Microstructural Anisotropy of Magnetocaloric Gadolinium Cylinders: Effect on the Mechanical Properties of the Material
Abstract
:1. Introduction
2. Experimental
2.1. Metallographic Analyses
2.2. XRD Phase Analysis
2.3. Mechanical Testing
3. Results
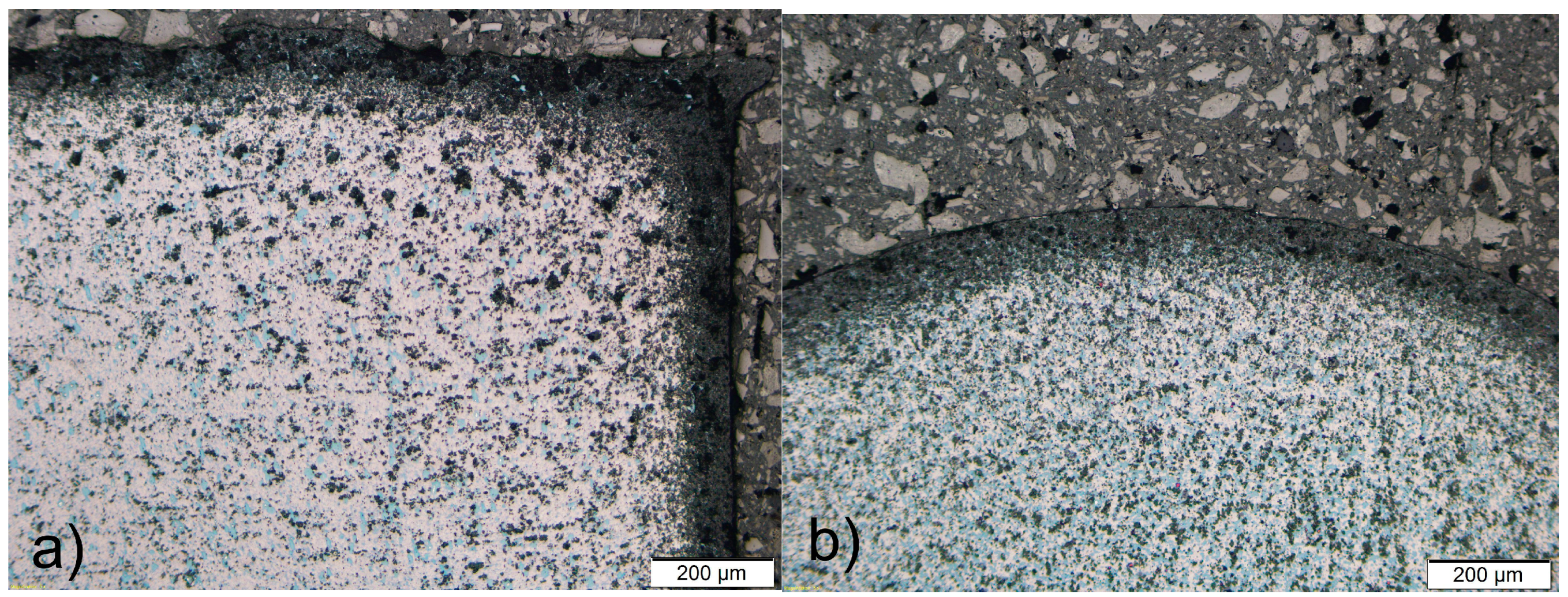
3.1. Metallography
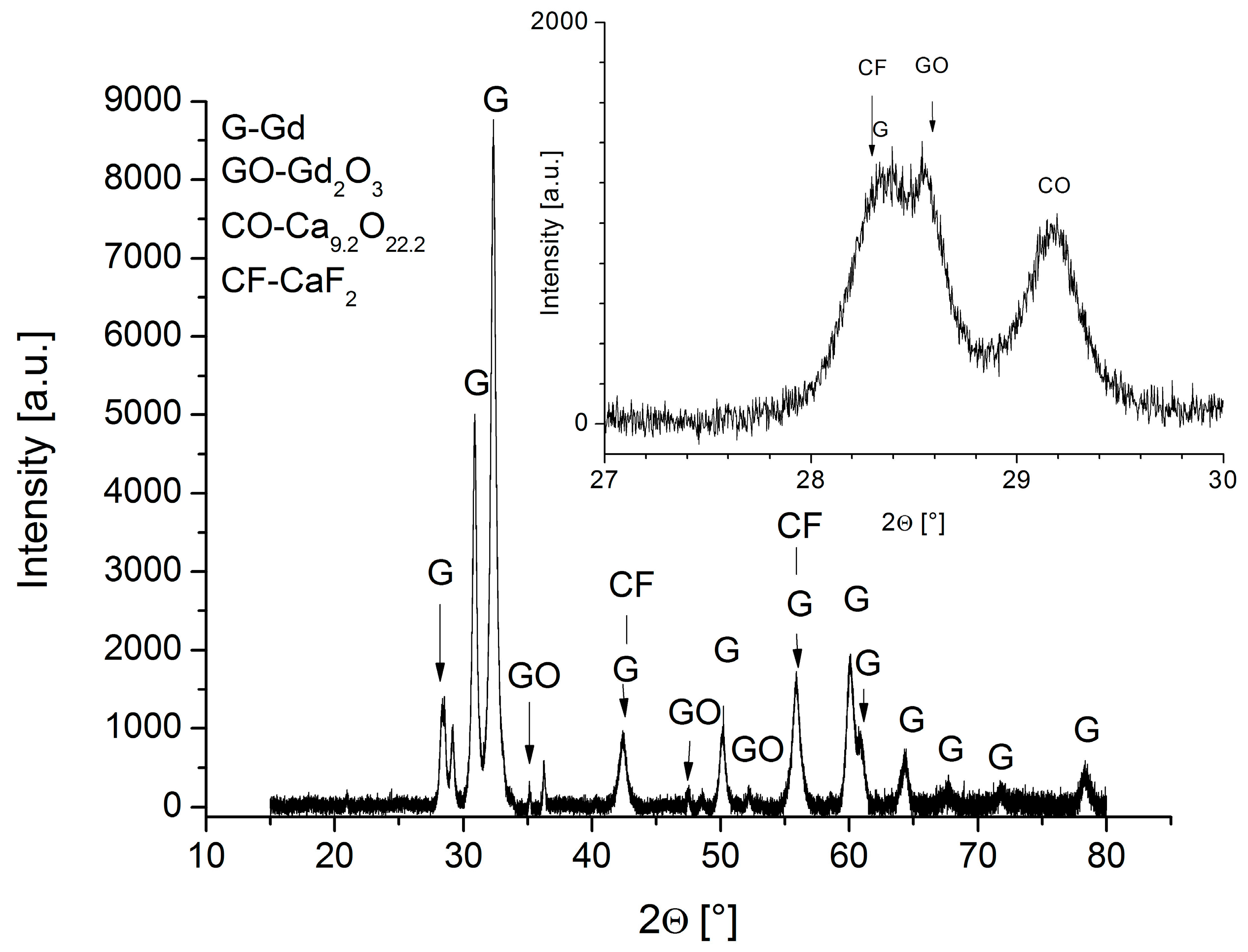
3.2. XRD Analysis
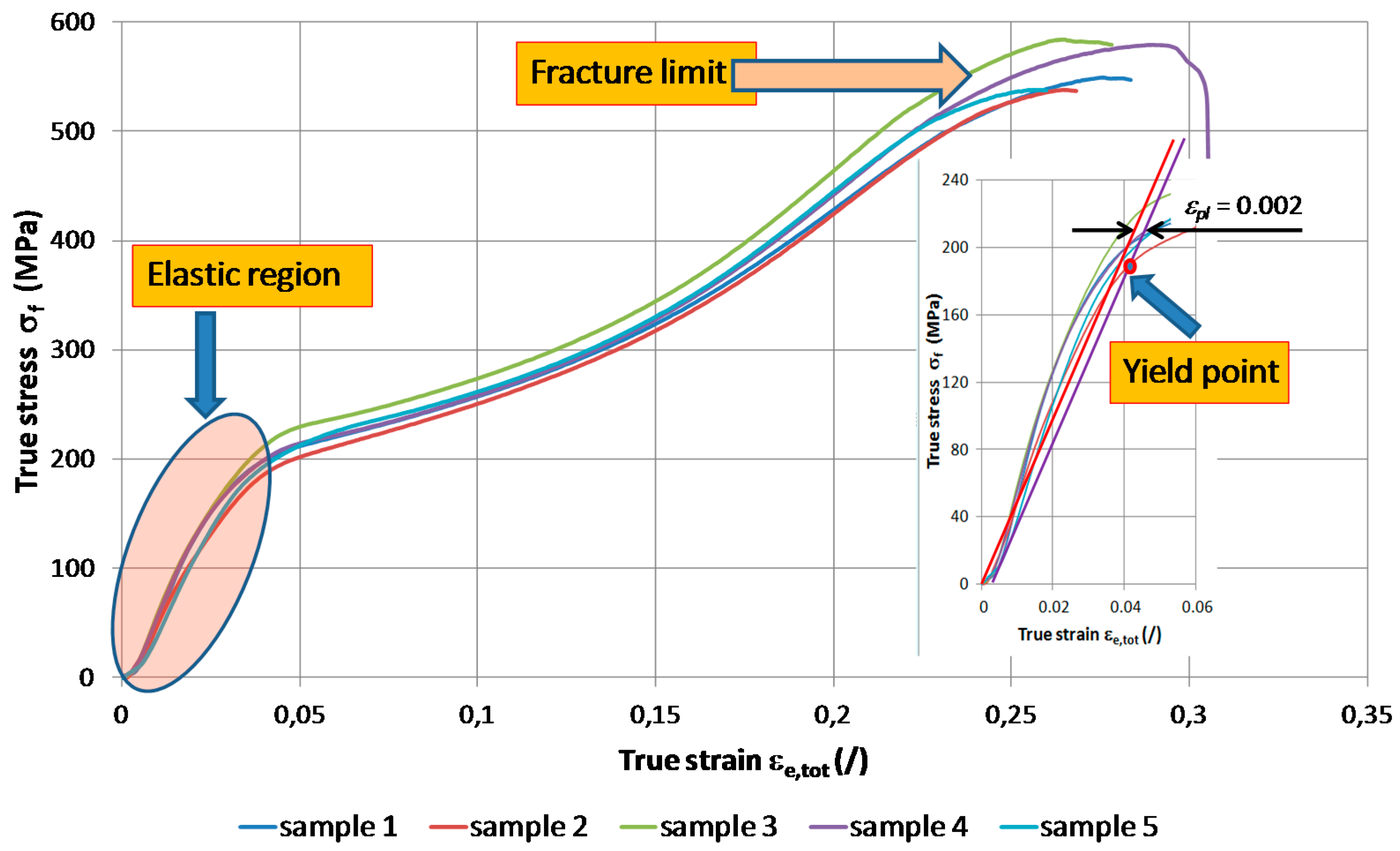
3.3. Mechanical Properties and Flow Curve
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005; p. 508. [Google Scholar]
- Brück, E.; Tegus, O.; Thanh, D.T.C.; Buschow, K.H.J. Magnetocaloric refrigeration near room temperature. J. Magn. Magn. Mater. 2007, 310, 2793–2799. [Google Scholar] [CrossRef]
- Rosario, L.; Rahman, M.M. Analysis of a magnetic refrigerator. Appl. Therm. Eng. 2011, 31, 1082–1090. [Google Scholar] [CrossRef]
- Sandeman, K.G. Magnetocaloric materials: The search for new systems. Scr. Mater. 2012, 67, 566–571. [Google Scholar] [CrossRef]
- Vuarnoz, D.; Kawanami, T. Numerical analysis of a reciprocating active magnetic regenerator made of gadolinium wires. Appl. Therm. Eng. 2012, 37, 388–395. [Google Scholar] [CrossRef]
- Wu, H.Y.; Liu, J.; Zhao, H.F.; Jiang, Q.; Xu, Y.; Xu, J. Effect of surface microstructure of aluminium coating on corrosion properties of magnetic refrigerant gadolinium. Trans. Nonfer. Met. Soc. 2013, 23, 3280–3285. [Google Scholar] [CrossRef]
- Plaznik, U.; Tušek, J.; Kitanovski, A.; Poredoš, A. Numerical and experimental analyses of different magnetic thermodynamic cycles with an active magnetic regenerator. Appl. Therm. Eng. 2013, 59, 52–59. [Google Scholar] [CrossRef]
- Tušek, J.; Kitanovski, A.; Zupan, S.; Prebil, I.; Poredoš, A. A comprehensive experimental analysis of gadolinium active magnetic regenerators. Appl. Therm. Eng. 2013, 53, 57–66. [Google Scholar] [CrossRef]
- Taskaev, S.V.; Kuz’min, M.D.; Skokov, K.P.; Karpenkov, D.Y.; Pellenen, A.P.; Buchelnikov, V.D.; Gutfleisch, O. Giant induced anisotropy ruins the magnetocaloric effect in gadolinium. J. Magn. Magn. Mater. 2013, 331, 33–36. [Google Scholar] [CrossRef]
- Brown, J.S.; Domanski, P.A. Review of alternative cooling technologies. Appl. Therm. Eng. 2014, 64, 252–262. [Google Scholar] [CrossRef]
- Ožbolt, M.; Kitanovski, A.; Tušek, J.; Poredoš, A. Electrocaloric vs. magnetocaloric energy. Int. J. Refrig. 2014, 37, 16–27. [Google Scholar] [CrossRef]
- Chennabasappa, M.; Chevalier, B.; Lahaye, M.; Labrugere, C.; Toulemonde, O. A core-shell phenomenon maintain the magnetocaloric properties of the ternary silicide Gd6Co1.67Si3 during water flux ageing. J. Alloy. Compd. 2014, 584, 34–40. [Google Scholar] [CrossRef]
- Forchelet, J.; Zamni, L.; El Maudni, S.E.; Hu, J.; Balli, M. Corrosion behaviour of gadolinium and La–Fe–Co–Si compounds in various heat conducting fluids. Int. J. Refrig. 2014, 37, 307–313. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between rare earth and transition metals working as magnetic materials in an AMR refrigerator in the room temperature range. Appl. Therm. Eng. 2015, 91, 767–777. [Google Scholar] [CrossRef]
- Kitanovski, A.; Tušek, J.; Tomc, T.; Plaznik, U.; Ožbolt, M.; Poredoš, A. Magnetocaloric Energy Conversion: From Theory to Applications; Springer: Basel, Switzerland, 2015; p. 456. [Google Scholar]
- Mansanares, A.M.; Gandra, F.C.G.; Soffner, M.E.; Guimaraes, A.O.; da Silva, E.C.; Vargas, H.; Marin, E. Anisotropic magnetocaloric effect in gadolinium thin films: Magnetization measurements and acoustic detection. J. Appl. Phys. 2013, 114, 163905. [Google Scholar] [CrossRef]
- Murokh, A.; Solovyov, V.; Agustsson, R.; O’Shea, F.H.; Chubar, O.; Chen, Y.; Grandsaert, T., II. Textured dysprosium and gadolinium poles for high-field, short period hybrid undulators. Nucl. Instr. Meth. Phys. Res. 2014, 735, 521–527. [Google Scholar] [CrossRef]
- Miao, R.; Zhang, X.; Zhu, Q.; Zhang, Z.; Wang, Z.; Yan, S.; Chen, D.; Zhou, L.; Li, Z. Impurities especially titanium in the rare earth metal gadolinium-before and after solid state electrotransport. J. Rare Earth 2014, 32, 1073–1079. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Fu, K.; Wang, C.; Zheng, J.; Xu, L.; Tian, W.; Li, X. Hydrogen in-situ refining method for preparing high purity gadolinium. J. Alloy. Compd. 2015, 648, 29–33. [Google Scholar] [CrossRef]
- Podmiljšak, B.; Škulj, I.; Markoli, B.; Žužek Rozman, K.; McGuiness, P.J.; Kobe, S. Microstructural changes in Fe-doped Gd5Si2Ge2. J. Magn. Magn. Mater. 2009, 321, 300–304. [Google Scholar] [CrossRef]
- Kuznetsova, Y.V.; Suponev, N.P.; Degteva, O.B.; Salev, P.S. Atomic force microscopy study of Gd(Co,Cu)5 single-crystal surface relief. J. Surf. Investig. 2011, 5, 1107–1111. [Google Scholar] [CrossRef]
- Penton-Madrigal, A.; de Oliveira, L.A.S.; Sinnecker, J.P.; Souza, D.M.; Grössinger, R.; Concepcion-Rosabal, B.; Estevez-Rams, E.; Diaz-Castanon, S. Coercitivity behavior in Gd(Co1-xCux)5 system as function of the microstructure evolution. Phys. B Condens. Matter. 2013, 414, 67–71. [Google Scholar] [CrossRef]
- Zeng, H.; Kuang, C.; Zhang, J.; Yue, M. Thermal properties and microstructure of bulk nanocrystalline Gd material. J. Mater. Sci. 2009, 44, 5509–5514. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, Y.; Zhang, J.; Kuang, C.; Yue, M.; Zhou, S. Grain size-dependent electrical resistivity of bulk nanocrystalline Gd metals. Prog. Nat. Sci. 2013, 23, 18–22. [Google Scholar] [CrossRef]
- Križan, J.; Mazaj, M.; Kavčič, V.; Bajsić, I.; Možina, J. Synthesis of Er- and Yb-doped gadolinium oxide polymorphs and influence of their structures on upconversion properties. Acta Chim. Slov. 2014, 61, 608–614. [Google Scholar] [PubMed]
- Chen, D.; Wang, Y.; Ma, E.; Yu, Y.; Liu, F. Partition, luminescence and energy transfer of Er3+/Yb3+ ions in oxyfluoride glass ceramic containing CaF2 nano-crystals. Opt. Mater. 2007, 29, 1693–1699. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, J.H.; Lim, K.S. Spatially selective Er/Yb-doped CaF2 crystal formation by CO2 laser exposure. Appl. Surf. Sci. 2014, 317, 98–102. [Google Scholar] [CrossRef]
- Imanieh, M.H.; Yekta, B.E.; Marghussian, V.; Shakhesi, S. Crystallization of nano calcium fluoride in CaF2-Al2O3-SiO2 system. Solid State Sci. 2013, 17, 76–82. [Google Scholar] [CrossRef]
- Taskaev, S.; Skokov, K.; Khovaylo, V.; Buchelnikov, V.; Pellenen, A.; Karpenkov, D.; Ulyanov, M.; Bataev, D.; Lyange, M.; Gutfleisch, O. Effect of severe plastic deformation on the specific heat and magnetic properties of cold rolled Gd sheets. J. Appl. Phys. 2015, 117, 123914. [Google Scholar] [CrossRef]
- Golchin, A.; Simmons, G.F.; Glavatskih, S.B. Break-away friction of PTFE materials in lubricated conditions. Tribol. Int. 2012, 48, 54–62. [Google Scholar] [CrossRef]
- ASM Materials Information. Available online: http://products.asminternational.org/hbk/index.jsp (accessed on 22 March 2016).
- Mortier, M.; Auzel, F. Rare-earth doped transparent glass-ceramics with high cross-sections. J. Non-Cryst. Solids 1999, 256, 361–365. [Google Scholar] [CrossRef]
- Luo, C. Modeling the Behavior of Inclusions in Plastic Deformation of Steels. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, May 2001. [Google Scholar]
- Auinger, M.; Müller-Lorenz, E.-M.; Rohwerder, M. Modelling and experiment of selective oxidation and nitridation of binary model alloys at 700 °C—The systems Fe, 1 wt. % {Al, Cr, Mn, Si}. Corros. Sci. 2015, 90, 503–510. [Google Scholar] [CrossRef]

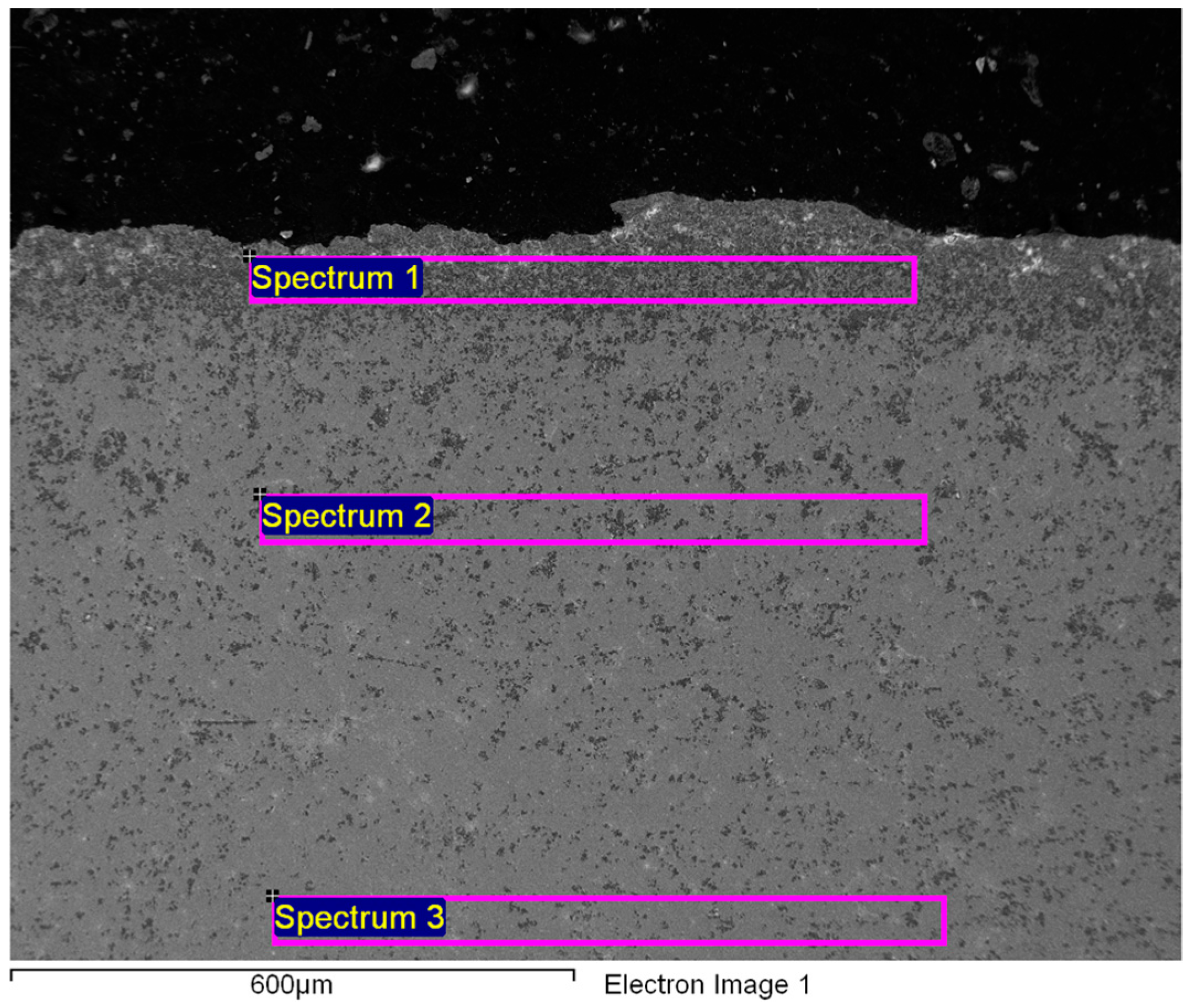


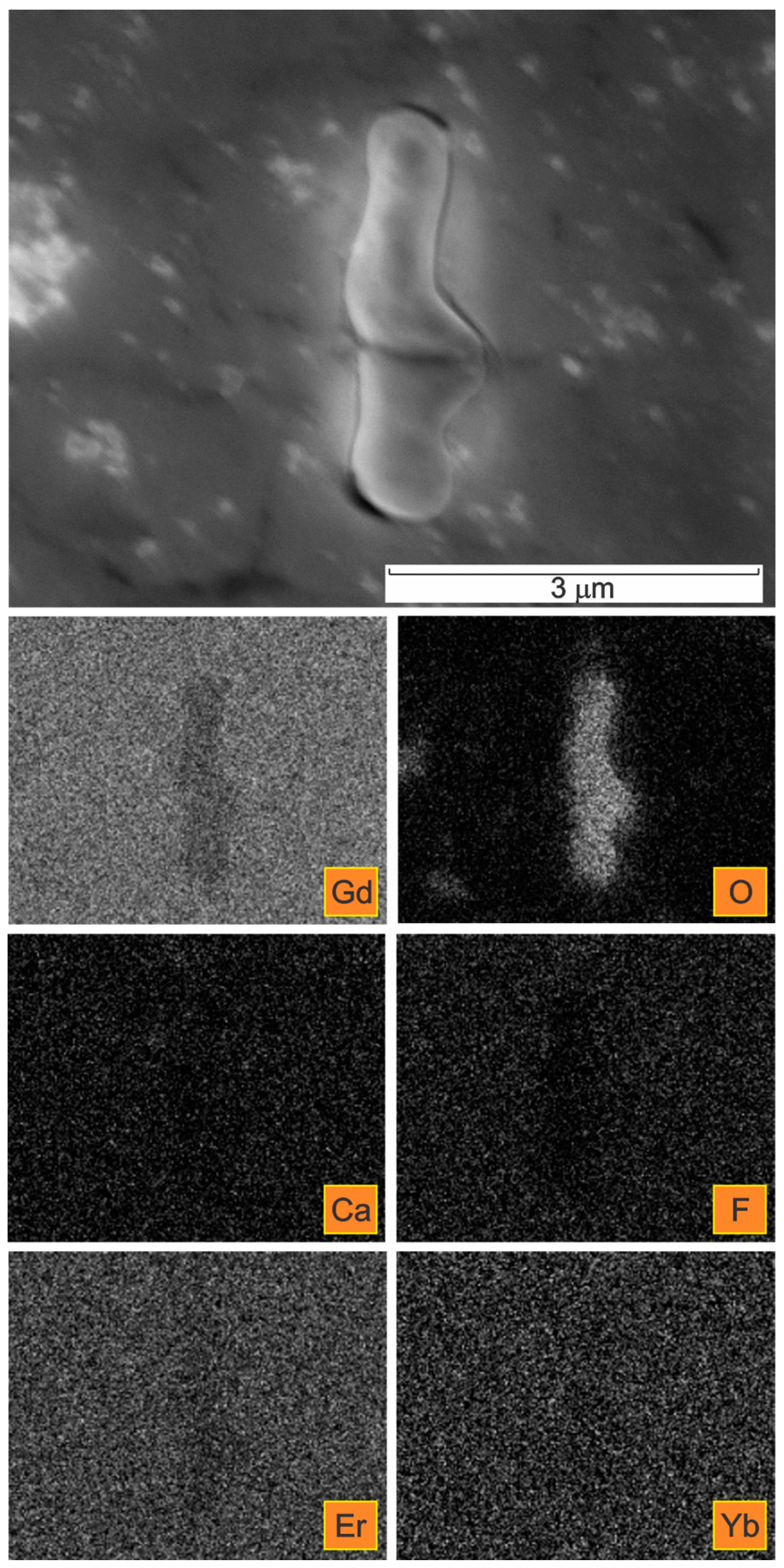
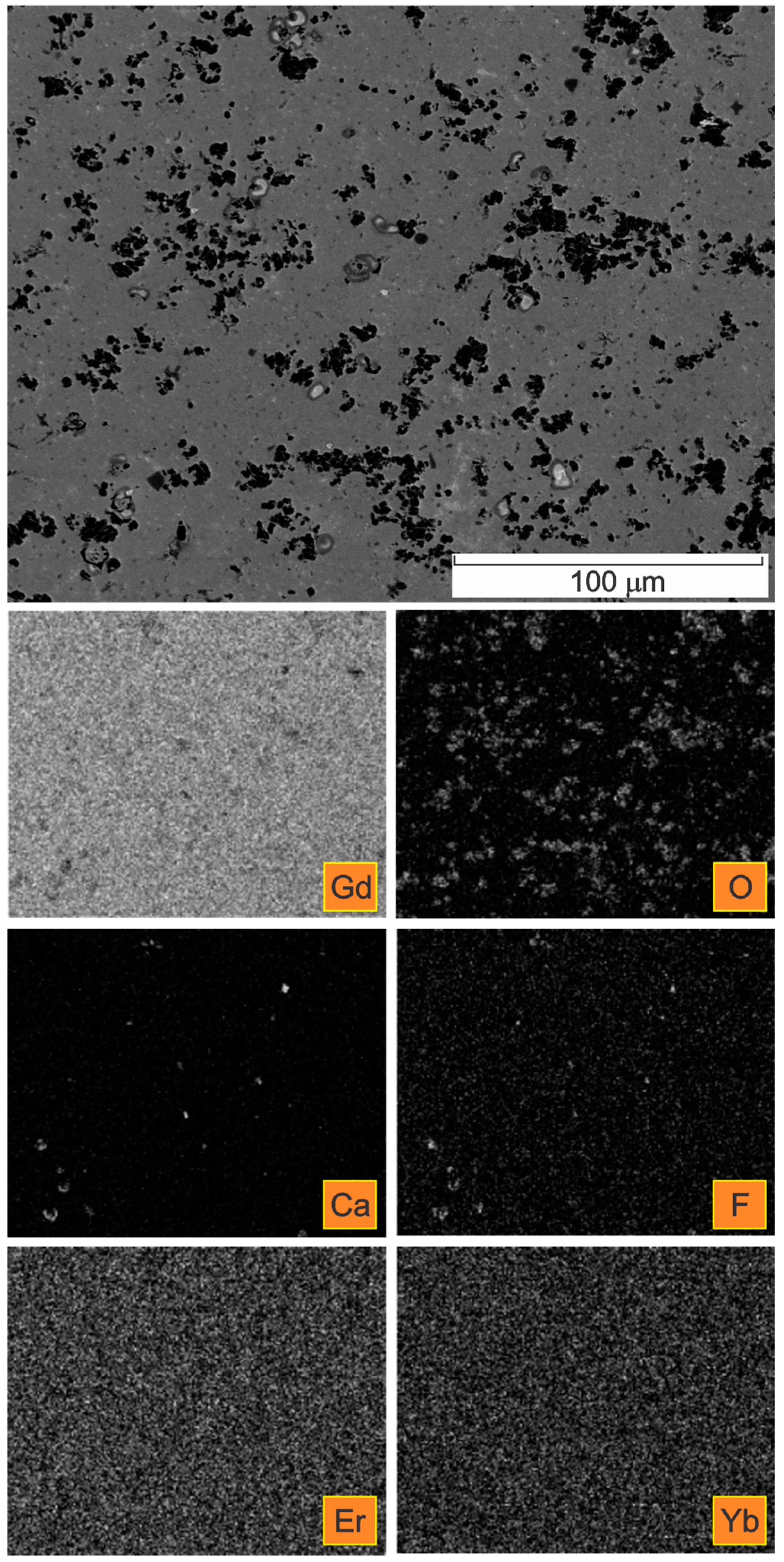
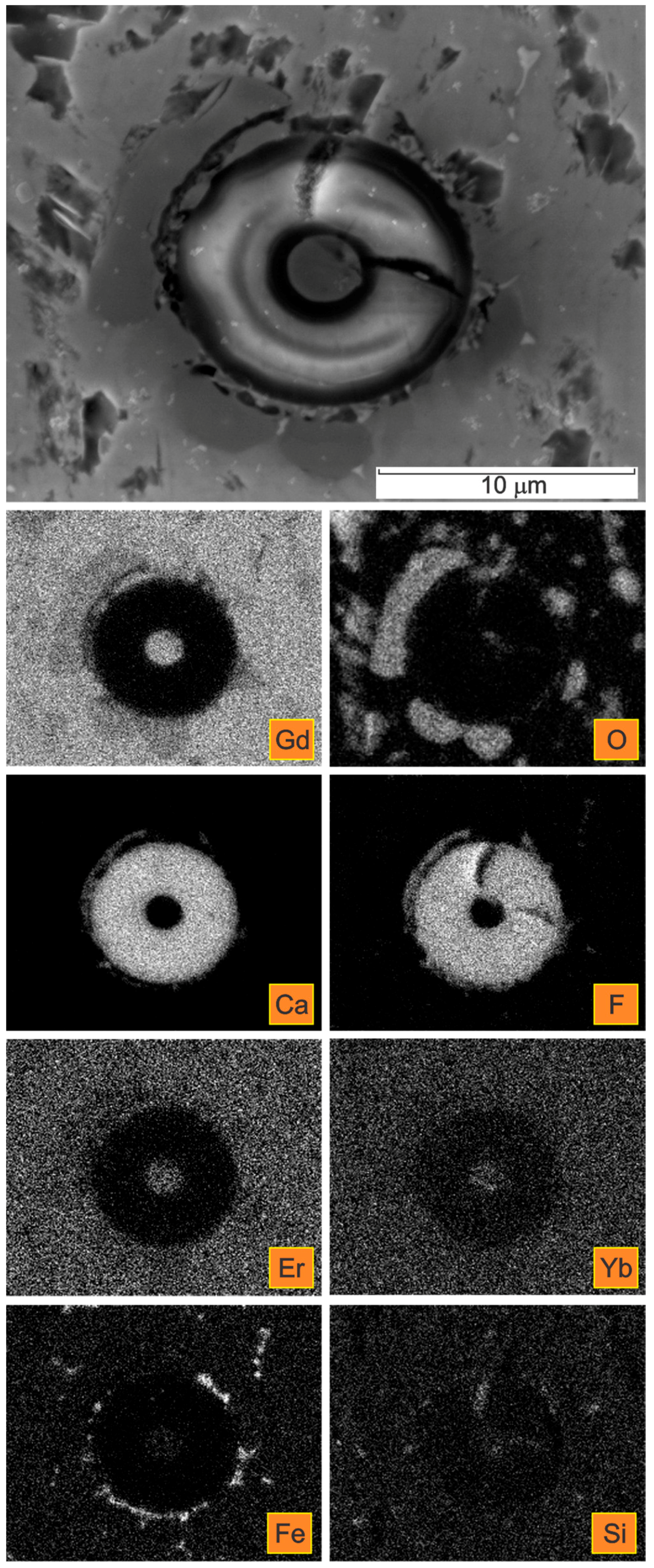
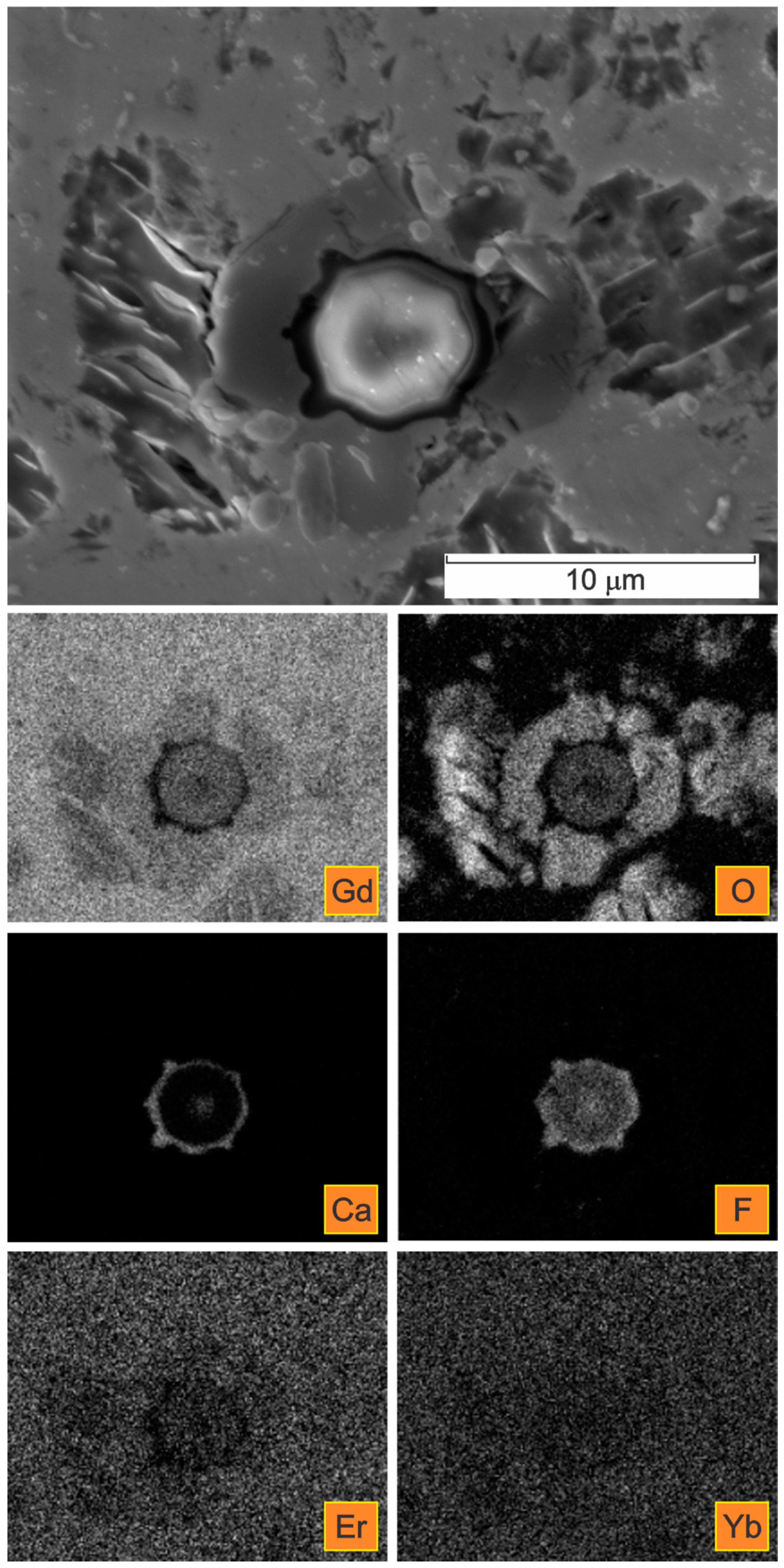
| Spectrum | Gd | Er | Yb | Si | F | Ca | O | C * |
|---|---|---|---|---|---|---|---|---|
| 1/wt. % | 76.41 | 0.00 | 0.00 | 0.69 | 0.00 | 0.00 | 15.81 | 7.09 |
| 2/wt. % | 86.38 | 4.00 | 2.99 | 0.00 | 0.00 | 0.00 | 4.22 | 2.41 |
| 3/wt. % | 91.75 | 0.00 | 1.87 | 0.00 | 0.00 | 0.00 | 2.43 | 2.15 |
| Spectrum | Gd | Er | Yb | W | F | Ca | O | C * |
|---|---|---|---|---|---|---|---|---|
| 1/wt. % | 85.00 | 4.87 | 2.26 | 1.17 | 0.00 | 0.00 | 4.03 | 2.67 |
| Parameter | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Average |
|---|---|---|---|---|---|---|
| E [MPa] | 5887 | 5020 | 5737 | 5510 | 5488 | 5528 |
| k [MPa] | −4.6 | −2.2 | −1.3 | 0.3 | −10.9 | −3.7 |
| σf0 [MPa] | 184 | 176 | 200 | 185 | 186 | 186 |
| R2 [/] | 0.9813 | 0.9924 | 0.9862 | 0.9805 | 0.9915 | 0.9864 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovič, D.S.; Šturm, R.; Naglič, I.; Markoli, B.; Pepelnjak, T. Microstructural Anisotropy of Magnetocaloric Gadolinium Cylinders: Effect on the Mechanical Properties of the Material. Materials 2016, 9, 382. https://doi.org/10.3390/ma9050382
Petrovič DS, Šturm R, Naglič I, Markoli B, Pepelnjak T. Microstructural Anisotropy of Magnetocaloric Gadolinium Cylinders: Effect on the Mechanical Properties of the Material. Materials. 2016; 9(5):382. https://doi.org/10.3390/ma9050382
Chicago/Turabian StylePetrovič, Darja Steiner, Roman Šturm, Iztok Naglič, Boštjan Markoli, and Tomaž Pepelnjak. 2016. "Microstructural Anisotropy of Magnetocaloric Gadolinium Cylinders: Effect on the Mechanical Properties of the Material" Materials 9, no. 5: 382. https://doi.org/10.3390/ma9050382






