Synthesis of AgInS2-xAg2S-yZnS-zIn6S7 (x, y, z = 0, or 1) Nanocomposites with Composition-Dependent Activity towards Solar Hydrogen Evolution
Abstract
:1. Introduction
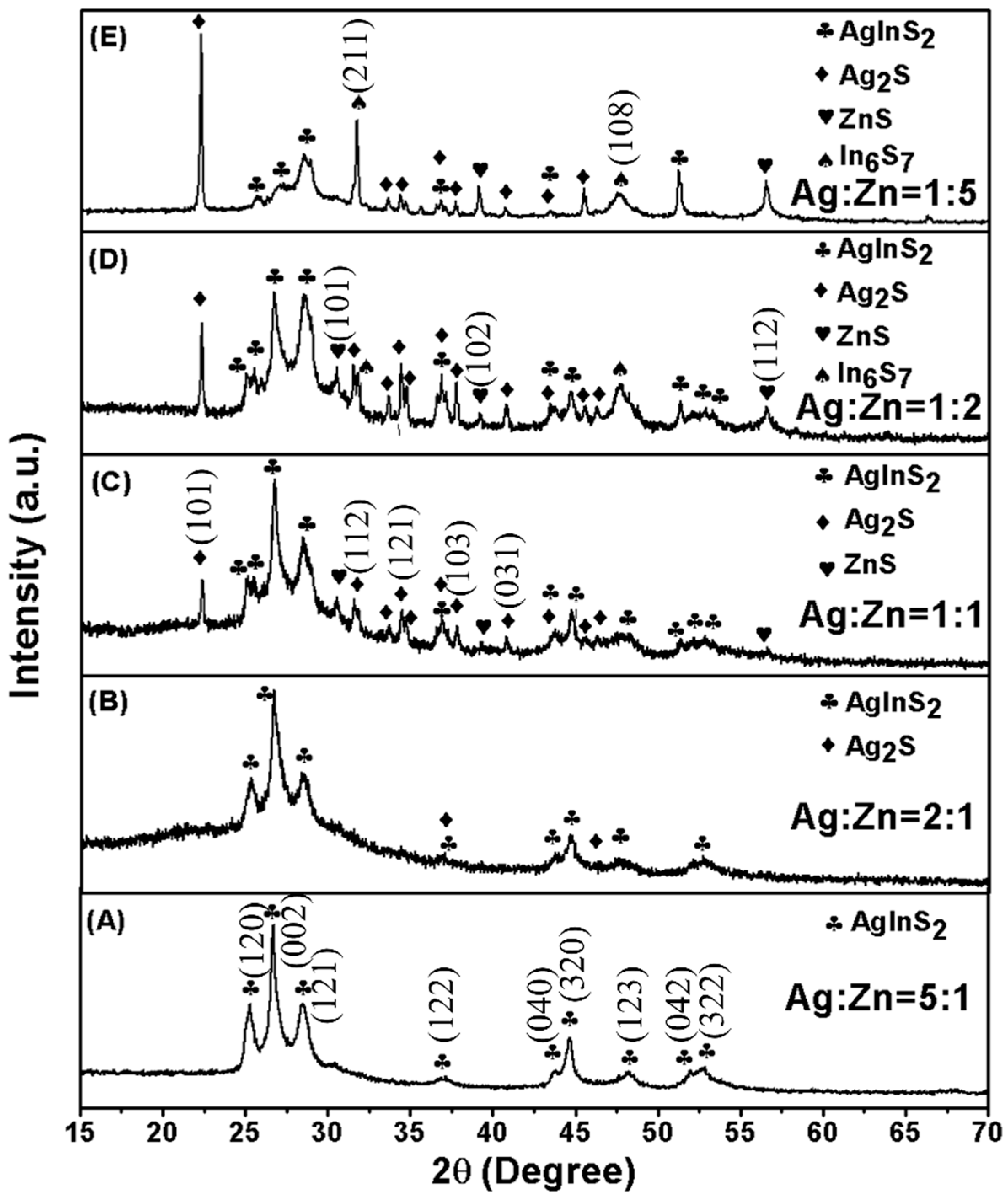
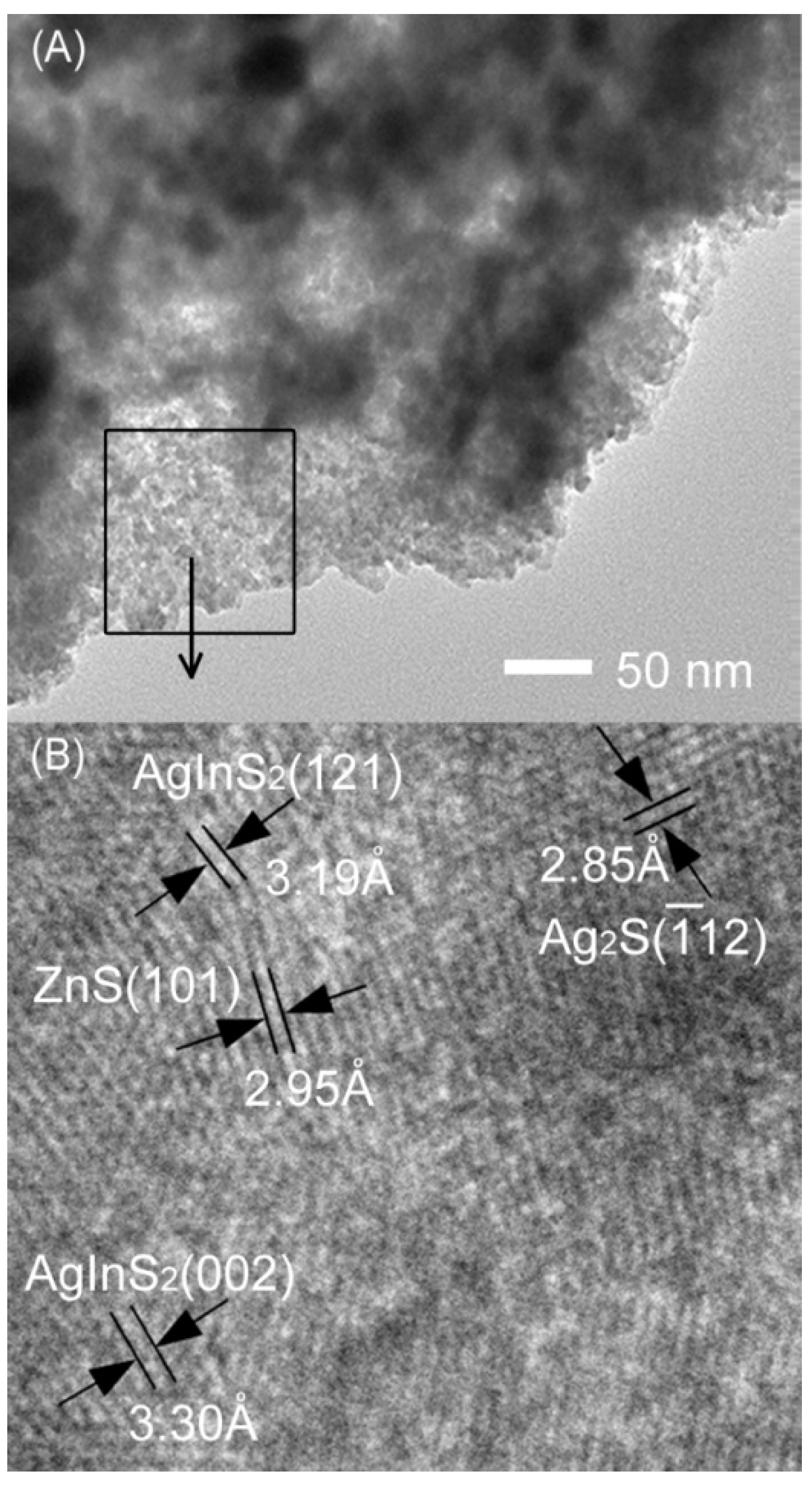
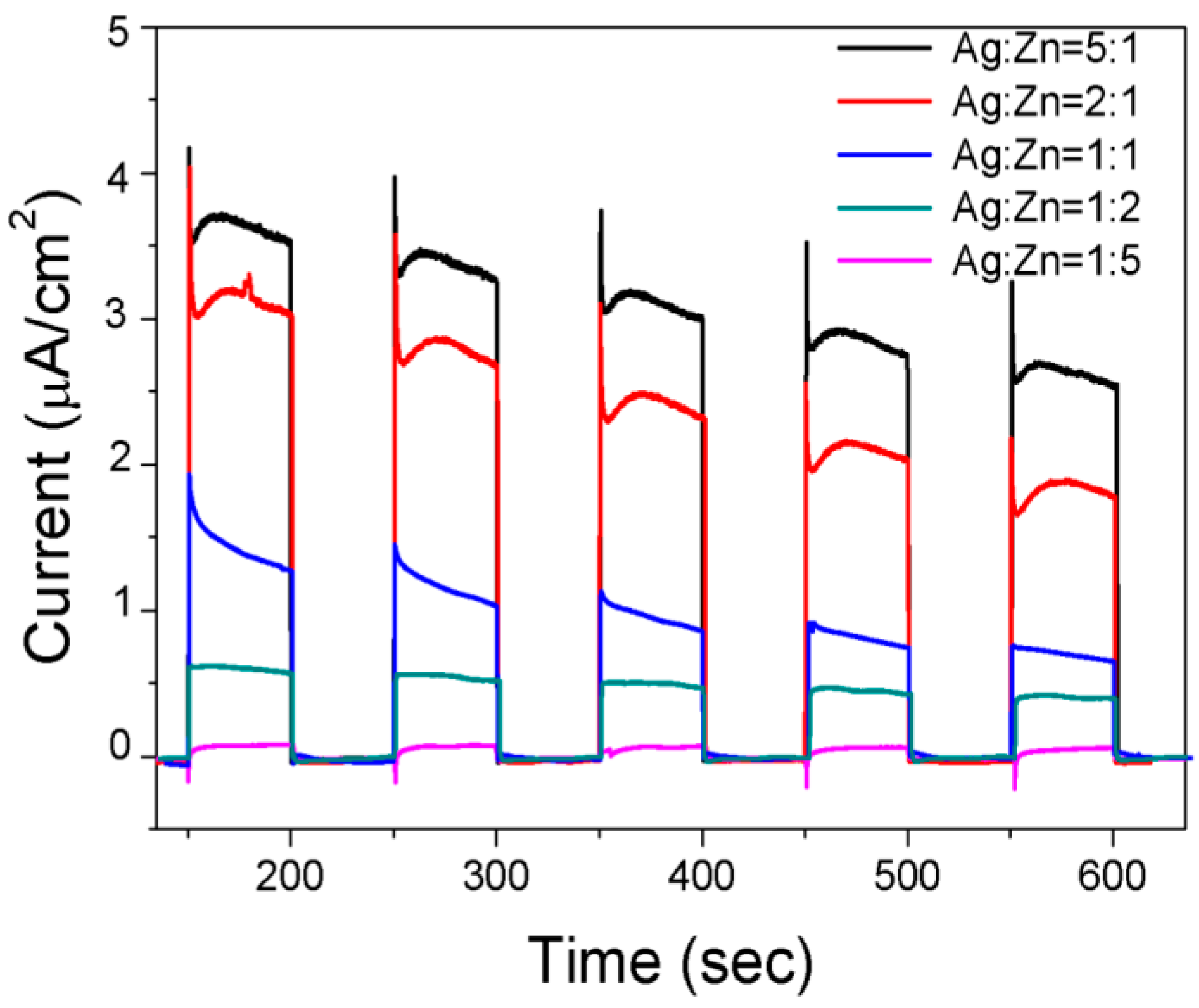
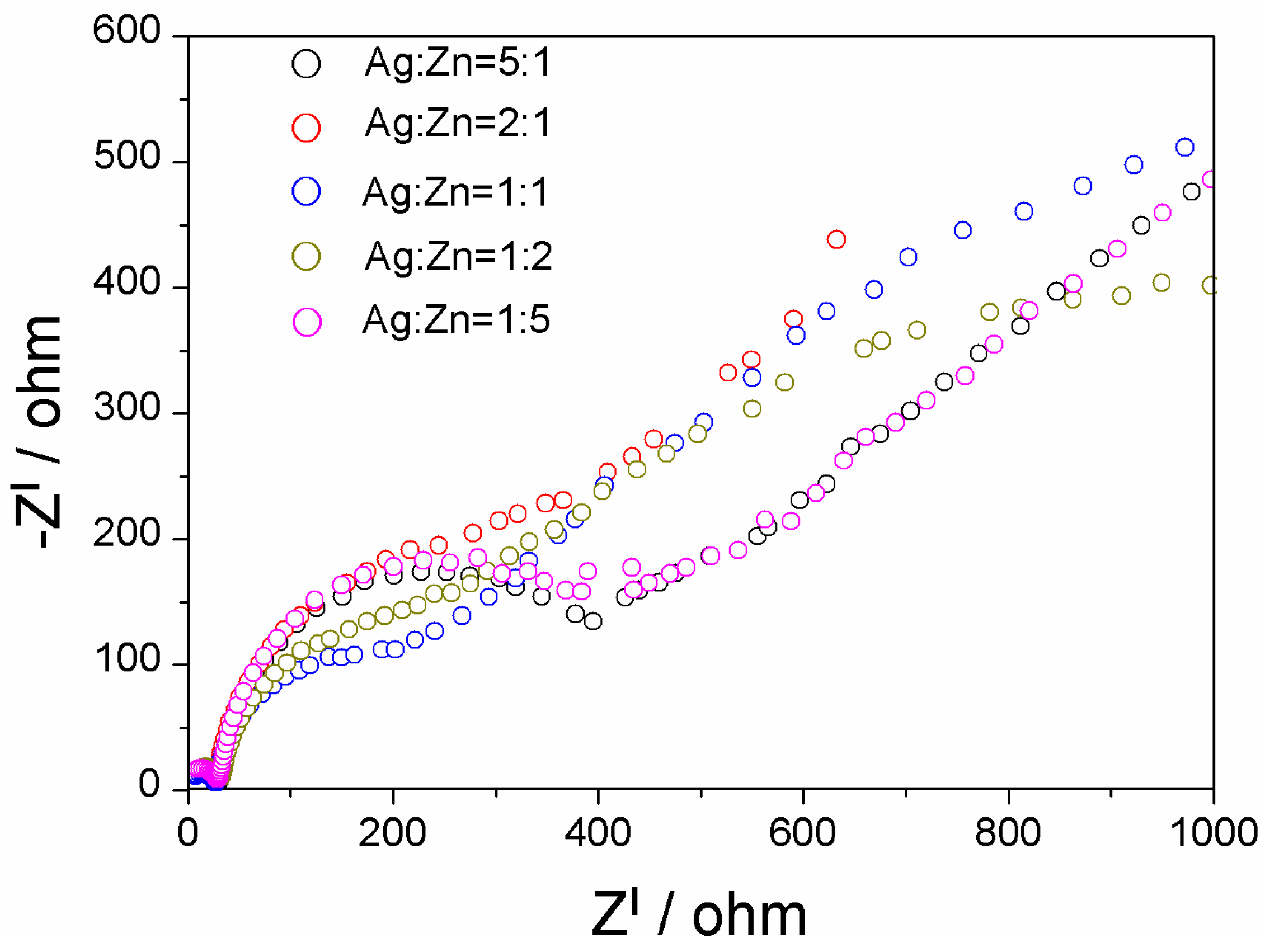
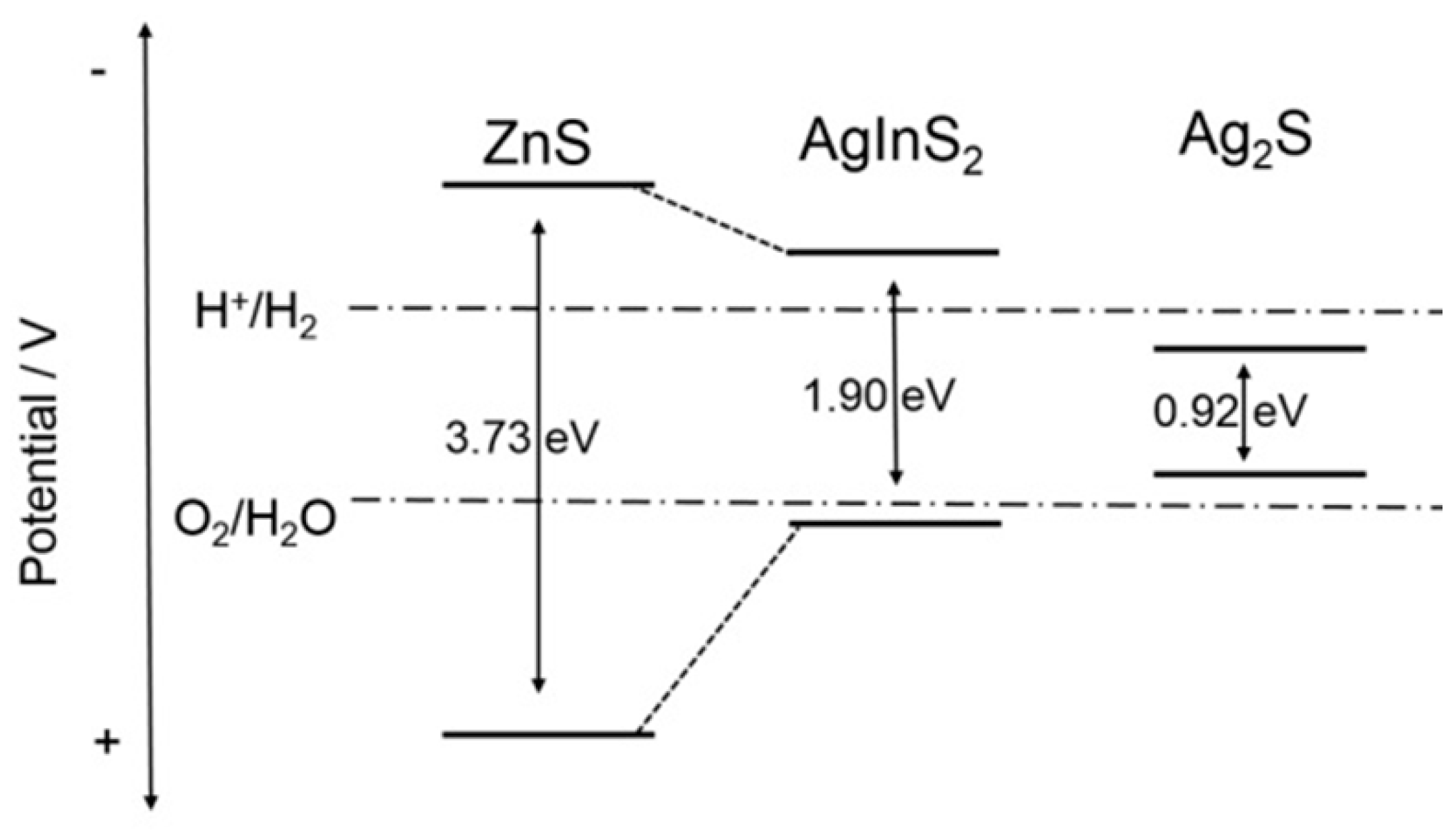
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis of AgI Polyhedrons
3.3. Synthesis of In(DDTC)3
3.4. Synthesis of AgInS2-Ag2S-ZnS Heterojunction Nanostructures
3.5. Characterization
3.6. Evaluation of Photo-Electrochemical Performance
3.7. Evaluation of Photocatalytic Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dahl, S.; Chorkendorff, I. Solar-fuel generation: Towards practical implementation. Nat. Mater. 2012, 11, 100–101. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Furukawa, S.; Saho, Y.; Tsubomura, H. Cadmium Sulfide photocatalyzed hydrogen production from aqueous solutions of sulfite: Effect of crystal structure and preparation method of the catalyst. J. Phys. Chem. 1985, 89, 1327–1329. [Google Scholar] [CrossRef]
- Reber, J.F.; Rusek, M. Photochemical hydrogen production with platinized suspensions of cadmium sulfide and cadmium zinc sulfide modified by silver sulfide. J. Phys. Chem. 1986, 90, 824–834. [Google Scholar] [CrossRef]
- Kershaw, S.V.; Susha, A.S.; Rogach, A.L. Narrow bandgap colloidal metal chalcogenide quantum dots: Synthetic methods, heterostructures, assemblies, electronic and infrared optical properties. Chem. Soc. Rev. 2013, 42, 3033–3087. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kudo, A. Photocatalytic Hydrogen Evolution on ZnS-CuInS2-AgInS2 Solid Solution Photocatalysts with Wide Visible Light Absorption Bands. Chem. Mater. 2006, 18, 1969–1975. [Google Scholar] [CrossRef]
- Mauser, C.; Da Como, E.; Baldauf, J.; Rogach, A.L.; Huang, J.; Talapin, D.V.; Feldmann, J. Spatio-temporal dynamics of coupled electrons and holes in nanosize CdSe-CdS semiconductor tetrapods. Phys. Rev. B 2010, 82, 081306. [Google Scholar] [CrossRef]
- Milliron, D.J.; Hughes, S.M.; Cui, Y.; Manna, L.; Li, J.; Wang, L.-W.; Alivisatos, A.P. Colloidal nanocrystal heterostructures with linear and branched topology. Nature 2004, 430, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides-efficient and viable materials for electro—And photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Weber, M.F.; Dignam, M.J. Splitting water with semiconducting photoelectrodes—Efficiency considerations. Int. J. Hydrogen Energy 1986, 11, 225–232. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Dong, S.-Y.; Sun, J.-H.; Feng, J.-L.; Wu, Q.-H.; Sun, S.-P. Microwave-assisted preparation, characterization and photocatalytic properties of a dumbbell-shaped ZnO photocatalyst. J. Hazard. Mater. 2010, 179, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, C.; Li, X.; Zhang, L.; Xue, H.; Wang, C.; Liu, Y. Electrospun Nanofibers of ZnO−SnO2Heterojunction with High Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 7920–7925. [Google Scholar] [CrossRef]
- Han, Z.; Qiu, F.; Eisenberg, R.; Holland, P.L.; Krauss, T.D. Robust photogeneration of H2 in water using semiconductor nanocrystals and a nickel catalyst. Science 2012, 338, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Seger, B.; Pedersen, T.; Laursen, A.B.; Vesborg, P.C.K.; Hansen, O.; Chorkendorff, I. Using TiO2 as a Conductive Protective Layer for Photocathodic H2 Evolution. J. Am. Chem. Soc. 2013, 135, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 Evolution Reaction from Aqueous Solutions over Band Structure-Controlled (AgIn)xZn2(1-x)S2 Solid Solution Photocatalysts with Visible-Light Response and Their Surface Nanostructures. J. Am. Chem. Soc. 2004, 126, 13406–13413. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kudo, A. Visible-Light-Induced H2 Evolution from an Aqueous Solution Containing Sulfide and Sulfite over a ZnS–CuInS2–AgInS2 Solid-Solution Photocatalyst. Angew. Chem. Int. Ed. 2005, 117, 3631–3634. [Google Scholar] [CrossRef]
- Iwashina, K.; Iwase, A.; Ng, Y.H.; Amal, R.; Kudo, A. Z-Schematic Water Splitting into H2 and O2 Using Metal Sulfide as a Hydrogen-Evolving Photocatalyst and Reduced Graphene Oxide as a Solid-State Electron Mediator. J. Am. Chem. Soc. 2015, 137, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, I.; Kato, H.; Kobayashi, H.; Kudo, A. Photocatalytic H2 Evolution under Visible-Light Irradiation over Band-Structure-Controlled (CuIn)xZn2(1-x)S2 Solid Solutions. J. Phys. Chem. B 2005, 109, 7323–7329. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Rutherford, M.; Peng, X. Formation of High-Quality I−III−VI Semiconductor Nanocrystals by Tuning Relative Reactivity of Cationic Precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.; Adrović, A.; Volbers, D.; Wyrwich, R.; Döblinger, M.; Susha, A.S.; Rogach, A.L. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Moore, D.; Li, J.; Wang, Z.L. Nanobelts, Nanocombs, and Nanowindmills of Wurtzite ZnS. Adv. Mater. 2003, 15, 228–231. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Wang, Z.; Zhong, Z.; Xu, R. Highly efficient and noble metal-free NiS/CdS photocatalysts for H2 evolution from lactic acid sacrificial solution under visible light. Chem. Commun. 2010, 46, 7631–7633. [Google Scholar] [CrossRef] [PubMed]
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily doped semiconductor nanocrystal quantum dots. Science 2011, 332, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.S.; Mirkovic, T.; Chuang, C.H.; Burda, C.; Scholes, G.D. Emergent Properties Resulting from Type-II Band Alignment in Semiconductor Nanoheterostructures. Adv. Mater. 2011, 23, 180–197. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Chuang, C.; Lu, F.; Sang, L.; Zhu, J.; Burda, C. Study of the Partial Ag-to-Zn Cation Exchange in AgInS2/ZnS Nanocrystals. J. Phys. Chem. C 2013, 117, 648–656. [Google Scholar] [CrossRef]
- Michalska, M.; Aboulaich, A.; Medjahdi, G.; Mahiou, R.; Jurga, S.; Schneider, R. Amine ligands control of the optical properties and the shape of thermally grown core/shell CuInS2/ZnS quantum dots. J. Alloy. Compd. 2015, 645, 184–192. [Google Scholar] [CrossRef]
- Manna, L.; Scher, E.C.; Li, L.S.; Alivisatos, A.P. Epitaxial growth and photochemical annealing of graded CdS/ZnS shells on colloidal CdSe nanorods. J. Am. Chem. Soc. 2002, 124, 7136–7145. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Piryatinski, A.; Nanda, J.; Tretiak, S.; Zavadil, K.R.; Wallace, W.O.; Werder, D.; Klimov, V.I. Type-II Core/Shell CdS/ZnSe Nanocrystals: Synthesis, Electronic Structures, and Spectroscopic Properties. J. Am. Chem. Soc. 2007, 129, 11708–11719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.C.; Zhang, W.-D. Charge transfer properties and photoelectrocatalytic activity of TiO2/MWCNT hybrid. Electrochim. Acta 2010, 56, 406–411. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.C.; Amal, R. Understanding the Enhancement in Photoelectrochemical Properties of Photocatalytically Prepared TiO2-Reduced Graphene Oxide Composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-Graphene Composite as a High Performance Photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef] [PubMed]
- He, B.-L.; Dong, B.; Li, H.-L. Preparation and electrochemical properties of Ag-modified TiO2 nanotube anode material for lithium–ion battery. Electrochem. Commun. 2007, 9, 425–430. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Y.; Ali, G.; Yoo, S.H.; Cho, S.O. Improved conversion efficiency of Ag2S quantum dot-sensitized solar cells based on TiO2 nanotubes with a ZnO recombination barrier layer. Nanoscale Res. Lett. 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.B.; Bennaceur, R. Electronic structure of the hexaindium heptasulfide In6S7. Physica B: Condens. Matter 2006, 382, 181–188. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, S.; Mhaisalkar, S.G.; Ramakrishna, S. Synthesis of AgInS2 nanocrystal ink and its photoelectrical application. Phys. Chem. Chem. Phys. 2012, 14, 8523–8529. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, T.; Tai, Y.; Yamamuro, S.; Hihara, T.; Peng, D.L.; Sumiyama, K. Synthesis of Zinc Sulfide Nanocrystals and Fabrication of Nanocrystal Superlattice. Mater. Trans. 2004, 45, 2650–2652. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, S.; Liu, J.; Jiang, W.; Zhou, Y.; An, C.; Zhang, J. Synthesis of AgInS2-xAg2S-yZnS-zIn6S7 (x, y, z = 0, or 1) Nanocomposites with Composition-Dependent Activity towards Solar Hydrogen Evolution. Materials 2016, 9, 329. https://doi.org/10.3390/ma9050329
Wang Z, Wang S, Liu J, Jiang W, Zhou Y, An C, Zhang J. Synthesis of AgInS2-xAg2S-yZnS-zIn6S7 (x, y, z = 0, or 1) Nanocomposites with Composition-Dependent Activity towards Solar Hydrogen Evolution. Materials. 2016; 9(5):329. https://doi.org/10.3390/ma9050329
Chicago/Turabian StyleWang, Zhaojie, Shutao Wang, Junxue Liu, Wen Jiang, Yan Zhou, Changhua An, and Jun Zhang. 2016. "Synthesis of AgInS2-xAg2S-yZnS-zIn6S7 (x, y, z = 0, or 1) Nanocomposites with Composition-Dependent Activity towards Solar Hydrogen Evolution" Materials 9, no. 5: 329. https://doi.org/10.3390/ma9050329




