging between 1400 and 1500 °C [
27]. Therefore, in order to reduce the environmental impact of the geopolymers, it is necessary to properly select the feedstock material and to limit the amount of sodium silicate in the mixture or to substitute it by less environmentally-impacting solutions, but still containing reactive amorphous silica. In this context, a point of interest is silica-rich wastes, such as rice husk ash (RHA). RHA is an industrial waste, generated by the combustion of rice husk (biomass used in co-generation plants for the production of green energy [
28]) and contains around 85%–90% SiO
2 mainly in the amorphous form [
12]. This waste has been used for the production of refractory materials, to synthesize silicon carbide, as a supplementary cementitious material for its pozzolanic features [
29,
30,
31,
32,
33,
34], as a component in the production of alkali-activated binders [
35] and, finally, as a point of interest in this contribution, as a precursor for the production of soluble sodium silicate [
36,
37]. Starting from our previous interesting results achieved with metakaolin-based geopolymers, activated with sodium and potassium silicate solutions [
38], this study assesses the feasibility to use two industrial wastes as raw materials for the production of geopolymer pastes, namely RHA and FA, on the ground of previous literature [
39]. As RHA is a suitable source of amorphous silica, this research aims to eliminate the use of commercial sodium/potassium silicate solution and of silica nanopowder that are generally used for geopolymer synthesis. Thus, RHA was dissolved in a KOH solution and used as an economical and green alternative to the commercial potassium silicate solution. Similarly, metakaolin was substituted with FA powder, which is a Si-Al-rich waste product and does not require any high temperature treatment prior to using.
Therefore, in this study, three typologies of dense samples were produced: (i) a calcined clay activated with potassium hydroxide and nanosilica, used as reference samples; (ii) calcined clay activated with RHA dissolved into KOH solution; and (iii) FA activated with the alkaline solution realized with RHA. The last formulation, containing FA as feedstock and RHA as the silica source for the silicate solution, can be considered particularly interesting because of the high recycled content. Furthermore, starting from the last two compositions, porous samples were realized, by surveying the role of aluminum powder on the foamability of the samples. The development of such porous materials was motivated by the increasing interest in lightened geopolymers, due to their high potential in the development of thermal insulator elements. For instance, Zhao
et al. [
40] developed fly ash-based geopolymer foams, showing a significantly low thermal conductivity (0.084 W/mK) and an excellent resistance when exposed to fire temperatures up to 1150 °C for 2 h. The development of new, thermal insulating building elements has great interest, since their use constitutes the most effective way of reducing heat losses in buildings, thus reducing the energy needs. These elements represent a very profitable business sector for the building material industry, which is destined for a further expansion in view of the achievement of the target of zero energy buildings [
1].
In our work, the porous samples realized with FA as the feedstock and RHA as the silica source for the silicate solution appear to be very innovative materials, without any previous reference in the literature and could be considered particularly interesting because of the high recycled content and of their physical-mechanical features. Finally, to quantify the benefit on the environmental impact associated with the use of waste materials in the studied samples, a preliminary evaluation of the global warming potential was done for the three formulations.
2. Materials and Methods
Geopolymers were realized using halloysite clay and fly ash as starting materials.
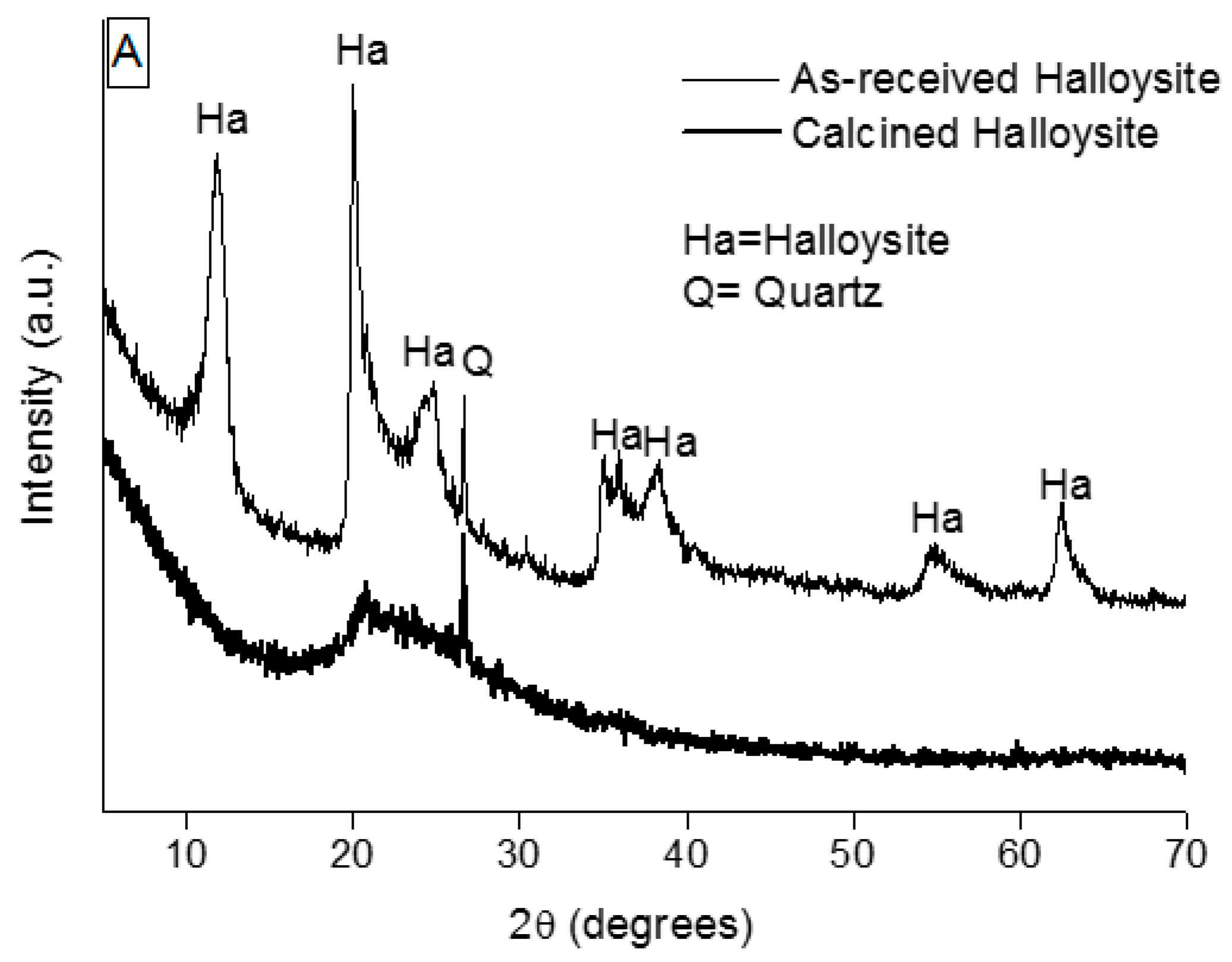
Halloysite clay, supplied by Applied Minerals Inc., was calcined at 650 °C for 3 h to dehydrate the hydroxide groups and to produce an almost amorphous powder (referred to as HL).
Fly ash (FA) was obtained from the ICIC/Enel SpA coal-fired power plant of La Spezia, Italy. It is characterized by an absolute density of 2.3 g/cm
3 and a water solubility of 1 g/L. Rice husk ash (RHA), a by-product of a biomass combustion plant (Idroblins srl, Crova, Italy, [
28]), was also investigated as a raw material for preparing the alkaline solution. It is a dry black powder, with a moisture content lower than 1% and specific weight of 0.17–0.24 g/cm
3.
X-ray diffraction (XRD) analysis was used to characterize the as-received powders and geopolymers. Spectra were recorded on a Philips PW 3800 apparatus operating with Cu Kα radiation (0.1541874 nm) and were acquired in the range 5°–70° 2θ, with a step size of 0.05° 2θ and an acquisition time per step of 5 s. Diffraction patterns were indexed by using the Powder Data File database (P.D.F. 2000, International Centre of Diffraction Data, Newtown Square, PA, USA).
X-ray fluorescence (XRF, Rigaku ZSX 100E, Tokyo, Japan) analysis was carried out on the powders, in order to assess their chemical composition.
The inductively-coupled plasma-atomic emission spectrometer (ICP-AES, Perkin Elmer, Optima 7000 DV, Waltham, MA, USA) was used to investigate the dissolution degree of RHA. First, the chemical composition of the starting RHA, sieved under 125 mesh, was determined. The powder was submitted to acid digestion in a microwave oven. Sample aliquots of 100 mg were treated with a mixture of 5 mL of aqua regia and 2 mL of hydrofluoric acid in polytetrafluoroethylene (PTFE) bombs. Four heating steps of 5 min each (250, 400, 600, 250 W power, respectively) followed by a ventilation step of 25 min, were applied. Then, 700 mg of boric acid were added, and the bombs were further heated for 5 min at 250 W, succeeded by 15 min of ventilation. At the end of the full treatment, the samples appeared completely dissolved.
The article size distribution of raw materials was determined by means of a laser granulometer (Fritsch Analyzette 22, Idar-Oberstein, Germany) after dispersion in ethanol and sonication in an ultrasonic bath for 10 min.
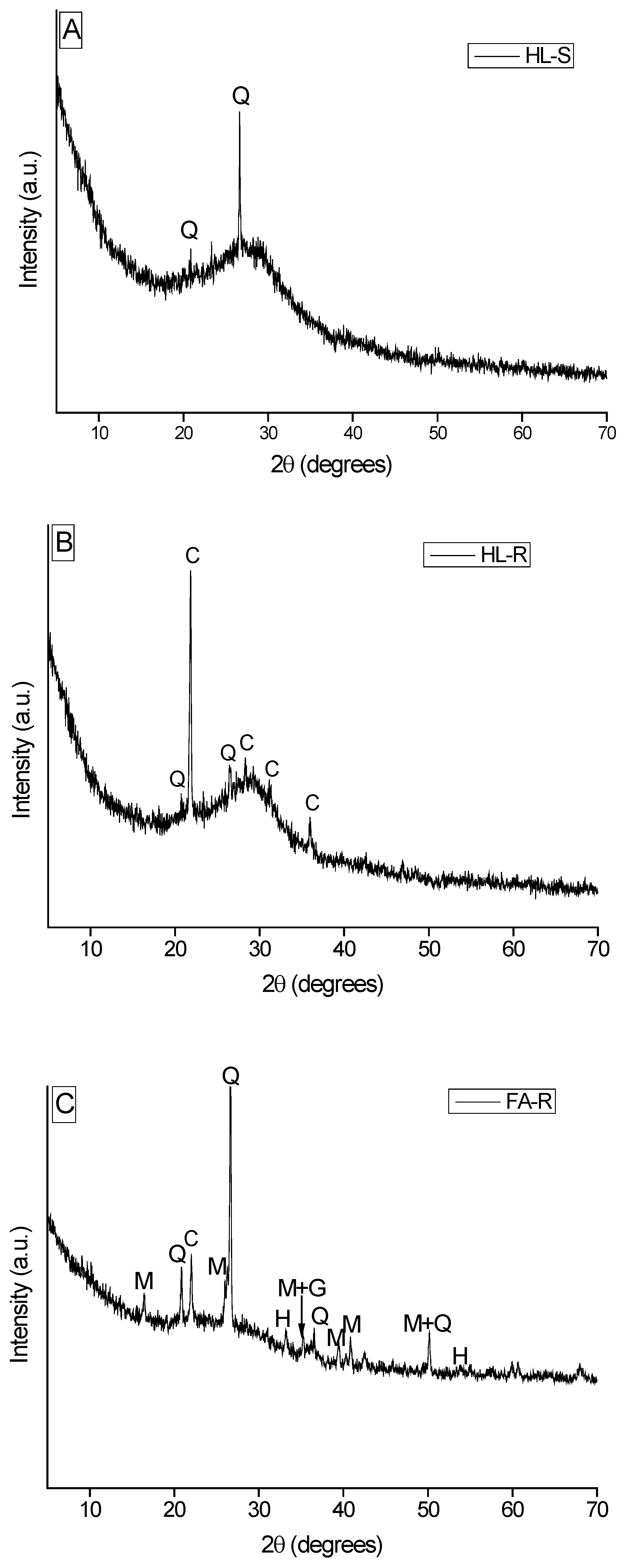
Three typologies of dense pastes were prepared, as reported in
Table 1, which also gives the designations used. In this study, HL-S is used as the reference material to assess the properties of the other two more innovative, waste-containing compositions,
i.e., HL-R and FA-R.
In order to prepare the alkaline silicate solutions, potassium hydroxide (KOH in pellets, analytical grade) and SiO
2 nanopowder (10–20 nm, 99.5% purity), all supplied by Sigma-Aldrich, were employed. The potassium silicate solution was prepared by adding amorphous silica nanopowder to a clear potassium hydroxide solution (8 M), kept under magnetic stirring for 24 h. After this stirring time, a clear solution was obtained, free from precipitates or silica particle residues, suggesting the complete dissolution of the silica nanoparticles. The SiO
2/K
2O and H
2O/K
2O molar ratios were fixed at 1.66 and 11, respectively, according to a previous work [
38] and to literature data [
20,
41].
A second alkaline solution was prepared using RHA instead of nanosilica powder: the ash was sieved under 125 mesh and added to a potassium hydroxide solution (8 M) in the same amount of silica nanopowder (leading to a ratio RHA:KOH:H2O in the solution equal to 1:1:2). In order to assess the best dissolution condition of RHA into the alkaline medium, three different solubility tests were carried out. Precisely, the RHA/KOH solution was stirred for 4, 24 or 168 h at room temperature. After each test, the solution was separated from the (eventual) precipitate and submitted to ICP-AES analysis. In this case, the suspensions were filtered, and the supernatants were diluted (1:100), stabilized with HNO3 (0.1%) and submitted to ICP-AES analysis. After that, calibrations with standard solutions prepared in aliquots of sample blanks were performed.
All of the dense geopolymer pastes were prepared by mechanically mixing raw materials (HL or FA) and the alkaline solution (KOH + SiO
2 or KOH + RHA) for about 15 min at 200 rpm, before casting into plastic prismatic molds (size of 20 × 20 × 80 mm
3). The mix design of the different compositions (expressed as wt % of each component in the mixture) is summarized in
Table 2.
Once the proper ratio between the components in the solutions was fixed (a SiO2/K2O molar ratio of 1.66 and a H2O/K2O molar ratio of 11, as previously explained), the ratio between the raw material and the potassium silicate solution was fixed on the ground of the paste workability, in order to have slurries sufficiently flowing to be easily cast, thus not requiring a de-airing process. Specifically, the apparent viscosity of the pastes was determined by means of a viscometer (Brookfield HBDV-II, Middleboro, MA, USA) and measured at 200 rpm (which corresponds to the mixing rate).
Starting from the two formulations containing industrial wastes (HL-R and FA-R), porous samples were also produced. To this aim, aluminum powder, supplied by Alfa Aesar
®, Karlsruhe, Germany, was used as the foaming agent and added at 0.05%–0.3% with respect to the total mass, as reported in
Table 2. The Al powder is characterized by 99.5% of purity and an average particle size of 7–15 μm, as declared by the supplier. After foaming agent addition, the slurry was mixed at high rpm for 1 min and then cast in prismatic molds. The foamed samples followed the same curing schedule as the dense materials.
The HL-based samples were cured in a sealed container at room temperature, whereas the samples realized with FA were cured at 50 °C for 24 h and then stored at room temperature until testing.
Bulk density was measured in accordance with EN 12390-7, after 14 days of curing.
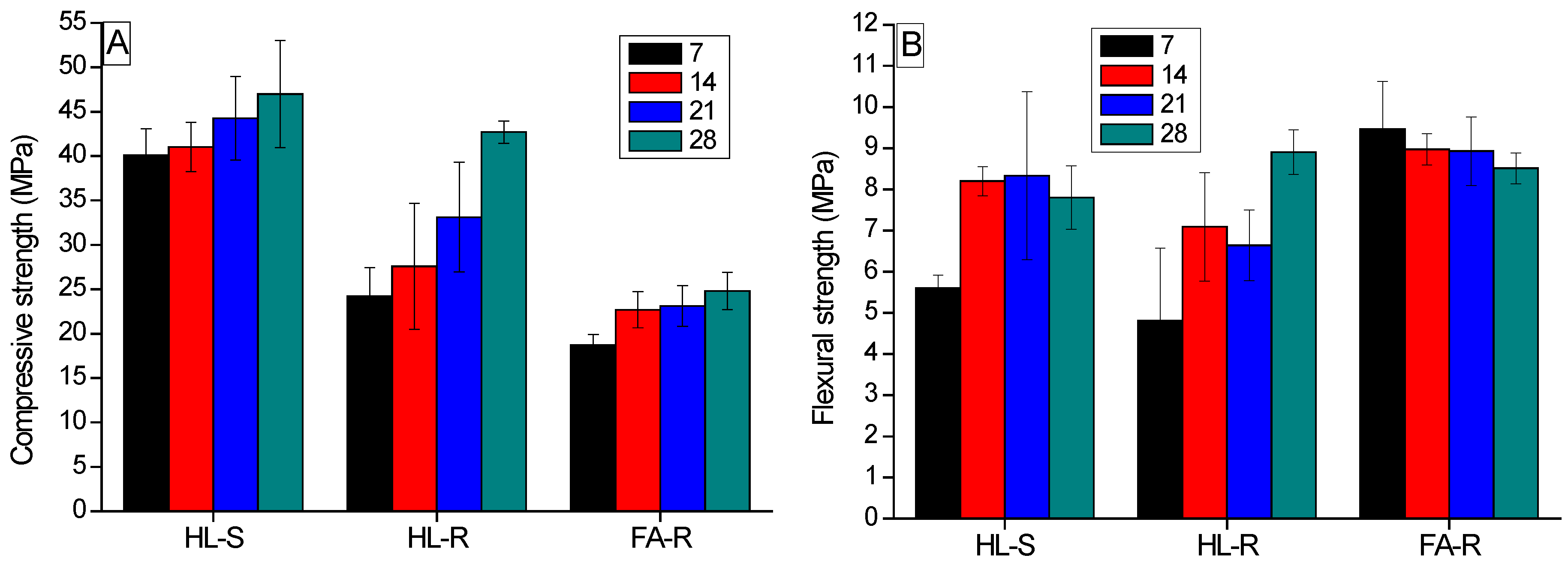
Flexural strength of the geopolymer samples was measured in three-point-bending, using an electromechanical testing system (Zwick Roell 2014, Ulm, Germany) with a maximum load capacity of 1 kN and standard length. Compressive strength was measured on the far edge of both residual pieces obtained from flexural tests according to the EN 196-1 standard, using a 50-kN closed-loop universal press by Zwick Roell, working in displacement control mode. Dense and foamed samples were submitted to mechanical tests after different curing times (7, 14, 21 and 28 days).
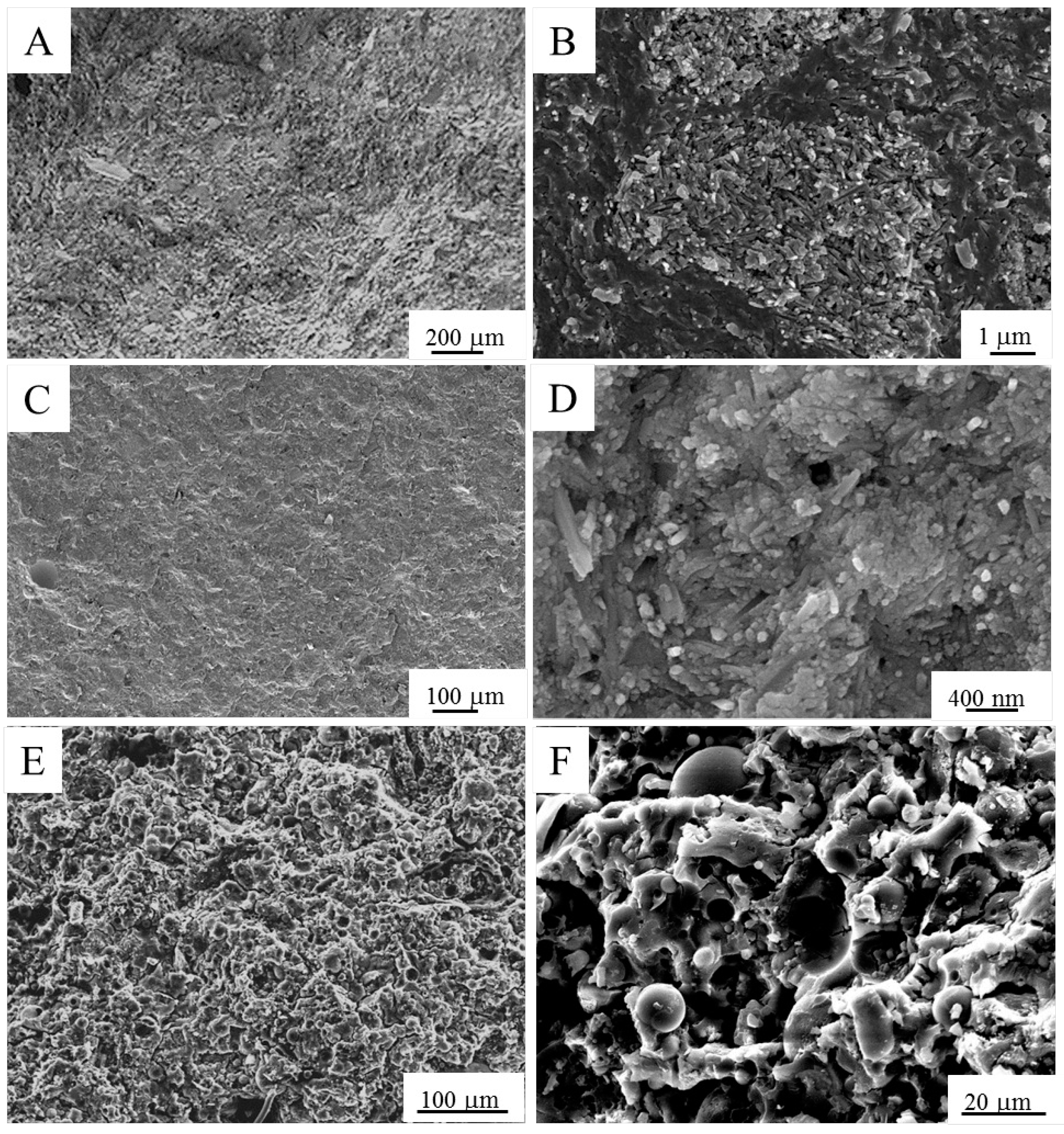
Field emission-scanning electron microscope (FE-SEM, Hitachi S4000, Tokyo, Japan) observations were performed on raw materials and on geopolymer pastes (observations were carried out on the fracture surfaces). The samples were gold sputtered (SPI, West Chester, PA, USA) prior to observations.
4. Conclusions
This paper demonstrated the feasibility of two industrial wastes, fly ash (FA) and rice husk ash (RHA), as raw materials for the production of innovative geopolymeric pastes. In particular, this study explores the use of RHA as a valuable alternative to silica nanopowder to prepare the activator solution.
Three typologies of samples were produced: (i) a calcined clay (Halloysite, HL) activated with potassium hydroxide and nanosilica, used as the reference sample (HL-S); (ii) halloysite activated with RHA dissolved into KOH solution (HL-R); (iii) FA activated with the alkaline solution realized with RHA (FA-R).
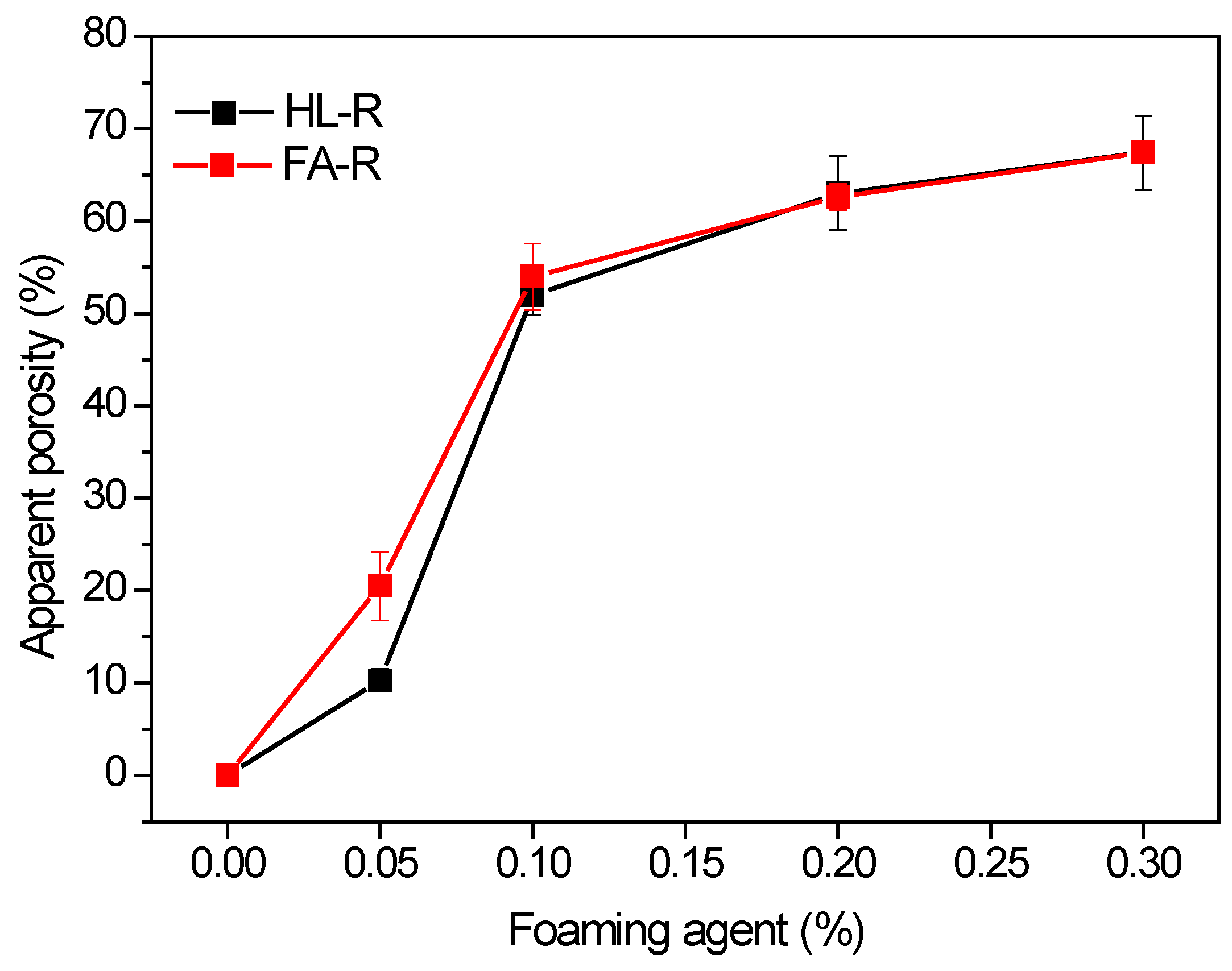
Substitution of the silica nanopowder with RHA in the starting potassium silicate solution led to a certain decrease (10%) of density. Instead, the higher density of FA-R could be related to the higher solid content of this mixture (S/L = 4.3) with respect to the HL-S and HL-R ones (S/L = 2.5). In fact, the rounded morphology of the fly ash powders showed a positive role in the mixture workability, as compared to the halloysite-based mixtures. Thus, the highest workability of FA-R allowed us to increase its solid loading, moving from the S/L value of 2.5 in HA-S and HA-R, to 4.3 in FA-R.
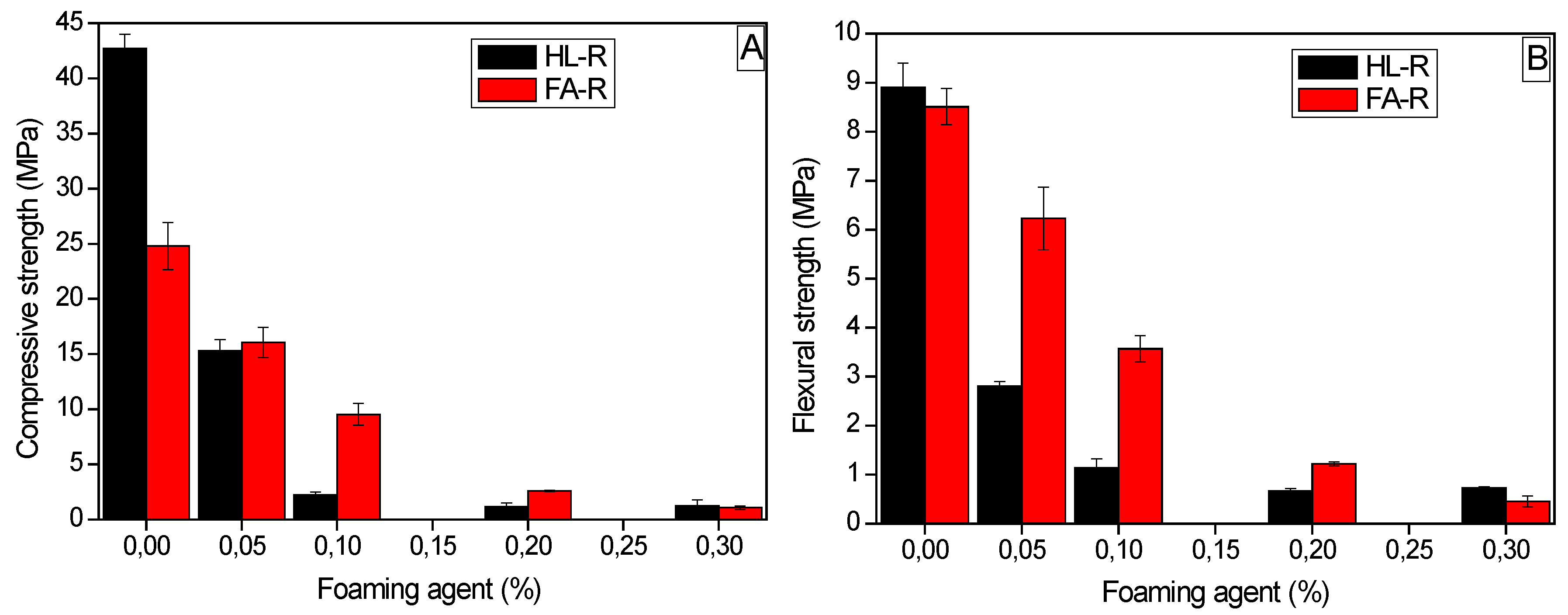
For HL-S, a relatively short curing time (7–14 days) was sufficient to reach high strength values, whereas HL-R required a longer time (28 days) to achieve comparable (under compression) or even higher (under bending) values. In fact, HL-R reached a flexural strength of about 9 MPa and a compressive strength of 43 MPa. On the contrary, the compressive strength of FA-R was significantly lower than the HL-R one, probably due to some cracks observed in the microstructure and to the Si/Al molar ratio, which was higher than the literature optimal values. However, when porous samples were concerned, FA-R showed comparable or even higher strength than HL-R. In this case, the macro-porosity of the samples governs the material’s failure, as observed with other engineering ceramic foams [
60]. Anyway, RHA addition to the geopolymeric composition proved to be effective either to produce dense or porous samples. In a future work, the thermal properties of the new, waste-derived porous materials will be assessed.
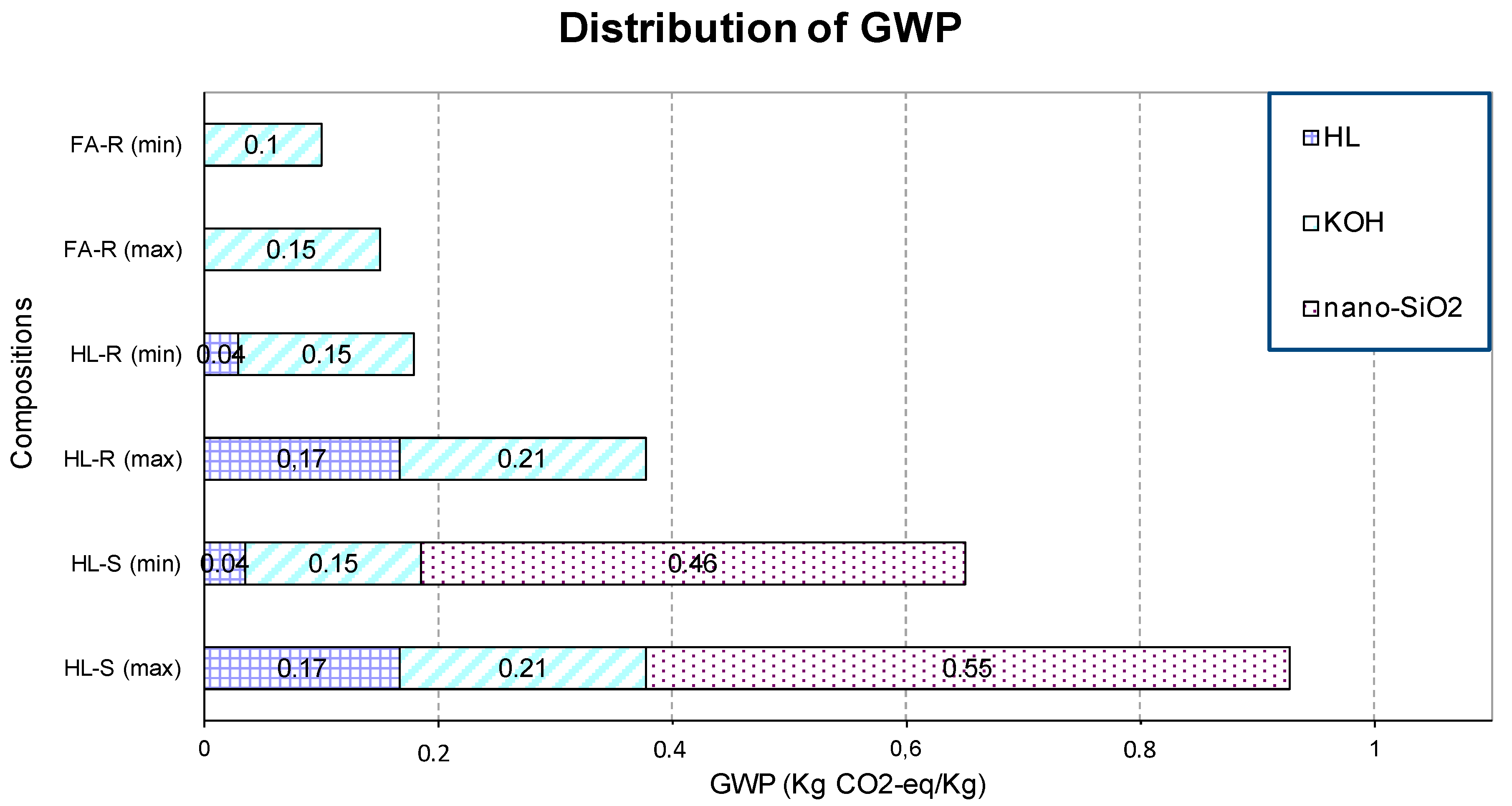
Finally, to quantify the benefit on the environmental impact associated with the use of waste materials in the production of geopolymers, a preliminary evaluation of the embodied carbon was calculated for the three investigated formulations. The composition HL-S presented the highest GWP due to the contribution of all the components, with values ranging between 0.65 and 0.9 kg CO2-eq/kg, depending on the data source. In particular, the important contribution of nanosilica was easily evidenced, contributing more than the sum of KOH and calcined clay emissions. When the nanosilica was substituted with RHA, the GWP values strongly decreased and ranged between 0.18 and 0.46 kg CO2-eq/kg. However, the lowest environmental impact was obtained when FA substituted halloysite as the raw material, RHA substituted the nanosilica powder in the solution and the amount of potassium silicate solution used was lower with respect to the previous two compositions, due to the highest workability of FA-R. At this point, only KOH gives a considerable contribution to the GWP, while the other components did not provide any impact. This composition reached a total value of 0.1–0.15 kg CO2-eq/kg emissions.