Lime as an Anti-Plasticizer for Self-Compacting Clay Concrete
Abstract
:1. Introduction
2. Materials
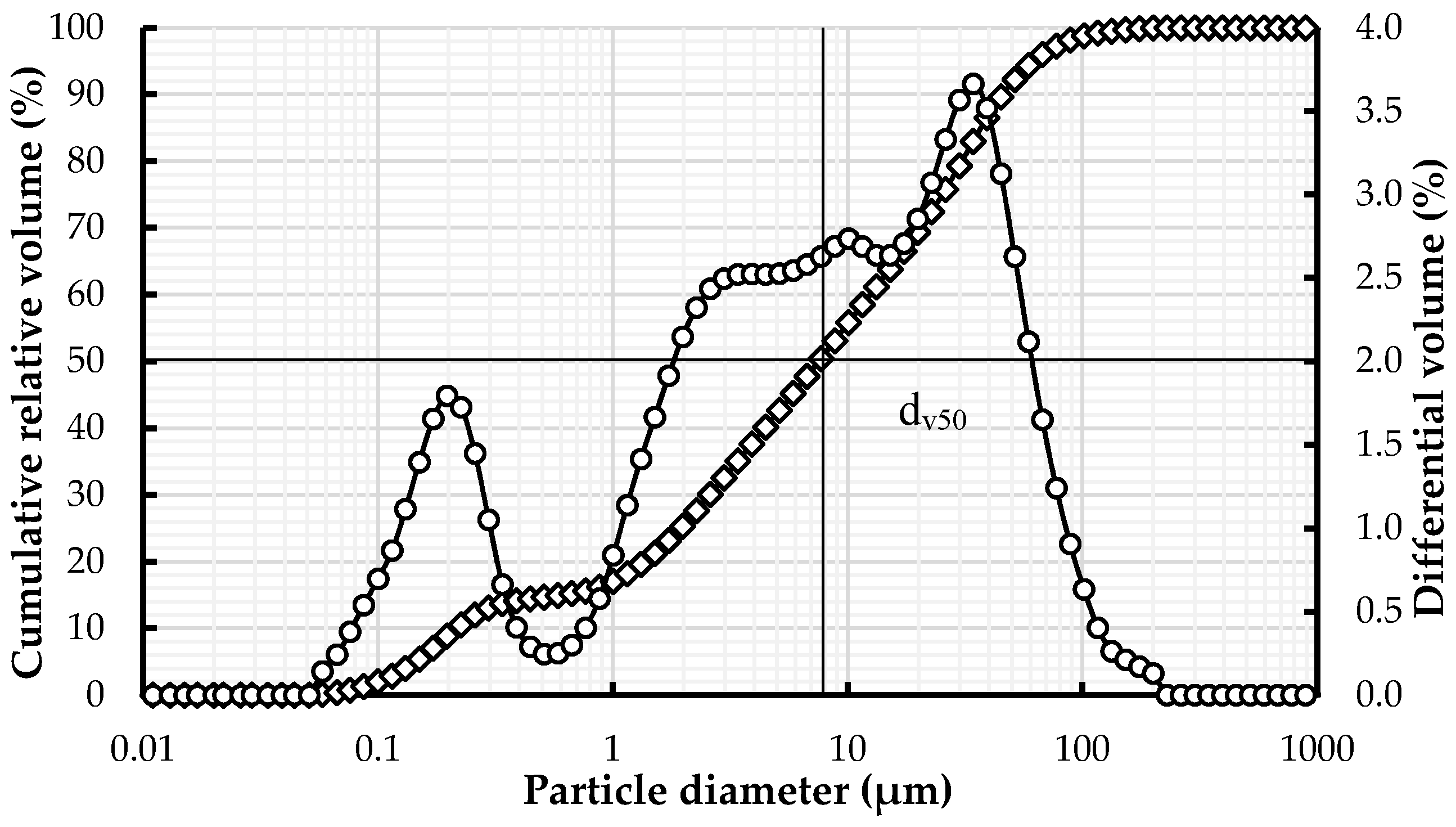
2.1. Earth
2.2. Dispersants-Deflocculants
2.3. Calcium Products
3. Measurement Procedures
3.1. Rheology Measurements
3.1.1. Yield Stress Measurements
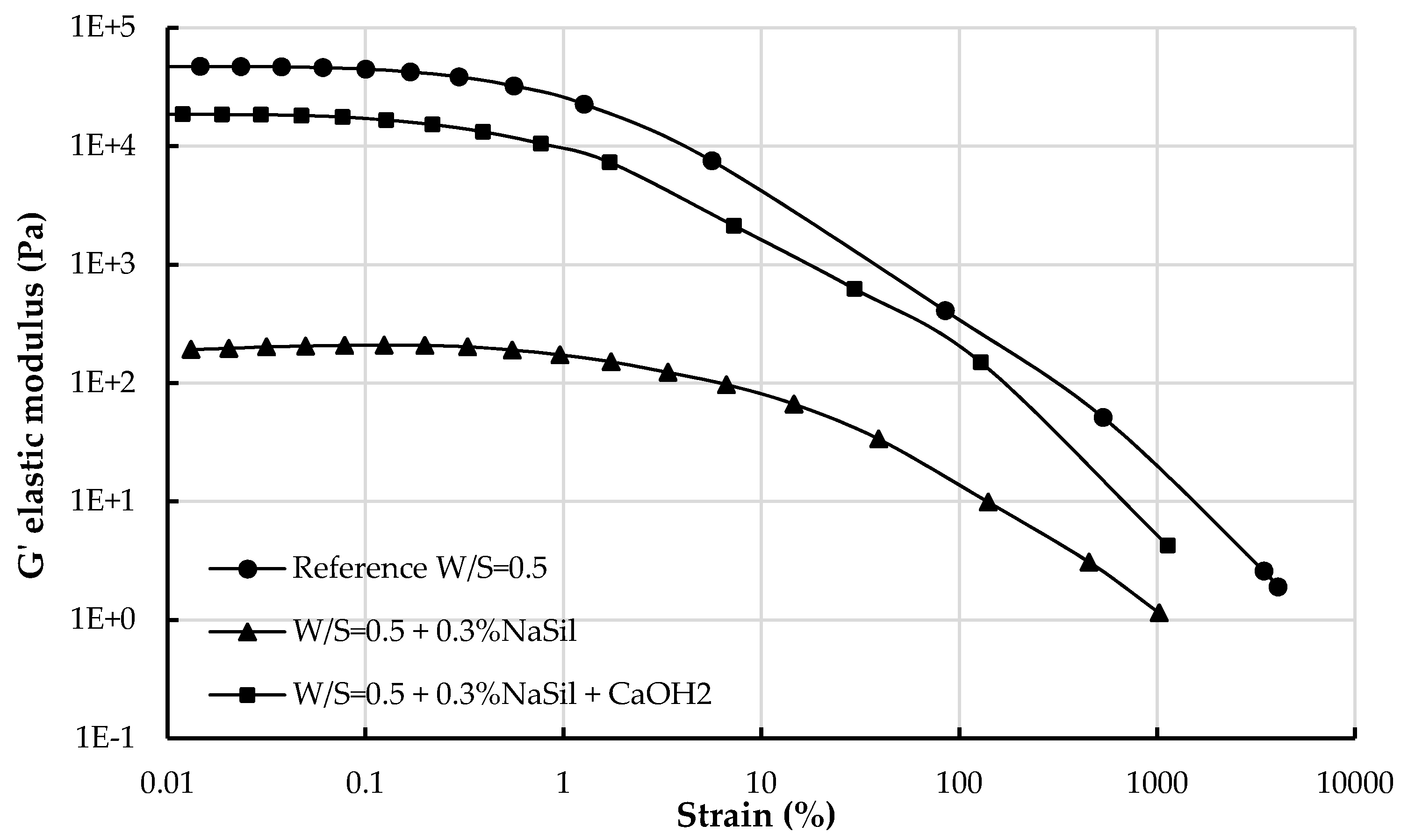
3.1.2. Viscoelastic Properties
3.2. Zeta Potential Measurements
3.3. Mineral Characterization
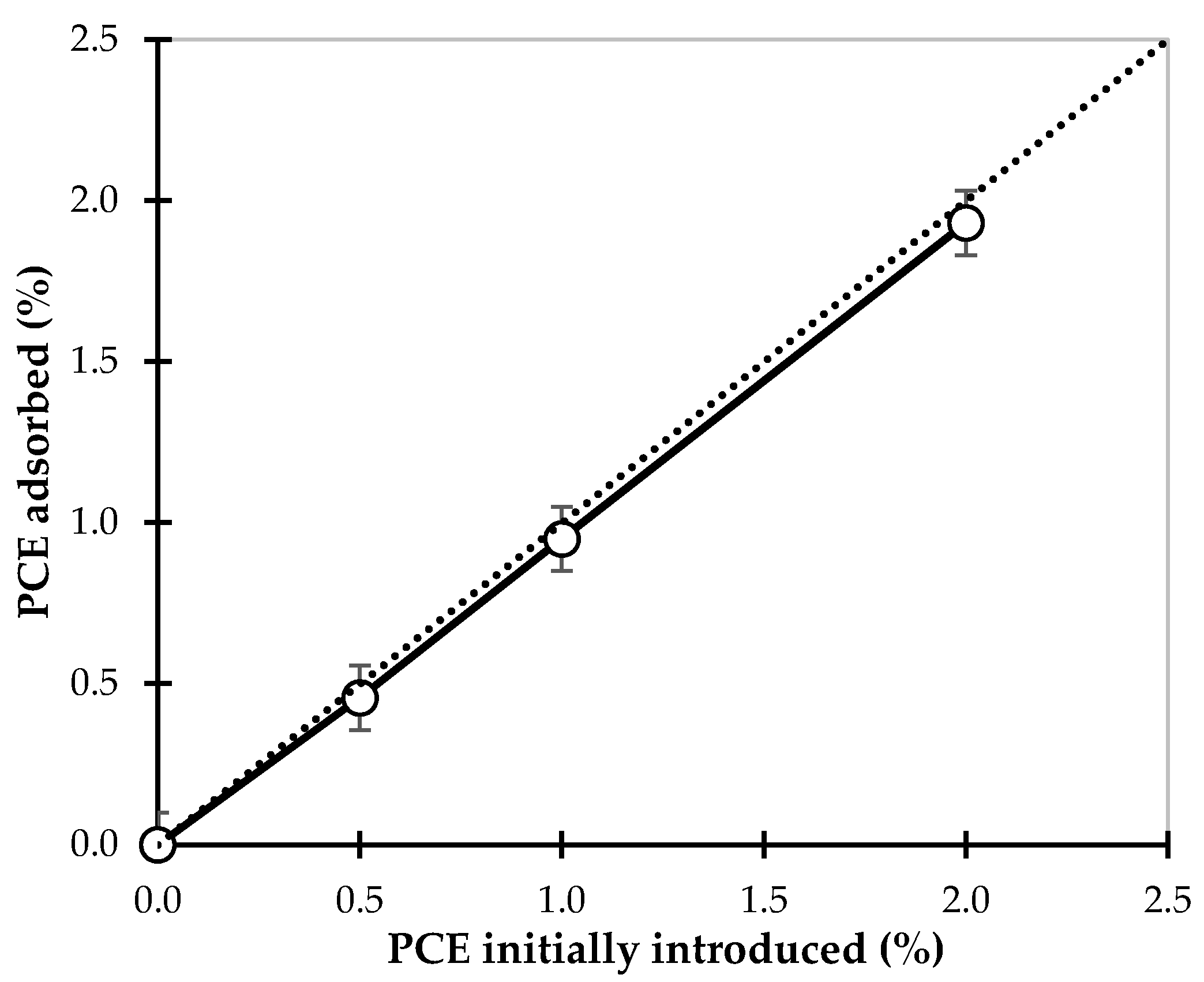
3.4. Adsorption Measurements
4. Experimental Results and Discussion
4.1. Effect of Dispersants on Earth Products
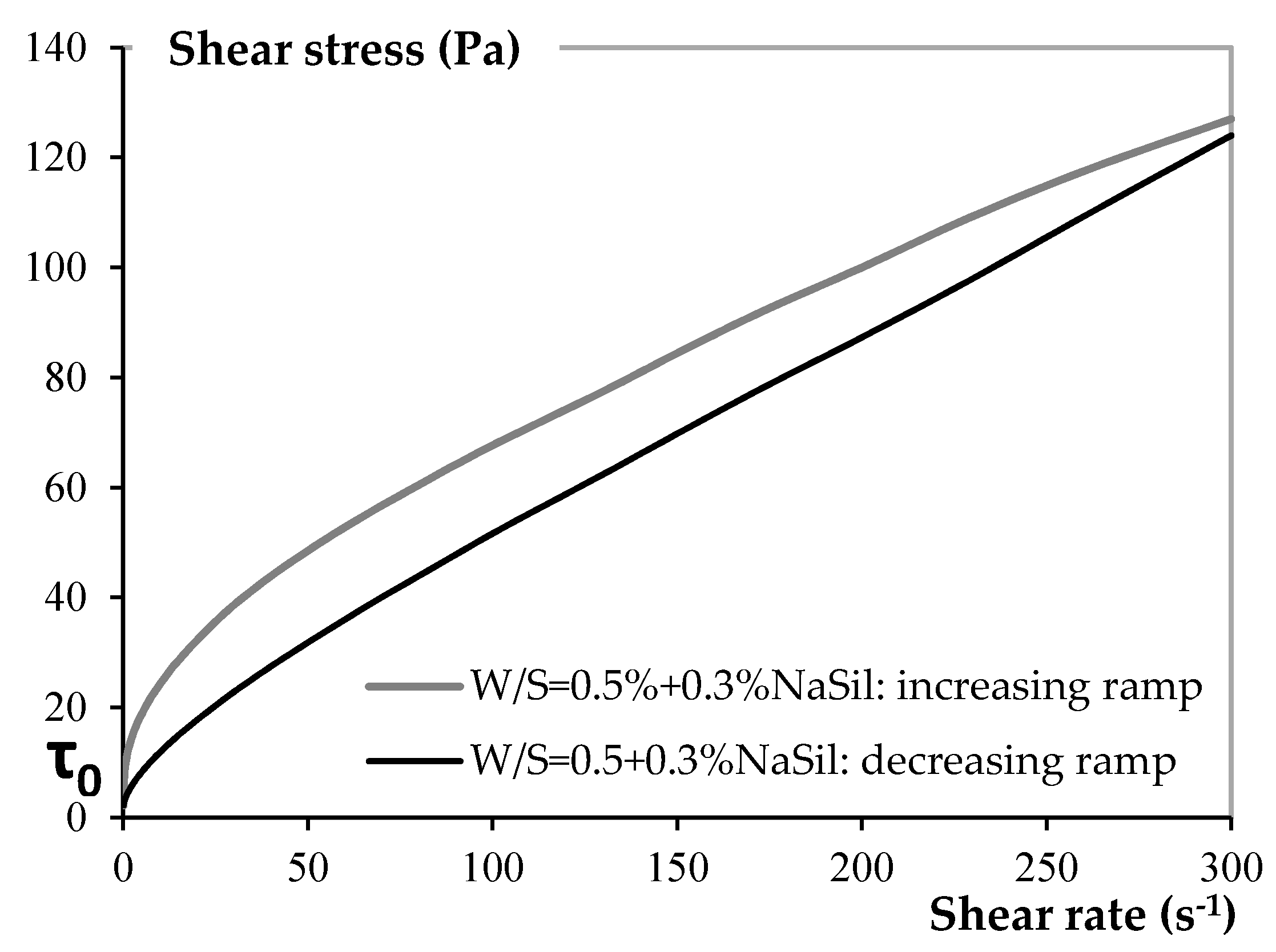
4.1.1. Rheology
4.1.2. Zeta Potential
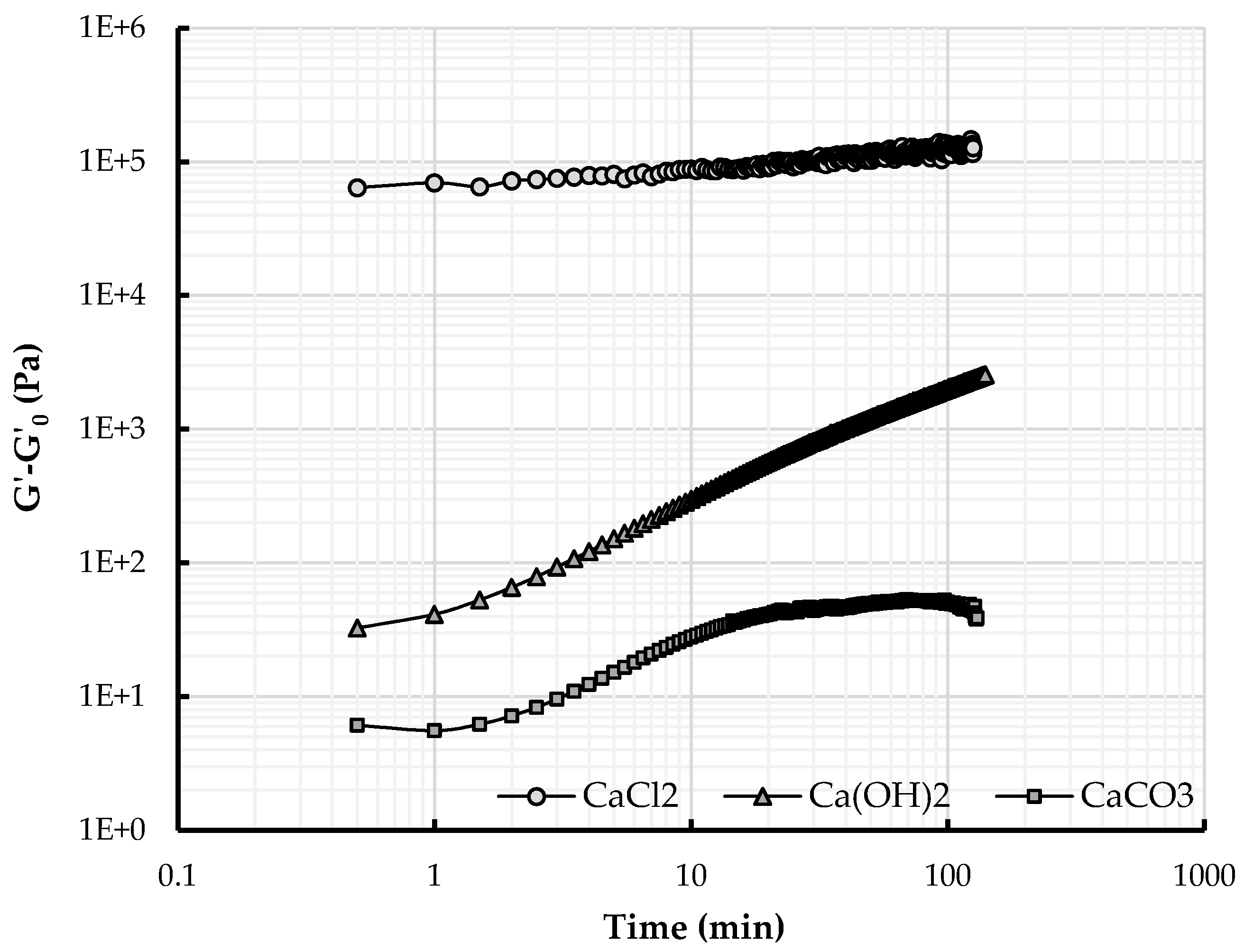
4.2. Effect of Releasing Calcium
4.2.1. Calcium Ions and Structuration over Time
4.2.2. Structuration and Nature of Interactions
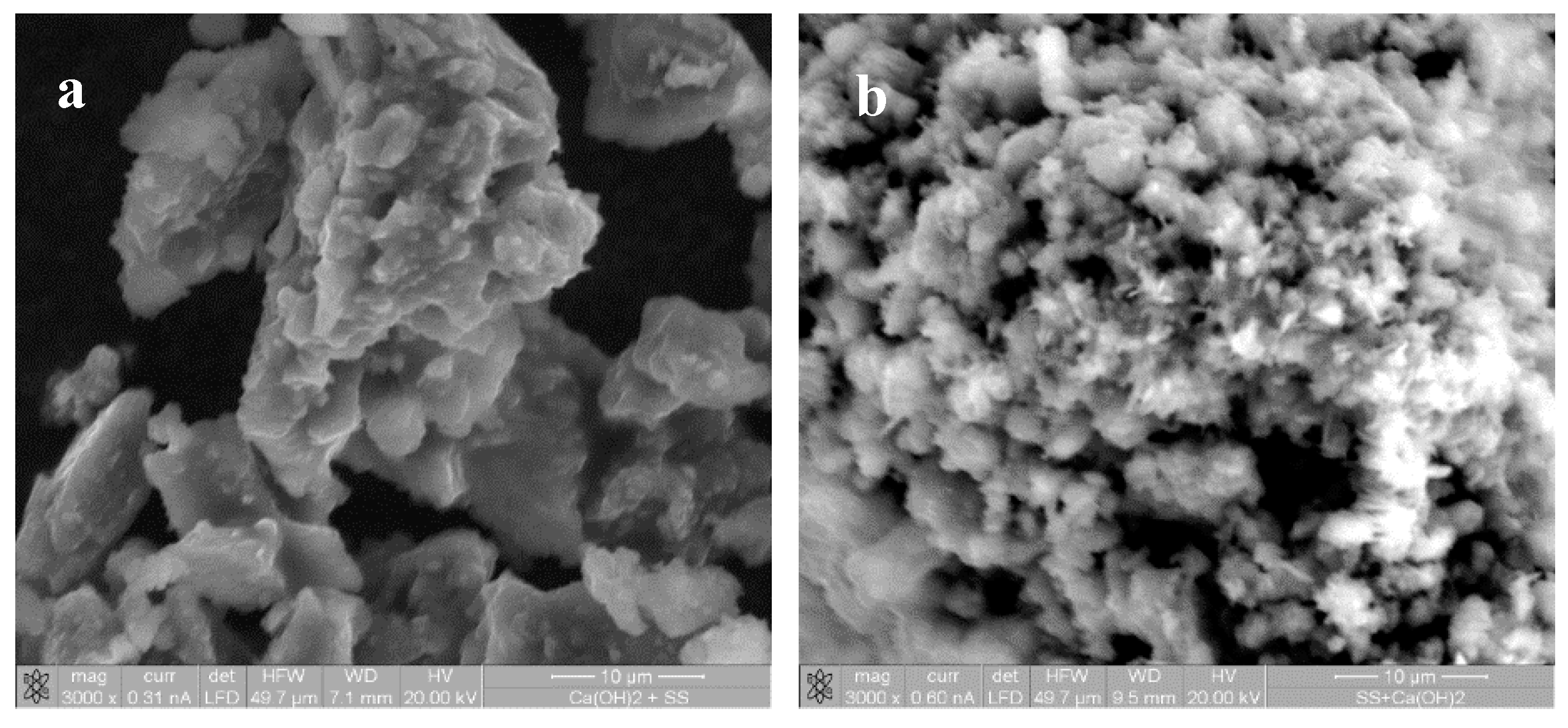
4.3. Identification of Reaction Products
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fontaine, L.; Anger, R. Building with Earth—From Sand Grain to Architecture; Belin/Cité des Sciences et de l’Industrie: Paris, France, 2009. [Google Scholar]
- Morel, J.C.; Pkla, A.; Walker, P. Compressive strength testing of compressed earth blocks. Constr. Build. Mater. 2007, 21, 303–309. [Google Scholar] [CrossRef]
- Shukla, A.; Tiwari, G.N.; Sodha, M.S. Embodied energy analysis of adobe house. Renew. Energy 2009, 34, 755–761. [Google Scholar] [CrossRef]
- Parra-Saldivar, M.L.; Batty, W. Thermal behaviour of adobe constructions. Build. Environ. 2006, 41, 1892–1904. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production-present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Moevus, M.; Fontaine, L.; Anger, R.; Doat, P. Environmental Clay Concrete (B.A.E); Scientific Report, Programme C2D2; Ministère de l’Ecologie, du Développement Durable et de l’Energie: Paris, France, 2013.
- Kang, N.; Hwang, H. Study on high strengthening of an earth wall using earth and high performance lime. In Proceedings of the International Conference on Earth Architecture in Asia, Mokpo, Korea, 11–14 October 2011.
- Pellenq, R.J.M.; Lequeux, N.; van Damme, H. Engineering the bonding scheme in C–S–H: The iono-covalent framework. Cem. Concr. Res. 2008, 38, 159–174. [Google Scholar] [CrossRef]
- Kovler, K.; Roussel, N. Properties of fresh and hardened concrete. Cem. Concr. Res. 2011, 41, 775–792. [Google Scholar] [CrossRef]
- Horn, R.G. Surface forces and their action in ceramic materials. J. Am. Ceram. Soc. 1990, 73, 1117–1135. [Google Scholar] [CrossRef]
- Van Damme, H.; Review of Horn, R.G. Surface forces and their action in ceramic materials. J. Am. Ceram. Soc. 1990, 73, 1117–1135. [Google Scholar]
- Yammine, J.; Chaouche, M.; Guerinet, M.; Moranville, M.; Roussel, N. From ordinary rheology concrete to self compacting concrete: A transition between frictional and hydrodynamic interactions. Cem. Concr. Res. 2008, 38, 890–896. [Google Scholar] [CrossRef]
- Roussel, N.; Lemaître, A.; Flatt, R.J.; Coussot, P. Steady state flow of cement suspensions: A micromechanical state of the art. Cem. Concr. Res. 2010, 40, 77–84. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 978-0-08-044183-2. [Google Scholar]
- Andreola, F.; Castellini, E.; Ferreira, J.M.F.; Olhero, S.; Romagnoli, M. Effect of sodium hexametaphosphate and ageing on the rheological behaviour of kaolin dispersions. Appl. Clay Sci. 2005, 31, 56–64. [Google Scholar] [CrossRef]
- Andreola, F.; Castellini, E.; Manfredinia, T.; Romagnoli, M. The role of sodium hexametaphosphate in the dissolution process of kaolinite and kaolin. J. Eur. Ceram. Soc. 2004, 24, 2113–2124. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Unuabonah, I.E.; Olu-Owolabi, B.I. Adsorption of some heavy metal ions on sulphate- and phosphate-modified kaolin. Appl. Clay Sci. 2005, 29, 145–148. [Google Scholar] [CrossRef]
- Andreola, F.; Castellini, E.; Lusvardi, G.; Menabue, L.; Romagnoli, M. Release of ions from kaolinite dispersed in deflocculant solutions. Appl. Clay Sci. 2007, 36, 271–278. [Google Scholar] [CrossRef]
- Rossington, K.R.; Senapati, U.; Carty, W.M. A critical evaluation of dispersants for clay-based systems. Ceram. Eng. Sci. Proc. 1998, 19, 77–85. [Google Scholar]
- Papo, A.; Piani, L.; Ricceri, R. Sodium tripolyphosphate and polyphosphate as dispersing agents for kaolin suspensions: Rheological characterization. Colloids Surfaces A 2002, 201, 219–230. [Google Scholar] [CrossRef]
- Castellini, E.; Berthold, C.; Malferrari, D.; Bernini, F. Sodium hexametaphosphate interaction with 2:1 clay minerals illite and montmorillonite. Appl. Clay Sci. 2013, 83–84, 162–170. [Google Scholar] [CrossRef]
- Worrall, W.E.; Worrall, M.D. Clays and Ceramic Raw Materials; Springer Science & Business Media: London, UK, 1986. [Google Scholar]
- Manfredini, T.; Pellacani, G.C.; Pozzi, P. Sodium silicates as deflocculating agents for clays. Ind. Ceram. 1987, 7, 85–87. [Google Scholar]
- Rossington, K.R.; Senapati, U.; Carty, W.M. A critical evaluation of dispersants: Part II. Effects on rheology, pH, and specific adsorption. Ceram. Eng. Sci. Proc. 1999, 20, 119–131. [Google Scholar]
- Penner, D.; Lagaly, G. Influence of anions on the rheological properties of clay mineral dispersions. Appl. Clay Sci. 2001, 19, 131–142. [Google Scholar] [CrossRef]
- Diz, H.M.M.; Rand, B. The mechanism of deflocculation of kaolinite by polyanions. Br. Ceram. Trans. J. 1990, 89, 77–82. [Google Scholar]
- Andreola, F.; Romagnoli, M. Role of the surface treatment in the deflocculation of kaolinite. J. Am. Ceram. Soc. 2006, 89, 1107–1109. [Google Scholar] [CrossRef]
- Amoros, J.L.; Beltran, V.; Sanz, V.; Jarque, J.C. Electrokinetic and rheological properties of highly concentrated kaolin dipersions: Influence of particle fraction and dispersant concentration. Appl. Clay Sci. 2010, 49, 33–43. [Google Scholar] [CrossRef]
- Zaman, A.A.; Demir, F.; Finch, E. Effects of process variables and their interactions on solubility of metal ions from crude kaolin particles: Results of a statistical design of experiments. Appl. Clay Sci. 2003, 22, 237–250. [Google Scholar] [CrossRef]
- Zaman, A.A.; Mathur, S. Influence of dispersing agents and solution conditions on the solubility of crude kaolin. J. Colloid Interface Sci. 2004, 271, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Garforth, W.L.; Pruett, R.J. Influence of dispersant on the solubility of calcined kaolin. Appl. Clay Sci. 1998, 13, 137–147. [Google Scholar] [CrossRef]
- Bergmann, J.; Kleeberg, R. Rietveld analysis of disordered layer silicates. In Proceedings of the 5th European Conference on Powder Diffraction (EPDIC 5), Materials Science Forum, Parma, Italy, 24–28 May 1997; pp. 300–305.
- Bish, D.L.; Plötze, M. X-ray powder diffraction with emphasis on qualitative and quantitative analysis in industrial mineralogy. In EMU Notes in Mineralogy Vol. 9, Advances in the Characterization of Industrial Minerals; Christidis, G.E., Ed.; EMU and Mineralogical Society: Jena, Germany, 2011; pp. 35–76. [Google Scholar]
- Gelardi, G.; Mantellato, S.; Marchon, D.; Palacios, M.; Eberhardt, A.B.; Flatt, R.J. Chemistry of chemical admixtures. In Science and Technology of Concrete Admixtures; Aïtcin, P.C., Flatt, R.J., Eds.; Elsevier: Cambridge, UK, 2016; pp. 149–218. [Google Scholar]
- Nguyen, Q.D.; Boger, D.V. Yield stress measurement for concentrated suspensions. J. Rheol. 1983, 27, 321–349. [Google Scholar]
- Nachbaur, L.; Mutin, J.C.; Nonat, A.; Choplin, L. Dynamic mode rheology of cement and tricalcium silicate pastes from mixing to setting. Cem. Concr. Res. 2001, 31, 183–192. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, R. Review of the measurement of zeta potentials in concentrated aqueous suspensions using electroacoustics. Adv. Colloid Interface Sci. 2003, 106, 55–81. [Google Scholar] [CrossRef]
- Au, P.; Leong, Y.K. Rheological and zeta potential behaviour of kaolin and bentonite composite slurries. Colloids Surfaces A 2013, 436, 530–541. [Google Scholar] [CrossRef]
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation kinetics of a bed of recycled concrete aggregates: A laboratory stud on model materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- Saak, A.W.; Jennings, H.M.; Shah, S.P. The influence of wall slip on yield stress and visco-elastic measurements of cement pastes. Cem. Concr. Res. 2001, 31, 205–212. [Google Scholar] [CrossRef]
- Roussel, N. A theoretical frame to study stability of fresh concrete. Mater. Struct. 2006, 39, 81–91. [Google Scholar] [CrossRef]
- Gelardi, G.; Flatt, R.J. Working mechanisms of water reducers and superplasticizers. In Science and Technology of Concrete Admixtures; Aïtcin, P.C., Flatt, R.J., Eds.; Elsevier: Cambridge, UK, 2016; pp. 257–278. [Google Scholar]
- Flatt, R.J. Superplasticizers and the rheology of concrete. In Understanding Rheology of Concrete; Roussel, N., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 144–208. [Google Scholar]
- Jeknavorian, A.A.; Jardine, L.A.; Koyata, H.; Ou, C.C.; Folliard, K. Interaction of superplasticizers with clay-bearing aggregates. In Proceedings of the 7th Canmet/ACI International Conference Superplasticizers and Other Chemical Admixtures in Concrete, Berlin, Germany, October 2003; pp. 1293–1316.
- Lei, L.; Plank, J. A study on the impact of different clay minerals on the dispersing force of conventional and modified vinyl ether based polycarboxylate superplasticizers. Cem. Concr. Res. 2014, 60, 1–10. [Google Scholar] [CrossRef]
- Ng, S.; Plank, J. Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem. Concr. Res. 2012, 42, 847–854. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Xu, Z.; Liu, Q.; Zeng, H. Effect of polycarboxylate ether comb-type polymer on viscosity and interfacial properties of kaolinite clay suspensions. J. Colloid Interface Sci. 2012, 378, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Marchon, D.; Mantellato, S.; Eberhardt, A.B.; Flatt, R.J. Adsorption of chemical admixtures. In Science and Technology of Concrete Admixtures; Aïtcin, P.C., Flatt, R.J., Eds.; Elsevier: Cambridge, UK, 2016; pp. 219–256. [Google Scholar]
- Kura, G.; Ohashi, S. Complex formation of cyclic phosphate anions with bivalent cations. J. Inorg. Nucl. Chem. 1974, 36, 1605–1609. [Google Scholar] [CrossRef]
- Maloney, C.E.; Lemaitre, A. Amorphous systems in athermal, quasistatic shear. Phys. Rev. E 2006, 74, 016118. [Google Scholar] [CrossRef] [PubMed]
- Zukoski, C.F. Particles and suspensions in chemical engineering: Accomplishments and prospects. Chem. Eng. Sci. 1995, 50, 4073–4079. [Google Scholar] [CrossRef]
- Roussel, N.; Ovarlez, G.; Garrault, S.; Brumaud, C. The origins of thixotropy of fresh cement pastes. Cem. Concr. Res. 2012, 42, 148–157. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicates hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Nonat, A. The structure and stoichiometry of C–S–H. Cem. Concr. Res. 2004, 34, 1521–1528. [Google Scholar] [CrossRef]
- Fabbri, M.; Celotti, G.C.; Ravaglioli, A. Hydroxyapatite-based porous aggregates: Physico-chemical nature, structure, texture and architecture. Biomaterials 1995, 16, 225–228. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
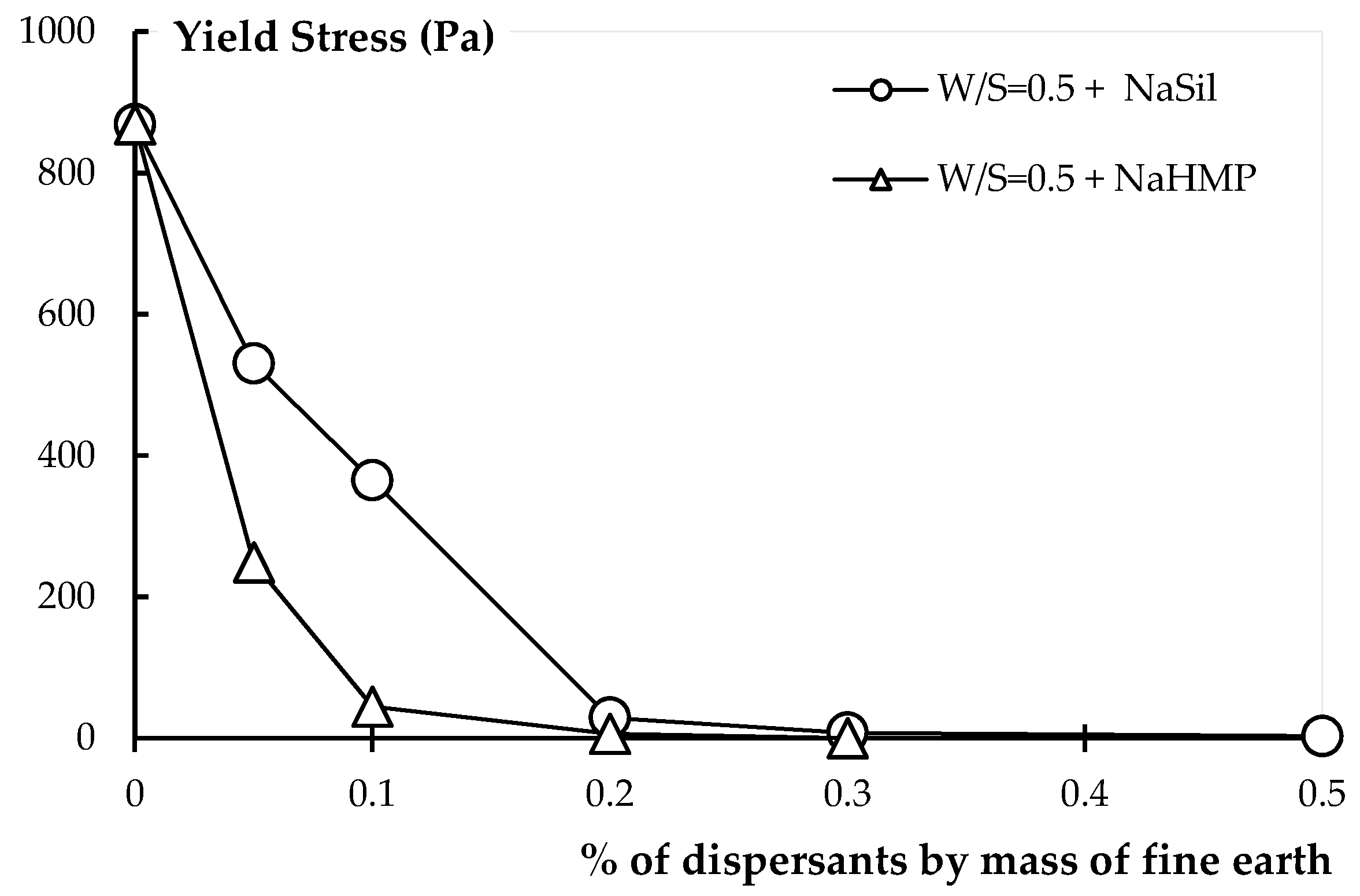




| SiO2 | TiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | Na2O | K2O | P2O5 | Cr2O3 | Ignition Loss |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 65.8 | 1.2 | 14.9 | 7.4 | 0.1 | 1.1 | 1.1 | 0.5 | 2.4 | 0.3 | 0.02 | 5.02 |
| Quartz | Smectite | Illite | Kaolinite | Muscovite | Plagioclase/Albite | Others | Total |
|---|---|---|---|---|---|---|---|
| 40.9 | 23.8 | 5.2 | 7.5 | 7.7 | 7.1 | 7.7 | 99.9 |
| Parameter | Reference | 2% PCE | 0.2% NaHMP | 0.3% NaSil |
|---|---|---|---|---|
| Average ζ (mV) | −4.6 | −4.5 | −13.4 | −9.7 |
| SD (mV) | ±0.1 | ±0.1 | ±0.4 | ±0.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landrou, G.; Brumaud, C.; Winnefeld, F.; Flatt, R.J.; Habert, G. Lime as an Anti-Plasticizer for Self-Compacting Clay Concrete. Materials 2016, 9, 330. https://doi.org/10.3390/ma9050330
Landrou G, Brumaud C, Winnefeld F, Flatt RJ, Habert G. Lime as an Anti-Plasticizer for Self-Compacting Clay Concrete. Materials. 2016; 9(5):330. https://doi.org/10.3390/ma9050330
Chicago/Turabian StyleLandrou, Gnanli, Coralie Brumaud, Frank Winnefeld, Robert J. Flatt, and Guillaume Habert. 2016. "Lime as an Anti-Plasticizer for Self-Compacting Clay Concrete" Materials 9, no. 5: 330. https://doi.org/10.3390/ma9050330






