Polystyrene-Poly(methyl methacrylate) Silver Nanocomposites: Significant Modification of the Thermal and Electrical Properties by Microwave Irradiation
Abstract
:1. Introduction
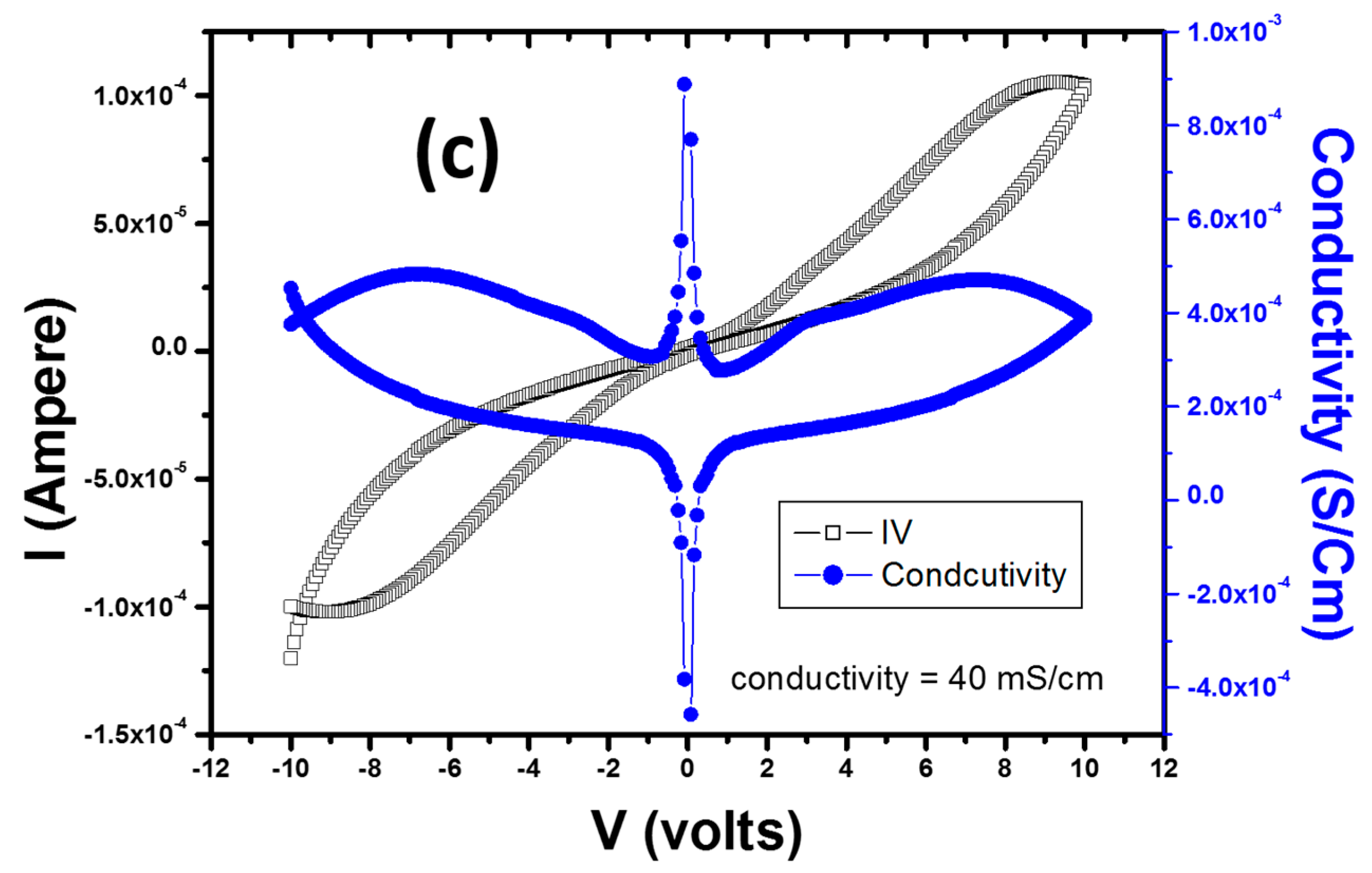
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. MWI Preparation of PS/Ag, PMMA/Ag, and PS–PMMA/Ag Nanocomposites
3.3. In Situ Preparation of PS/Ag, PMMA/Ag, and PS–PMMA/Ag Nanocomposites
3.4. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Kampf, G.; Dietze, B.; Große-Siestrup, C.; Wendt, C.; Martiny, H. Microbicidal activity of a new silver-containing polymer, SPI-ARGENT II. Antimicrob. Agents Chemother. 1998, 42, 2440–2442. [Google Scholar] [PubMed]
- Fang, N.; Lee, H.; Sun, C.; Zhang, X. Sub-diffraction-limited optical imaging with a silver superlens. Science 2005, 308, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gates, B.; Mayers, B.; Xia, Y. Crystalline silver nanowires by soft solution processing. Nano Lett. 2002, 2, 165–168. [Google Scholar] [CrossRef]
- Nicewarner-Pena, S.R.; Freemean, R.G.; Reiss, B.D.; He, L.; Pena, D.J.; Walton, I.D.; Cromer, R.; Keating, C.D.; Natan, M.J. Submicrometer metallic barcodes. Science 2001, 294, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Oldenburg, S.J.; Smith, D.R.; Schultz, D.A.; Schultz, S. Composite plasmon resonant nanowires. Nano Lett. 2002, 5, 465–469. [Google Scholar] [CrossRef]
- Mailard, M.; Huang, O.; Brus, L. Silver Nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+]. Nano Lett. 2003, 3, 1611–1615. [Google Scholar] [CrossRef]
- Haes, A.J.; Stuart, D.A.; Nie, S.; van Dyyene, R.P. Using solution-phase nanoparticles, surface-confined nanoparticle arrays and single nanoparticles as biological sensing platforms. J. Fluoresc. 2004, 14, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Jeong, S.H. Preparation and characterization of polypropylene/silver nanocomposite fibers. Polym. Int. 2003, 52, 1053–1057. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. A 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Gupta, A.; Silver, S.S. Molecular genetics: Silver as a biocide: Will resistance become a problem? Nat. Biotechnol. 1998, 16, 888. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Gubin, S.P. Metalcontaining nano-particles within polymeric matrices: Preparation, structure, and properties. Colloids Surf. A 2002, 202, 155–163. [Google Scholar] [CrossRef]
- Hong, W.; Woo, H.-J.; Choi, H.-W.; Kim, Y.-S.; Kim, G.-D. Optical property modification of PMMA by ion-beam implantation. Appl. Surf. Sci. 2001, 169–170, 428–432. [Google Scholar] [CrossRef]
- Johnson, P.B.; Christy, R.W. Optical constants of the noble metals. Phys. Rev. B 1972, 16, 4370–4379. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Alsharaeh, E.; El-Shall, M.S. Catalyzed radical polymerization of styrene vapor on nanoparticle surfaces and the incorporation of metal and metal oxide nanoparticles within polystyrene polymers. J. Phys. Chem. B 2006, 110, 19100–19103. [Google Scholar] [CrossRef] [PubMed]
- Alsharaeh, E.H.; Othman, A.A. Microwave irradiation synthesis and characterization of RGO-AgNPs/polystyrene nanocomposites. Polym. Compos. 2014, 35, 2318–2323. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Othman, A.A.; Aldosari, M.A. Microwave irradiation effect on the dispersion and thermal stability of rgo nanosheets within a polystyrene matrix. Materials 2014, 7, 5212–5224. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Rogosic, M.; Sipusic, J. Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym. Degrad. Stab. 2009, 94, 95–101. [Google Scholar] [CrossRef]
- Coskun, M.; Seven, P. Synthesis, characterization and investigation of dielectric properties of two-armed graft copolymers prepared with methyl methacrylate and styrene onto PVC using atom transfer radical polymerization. React. Funct. Polym. 2011, 71, 395–401. [Google Scholar] [CrossRef]
- Wei, H.; Eilers, H. Electrical conductivity of thin-film composites containing silver nanoparticles embedded in a dielectric fluoropolymer matrix. Thin Solid Films 2008, 517, 575–581. [Google Scholar] [CrossRef]
- Du, B.D.; Phu, D.V.; Duy, N.N.; Lan, N.T.K.; Lang, V.T.K.; KeThanh, N.V.; Phong, N.T.P.; QuocHien, N. Preparation of colloidal silver nanoparticles in poly(N-vinylpyrrolidone) by γ-irradiation. J. Exp. Nanosci. 2008, 3, 207–213. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.Y.; Wu, S.X.; Qi, X.Y.; He, Q.Y.; Zhang, Q.C.; Yan, Q.Y.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, M.; Othman, A.; Alsharaeh, E. Synthesis and Characterization of the in Situ Bulk Polymerization of PMMA Containing Graphene Sheets Using Microwave Irradiation. Molecules 2013, 18, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A.; Abdelsayed, V.; Khder, A.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Zedan, A.F.; Sappal, S.; Moussa, S.; El-Shall, M.S. Ligand-controlled microwave synthesis of cubic and hexagonal CdSe nanocrystals supported on graphene. Photoluminescence quenching by graphene. J. Phys. Chem. C 2010, 114, 19920–19927. [Google Scholar] [CrossRef]
- Siamaki, A.R.; Khder, A.E.R.S.; Abdelsayed, V.; El-Shall, M.S.; Gupton, B.F. Microwave-assisted synthesis of palladium nanoparticles supported on graphene: A highly active and recyclable catalyst for carbon–carbon cross-coupling reactions. J. Catal. 2011, 279, 1–11. [Google Scholar] [CrossRef]
- Herring, N.; Almahoudi, S.; Olson, C.; El-Shall, M.S. Enhanced photocatalytic activity of ZnO–graphene nanocomposites prepared by microwave synthesis. J. Nanopart. Res. 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Chung, D.D.L. Electrical applications of carbon materials. J. Mater. Sci. 2004, 39, 2645–2661. [Google Scholar] [CrossRef]
- Vodnik, V.; Božanić, D.K.; Džunuzović, J.V.; Vukoje, I.; Nedeljković, J. Silver/polystyrene nanocomposites: Optical and thermal properties. Polym. Compos. 2012, 33, 782–788. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.; Kim, K.; Lee, B.H.; Choe, S. Polyaniline effect on the conductivity of the PMMA/Ag hybrid composite. Colloids Surf. A 2012, 396, 195–202. [Google Scholar] [CrossRef]
- White, S.I.; Mutiso, R.M.; Vora, P.M.; Jahnke, D.; Hsu, S.; Kikkawa, J.M.; Li, J.; Fischer, J.E.; Winey, K.I. Electrical percolation behavior in silver nanowire-polystyrene composites: Simulation and experiment. Adv. Funct. Mater. 2010, 20, 2709–2716. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray Diffraction; Addison Wesley Publishing Co.: London, UK, 1969. [Google Scholar]
- NIST-XPS Database. Available online: http://srdata.nist.gov/xps/2000 (accessed on 8 December 2009).
- Gröning, P.; Collaud, M.; Dietler, G.; Schlapbach, L. Plasma modification of polymethylmethacrylate and polyethyleneterephthalate surfaces. J. Appl. Phys. 1994, 76, 887–892. [Google Scholar] [CrossRef]
- Burkstrand, J.M. ’’Hot’’ atom interactions with polymer surfaces. J. Vac. Sci. Technol. 1982, 21, 70–73. [Google Scholar] [CrossRef]
- Burkstrand, J.M. Metal-polymer interfaces: Adhesion and X-ray photoemission studies. J. Appl. Phys. 1981, 52, 4795–4800. [Google Scholar] [CrossRef]
- Henneuse-Boxus, C.; Poleunis, C.; Ro, A.D.; Adriaensen, Y.; Bertrand, P.; Marchand-Brynaert, J. Surface functionalization of PEEK films studied by time-of-flight secondary ion mass spectrometry and X-ray photoelectron spectroscopy. Surf. Interface Anal. 1999, 27, 142–152. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Kimura, K. Superlattice formation from polydisperse Ag nanoparticles by a VAPor-diffusion method. Angew. Chem. Int. Ed. 2006, 45, 5662–5665. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.R.; Prasad, B.L.V.; Gopinath, C.S.; Badiger, M.V. A nanocomposite of silver and thermo-associating polymer by a green route: A potential soft–hard material for controlled drug release. RSC Adv. 2014, 4, 10261–10268. [Google Scholar] [CrossRef]
- Mulvaney, P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir 1996, 12, 788–800. [Google Scholar] [CrossRef]
- Carotenuto, G.; Palomba, M.; Nicolais, L. Nanocomposite synthesis by thermolysis of [Ag(hfac)(COD)] in amorphous polystyrene. Sci. Eng. Compos. Mater. 2012, 19, 195–197. [Google Scholar] [CrossRef]
- Singho, N.; Johan, M.; Lah, N.A. Temperature-dependent properties of silver-poly(methylmethacrylate) nanocomposites synthesized by in-situ technique. Nanoscale Res. Lett. 2014, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Nobel, M.L.; Mendes, E.; Picken, S.J. Acrylic-based nanocomposite resins for coating applications. J. Appl. Polym. Sci. 2007, 104, 2146–2156. [Google Scholar] [CrossRef]
- Van Hamersveld, E.M.S.; van Es, J.J.G.S.; German, A.L.; Cuperus, F.P.; Weissenborn, P.; Hellgren, A.C. Oil-acrylic hybrid latexes as binders for waterborne coatings. Prog. Org. Coat. 1999, 35, 235–246. [Google Scholar] [CrossRef]
- Tigli, R.S.; Evren, V. Synthesis and characterization of pure poly(acrylate) latexes. Prog. Org. Coat. 2005, 52, 144–150. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G.; Greenwell, C.H.; Boulet, P.; Coveney, P.V.; Bowden, A.A.; Whiting, A.A. A critical appraisal of polymer–clay nanocomposites. Chem. Soc. Rev. 2008, 37, 568–594. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.S.; Ishak, Z.A.M. Effects of compatibilizers and testing speeds on the mechanical properties of organophilic montmorillonite filled polyamide 6/polypropylene nanocomposites. Polym. Lett. 2007, 1, 77–83. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, P.D.; Vries, A. The MARTINI Force Field: Coarse Grained Model for Biomolecular Simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [PubMed]
- Manoudis, P.; Papadopoulou, S.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Panayiotou, C. Polymer-Silica nanoparticles composite films as protective coatings for stone-based monuments. J. Phys. Conf. Ser. 2007, 61, 1361–1365. [Google Scholar] [CrossRef]
- Desai, T.; Keblinski, P.; Kumar, S.K. Molecular dynamics simulations of polymer transport in nanocomposites. J. Chem. Phys. 2005, 122, 134910. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R., Jr.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer−layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; vanSittert, C.G.C.E.; Nedeljković, J.M.; Djoković, V.; Luyt, A.S. Fabrication and characterization of silver−polyvinyl alcohol nanocomposites. J. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Periodic Table of Elements and Chemistry. Available online: http://www.chemicool.com/elements/silver.html (accessed on 1 March 2011).
- Stauffer, D. Introduction to Percolation Theory; Taylor and Francis: London, UK, 1985. [Google Scholar]
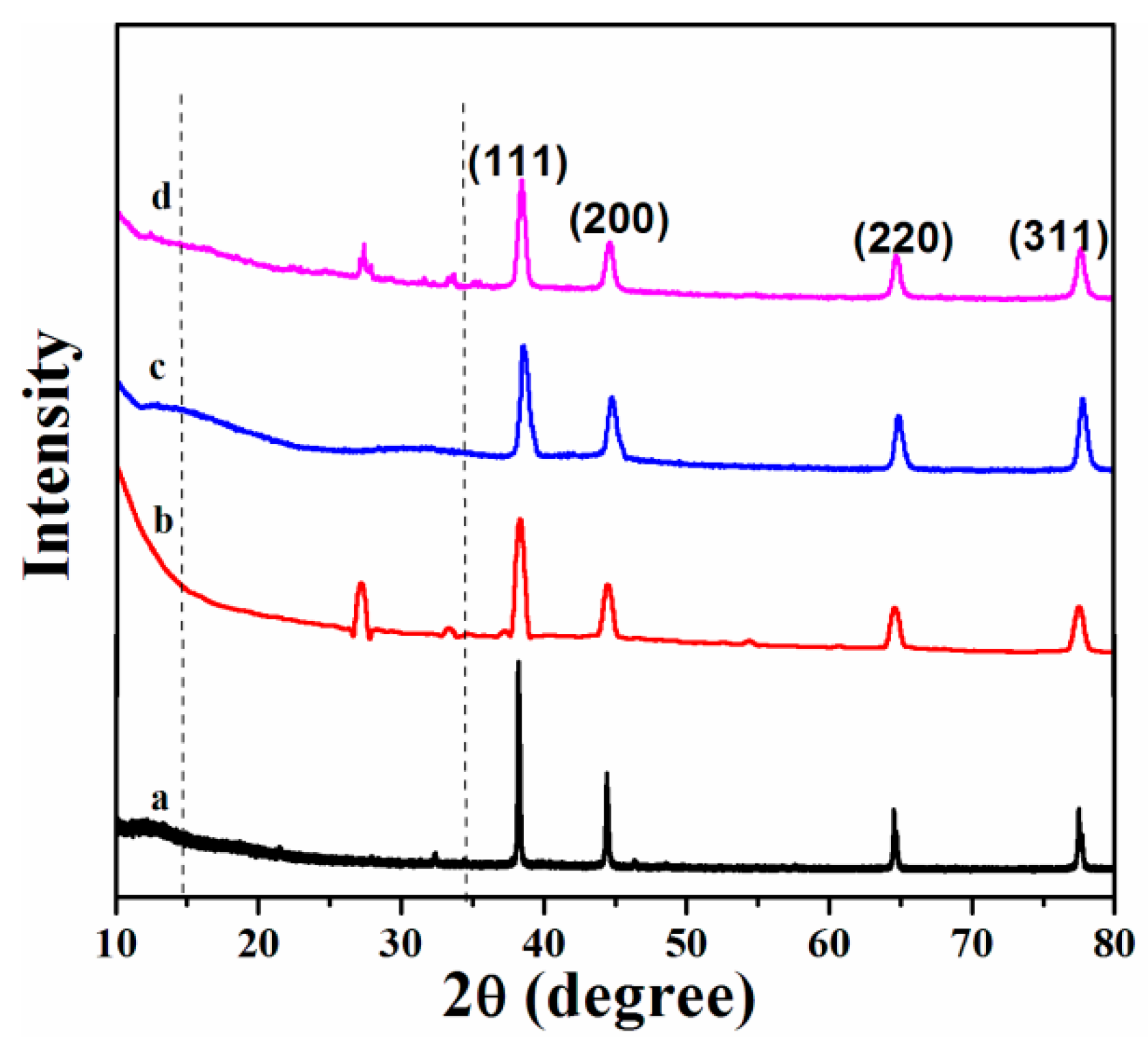

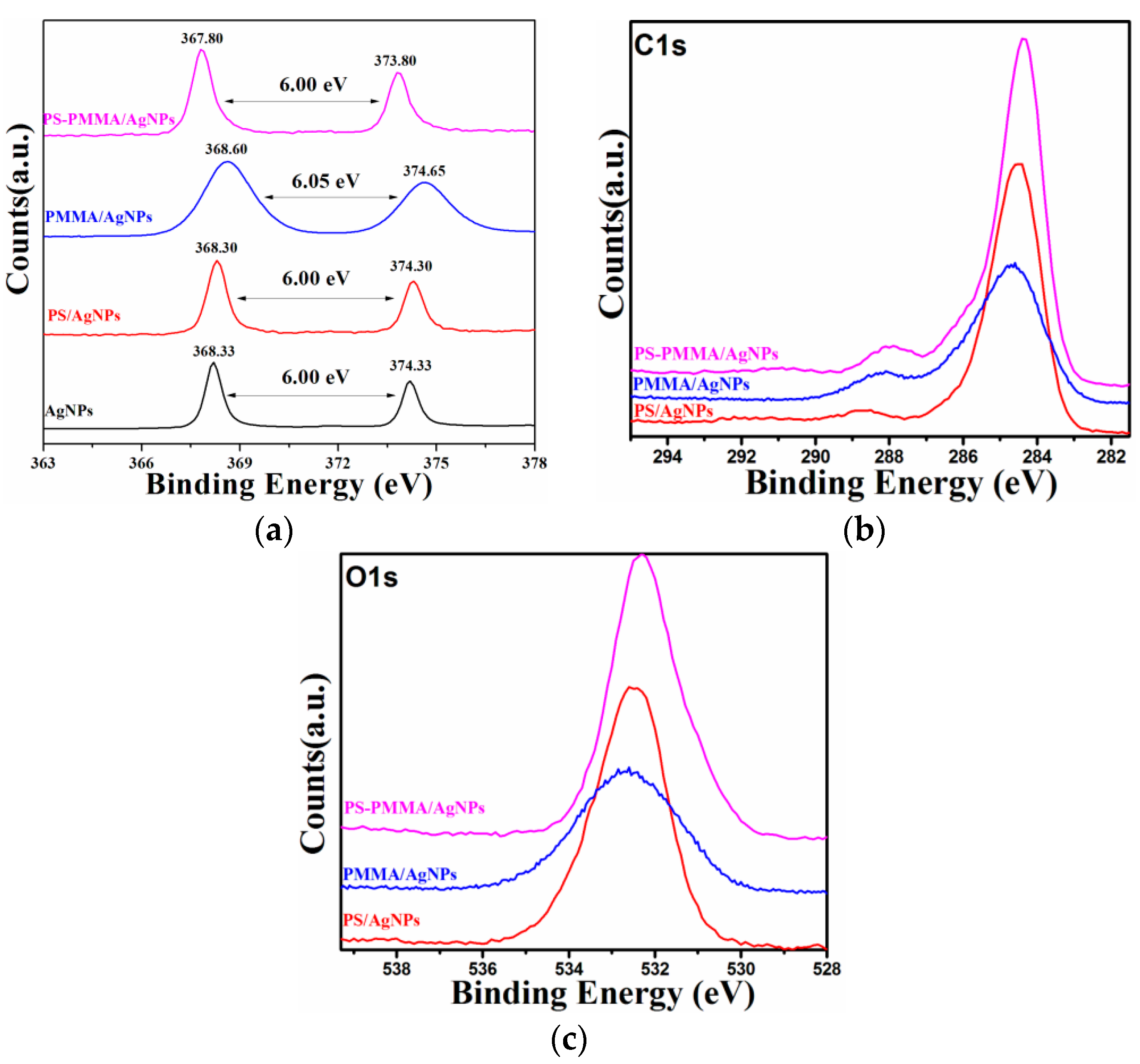
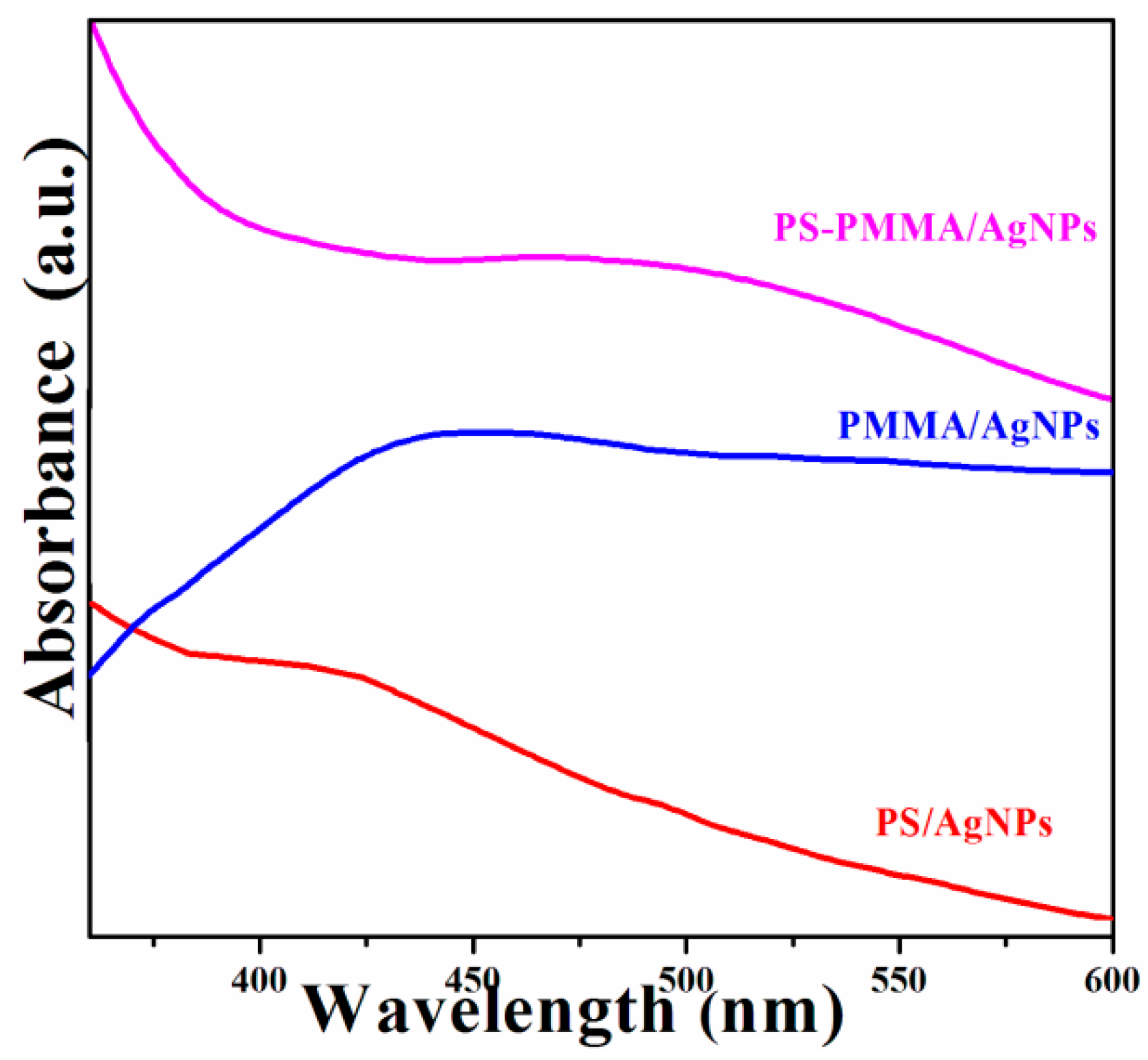

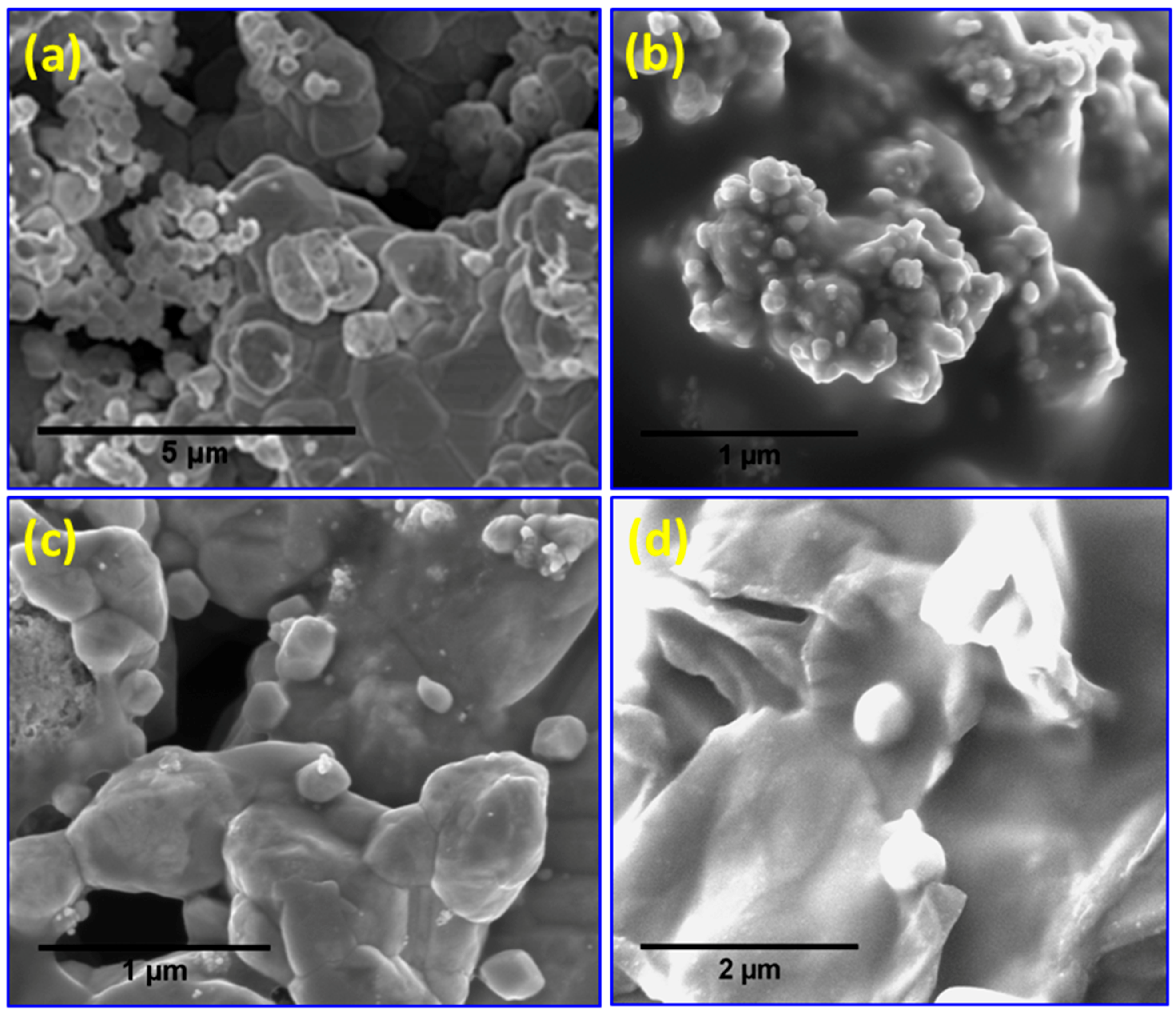
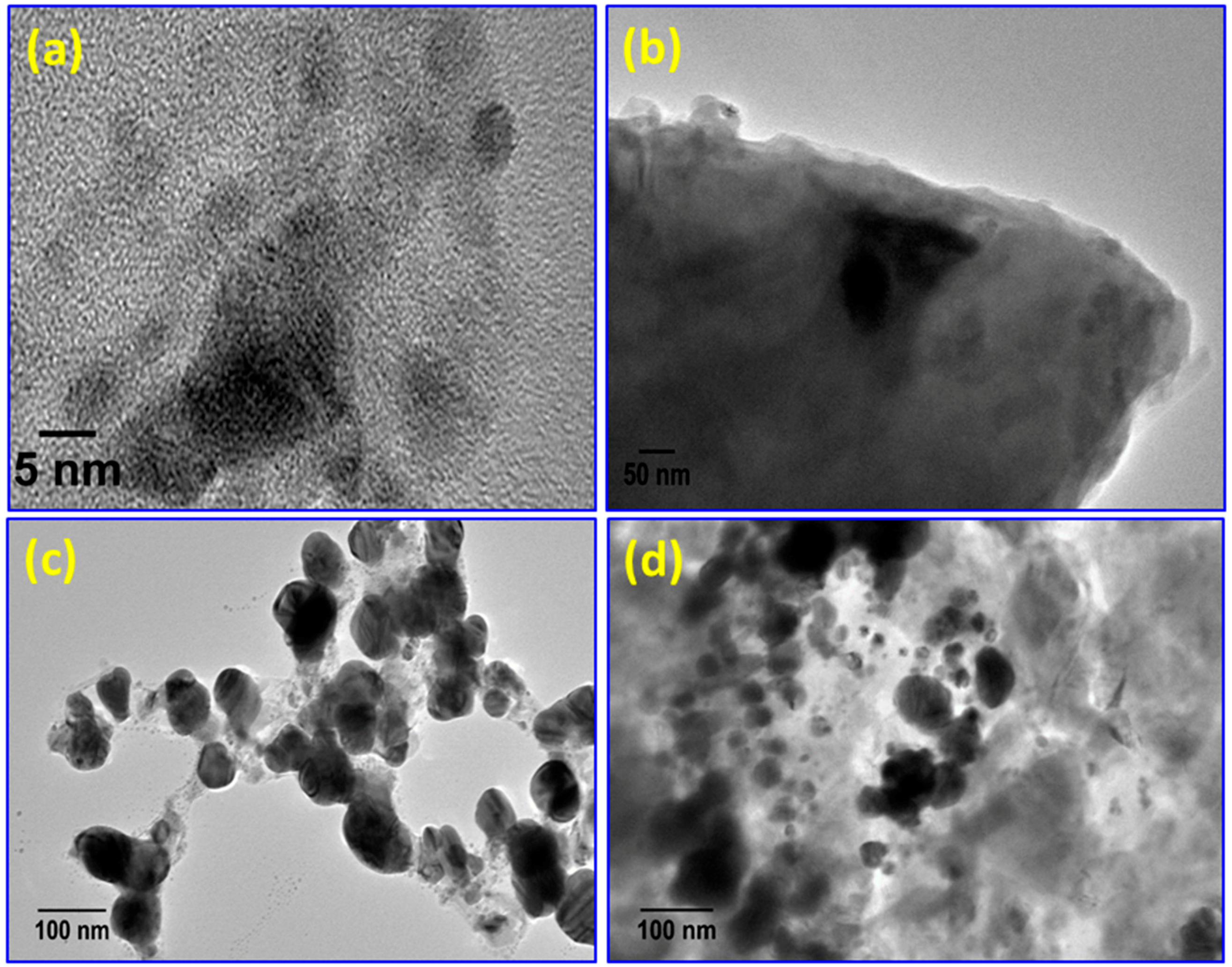
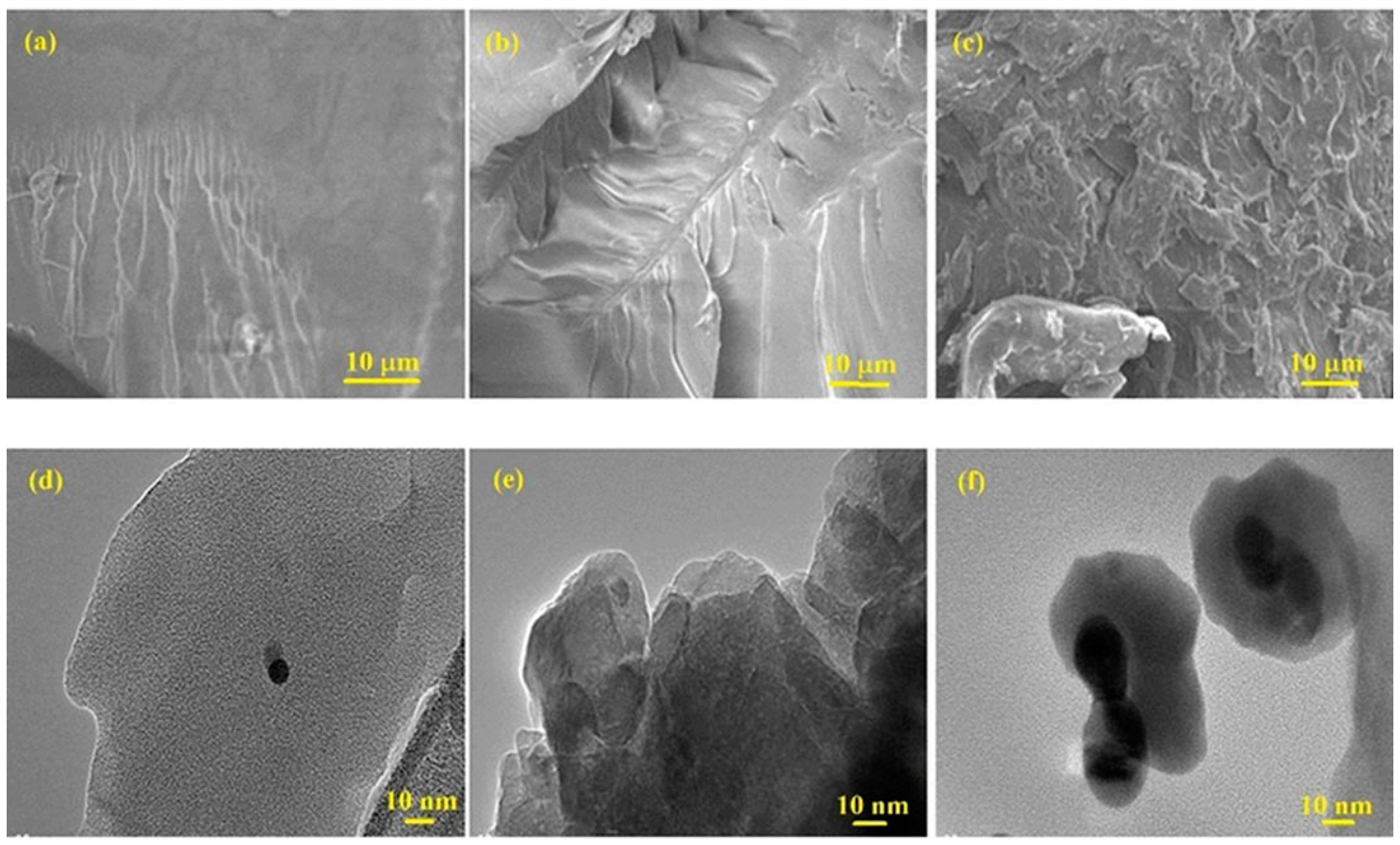

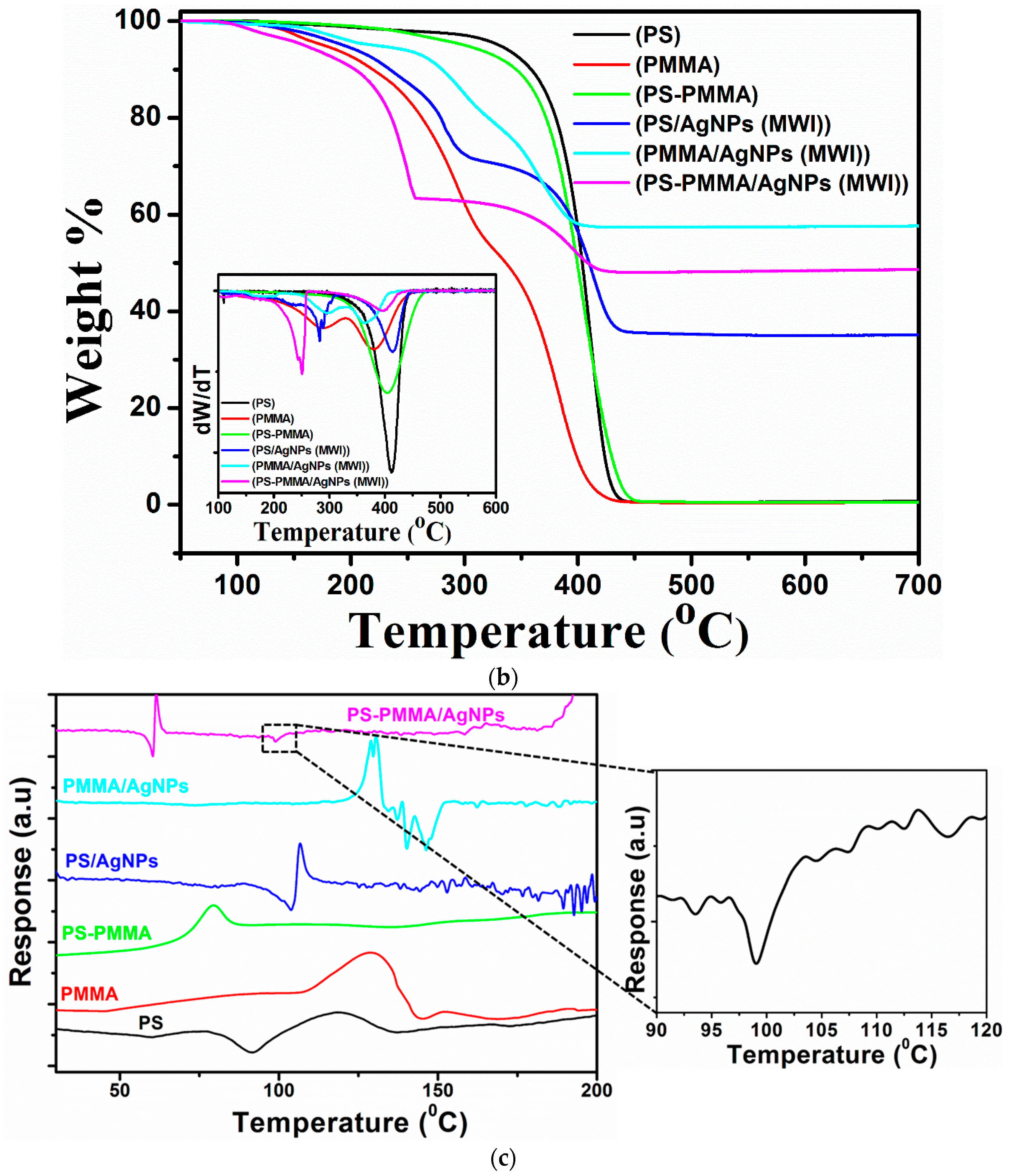
| Sample | Tdeg a (MWI, °C) | Tdeg a (in situ, °C) | Tg b (MWI, °C) |
|---|---|---|---|
| PS/AgNPs | 191 | 334 | 107 |
| PMMA/AgNPs | 232 | 202 | 130 |
| PS-PMMA/AgNPs | 159 | 198 | 110 |
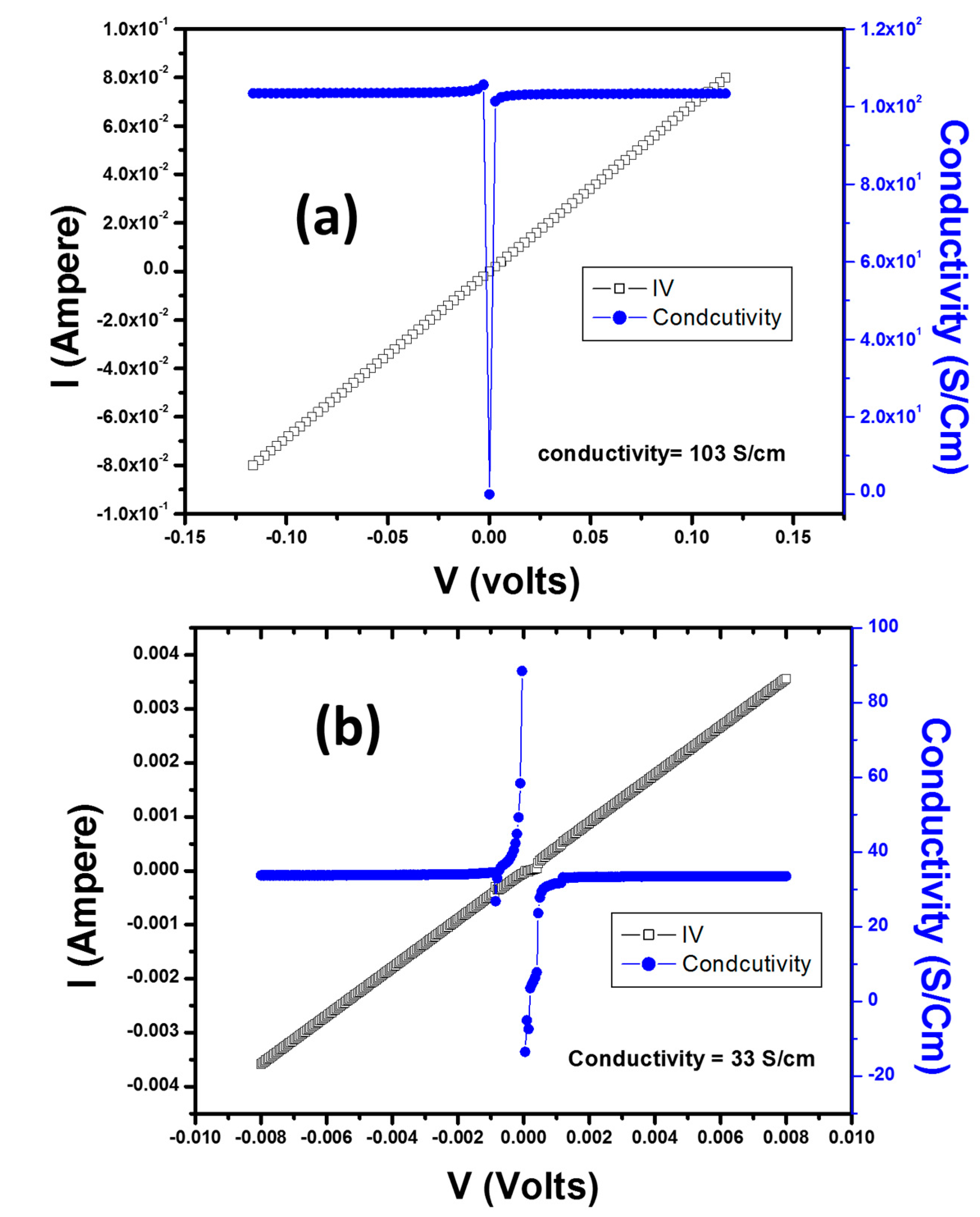
| Nanocomposites | Resistance (Ohm) | Conductivity (S/cm) | References |
|---|---|---|---|
| Ag | 10−5−5 | 6 × 105 | [52] |
| PS | 102–107 | 10−2 to 10−7 | [53] |
| PMMA | 1014–1016 | 10−14 to 10−16 | [53] |
| PS/Ag nanowires (A.R = 8) | 102 | 1 × 10−2 | [53] |
| PS/Ag nanowires (A.R = 31) | 103 | 1 × 10−3 | [53] |
| PS/Ag | 10−1 | 1.4 × 10−1 | [54] |
| PMMA/Ag | 1014–107 | 10−14–10−7 | [55] |
| PANI-PMMA/Ag | 109–10−4 | 10−9–104 | [55] |
| PS/Ag | 1.46 | 1.03 × 102 | Our Work |
| PMMA/Ag | 10−1 | 3.3 × 10 | Our Work |
| PS-PMMA/Ag | 73.98 × 103 | 40 × 10−3 | Our Work |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharaeh, E.H. Polystyrene-Poly(methyl methacrylate) Silver Nanocomposites: Significant Modification of the Thermal and Electrical Properties by Microwave Irradiation. Materials 2016, 9, 458. https://doi.org/10.3390/ma9060458
Alsharaeh EH. Polystyrene-Poly(methyl methacrylate) Silver Nanocomposites: Significant Modification of the Thermal and Electrical Properties by Microwave Irradiation. Materials. 2016; 9(6):458. https://doi.org/10.3390/ma9060458
Chicago/Turabian StyleAlsharaeh, Edreese H. 2016. "Polystyrene-Poly(methyl methacrylate) Silver Nanocomposites: Significant Modification of the Thermal and Electrical Properties by Microwave Irradiation" Materials 9, no. 6: 458. https://doi.org/10.3390/ma9060458





