1-D Compression Behaviour of Acid Sulphate Soils Treated with Alkali-Activated Slag
Abstract
:1. Introduction
2. Laboratory Investigation
2.1. Materials
2.2. Additives
2.3. Sample Preparation
2.4. 1-D Compression Tests
2.5. X-ray Diffraction(XRD) Analysis
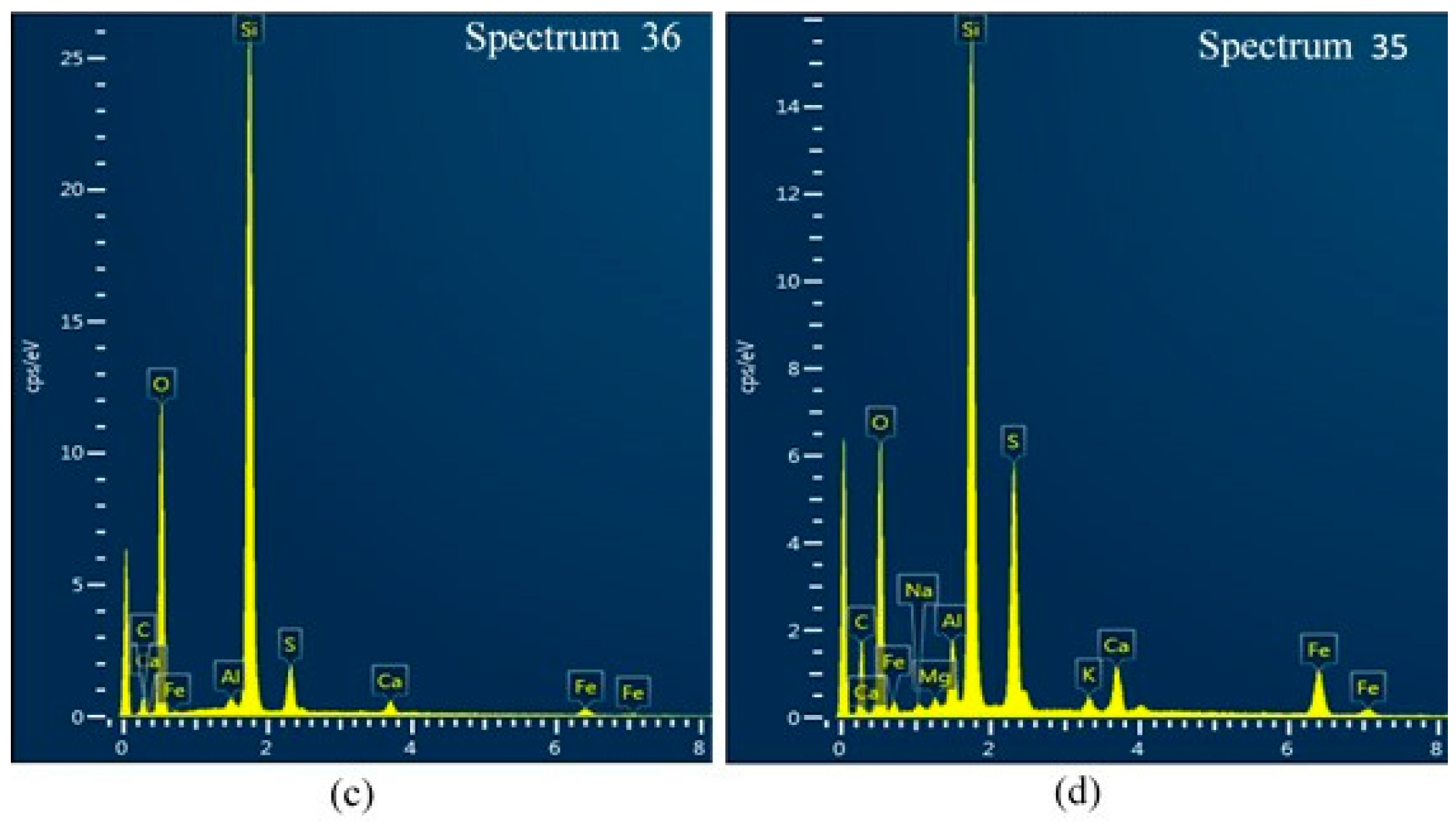
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
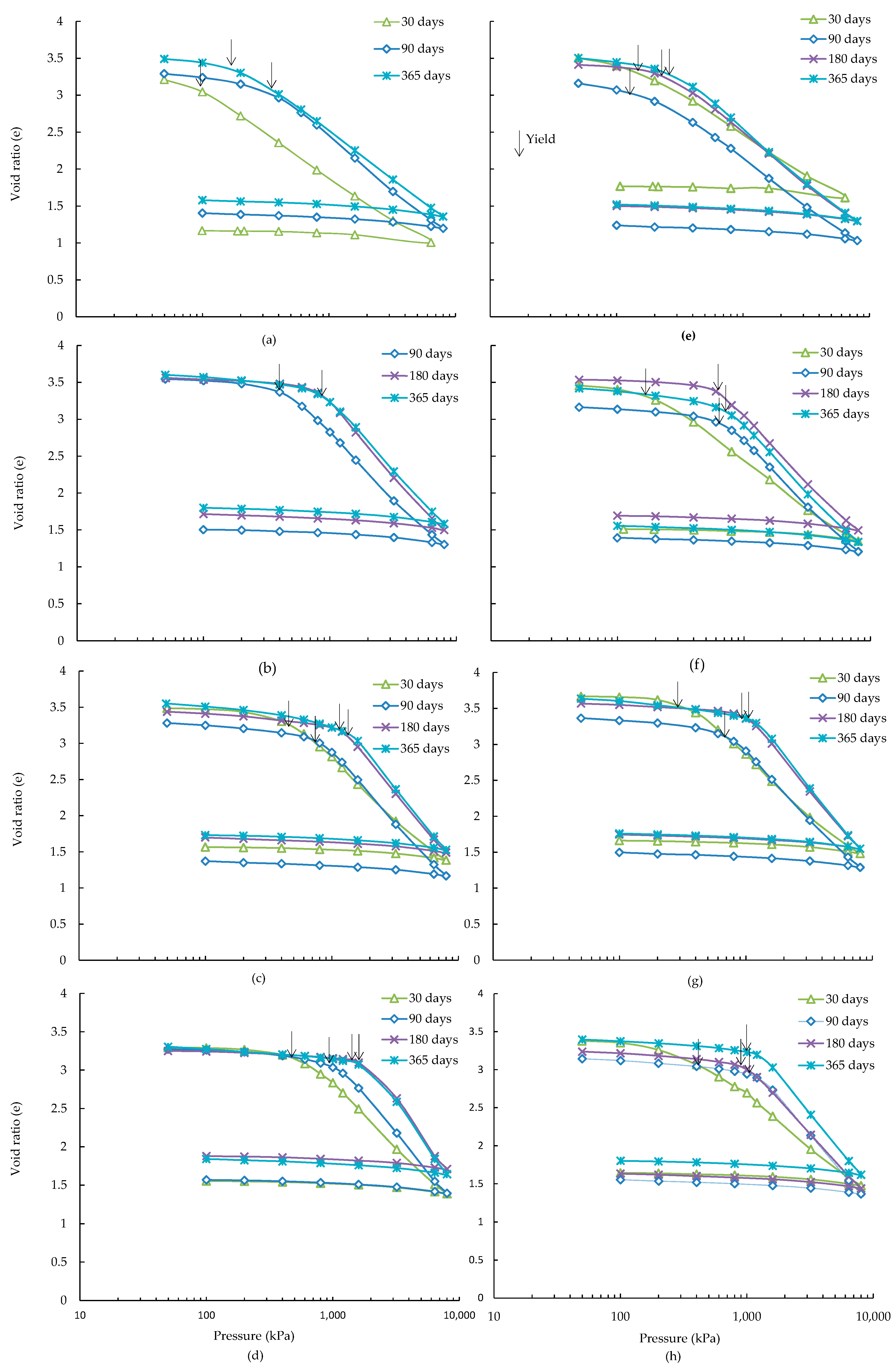
3.1. e-logσ′ Behaviour
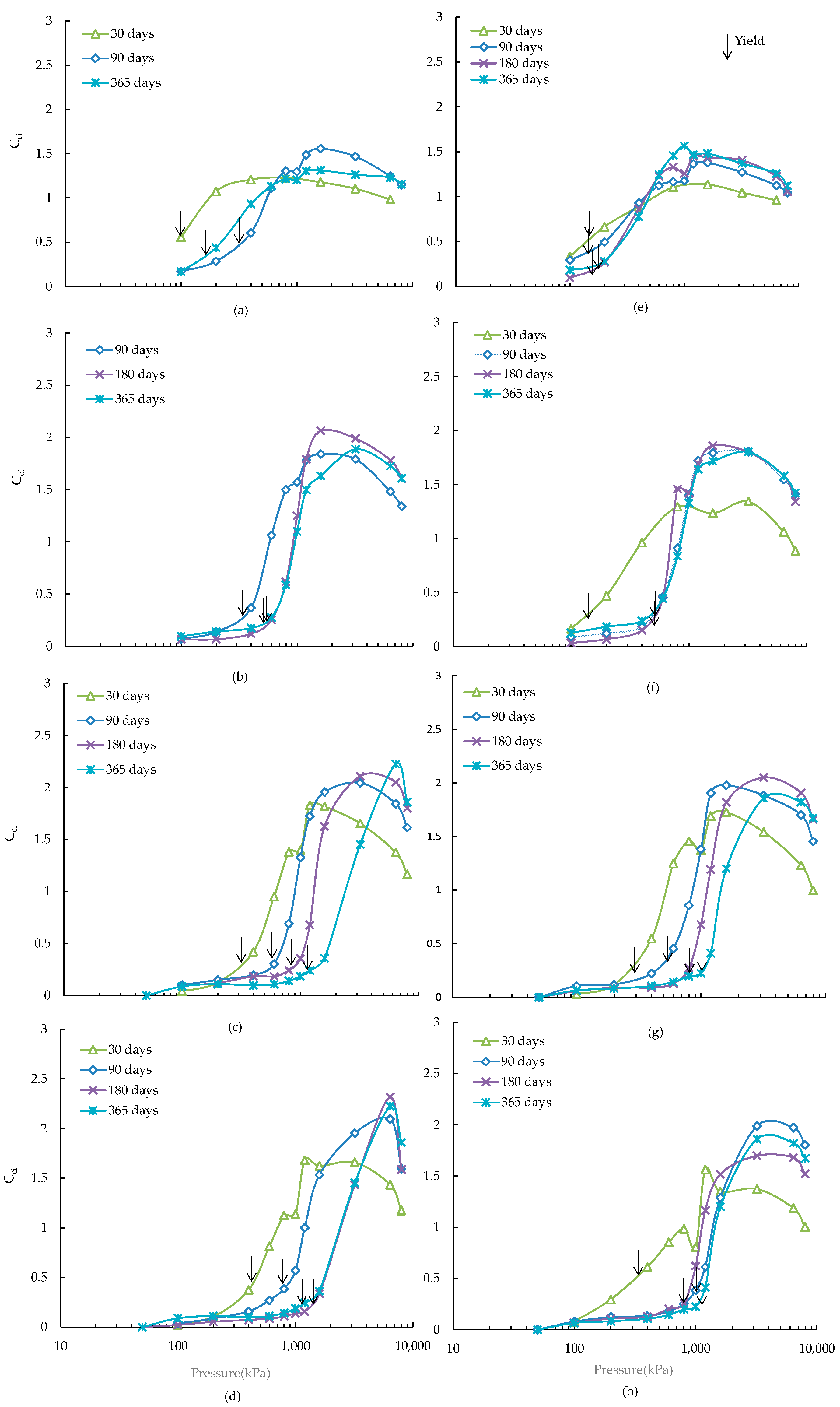
3.2. Destructuration Behaviour
3.3. Mineralogical Quantification
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
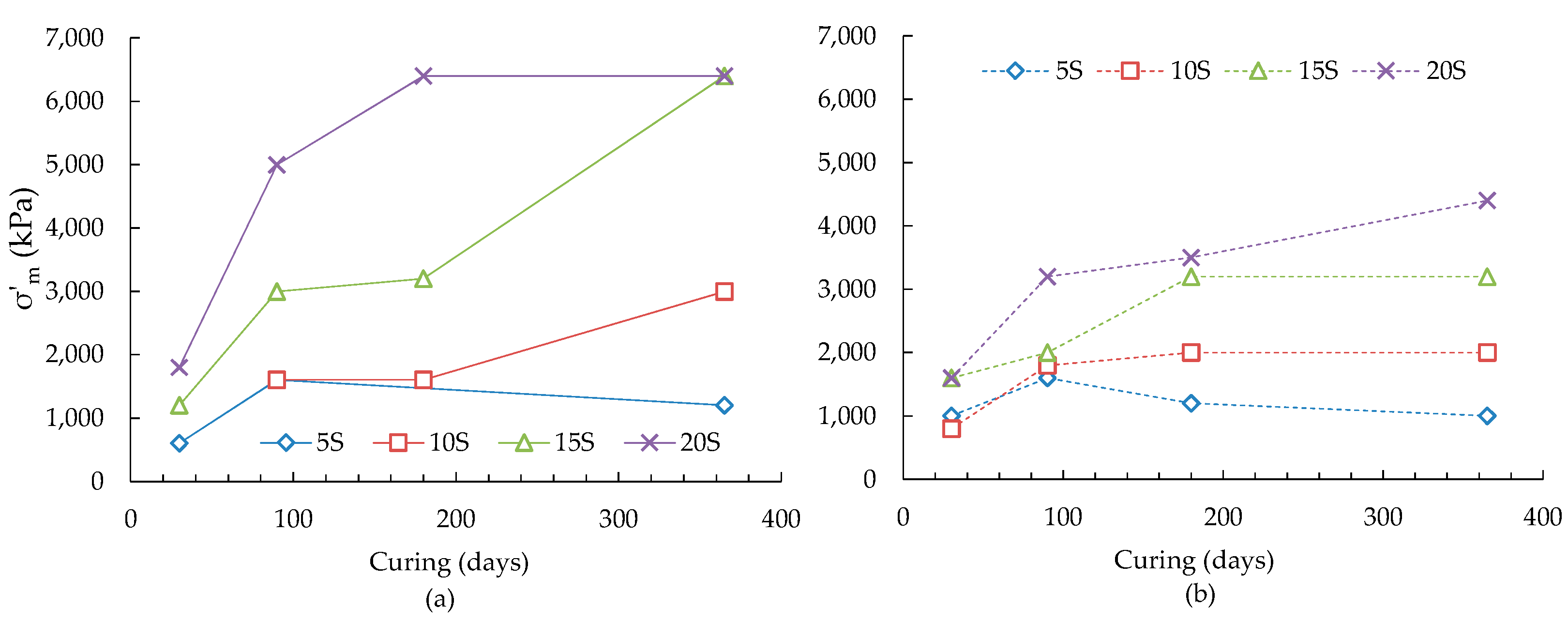
- In general, the void ratio-logarithm of effective stress plots of ASS showed an increase of slope of virgin compression line and a reduction of slope of recompression line with increased curing time and slag contents. Similar trend was observed for 4 wt % additional pyrite containing ASS but with an overall reduction of values of these slopes. The yield stress of treated ASS was found to increase with the increase of curing and slag contents, when the slag to lime ratios were 0.67 and 1.0. A higher slag to lime ratios (1.0, 1.33) was required for increasing yield stresses of 4 wt % additional pyrite containing ASS. The increase of yield stresses was associated with the increase of amorphous mineral quantity resulting from the formation of poorly crystalline cementitious reaction products.
- The maximum values of instantaneous slope (Cci) of the e-logσ′ plots were found to increase with the increase of slag and curing periods. The presence of additional amount of pyrite was found to have a minimal effect on the maximum value of Cci except for the 20 wt % slag at curing 180 and 365 days, where a drop of maximum Cci was observed. The drop could be due to the degradation of soil structure resulting from the formation of deleterious minerals (thaumasite-ettringite) as identified through scanning electron microscopy. The stress that causing maximum destructuration was found to increase with the increase of curing time and slag content for the ASS. An overall reduction of maximum destructuration stresses was observed for 15 and 20 wt % slag treated ASS containing 4 wt % additional pyrite.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mitchell, J.K. Fundamentals of Soil Behaviour; John Willey & Sons Inc.: New York, NY, USA, 1976. [Google Scholar]
- Burland, J.B. On the compressibility and shear strength of natural clays. Géotechnique 1990, 40, 327–378. [Google Scholar] [CrossRef]
- Leroueil, S.; Vaughan, P.R. The general and congruent effects of structure in natural soils and weak rocks. Géotechnique 1990, 40, 467–488. [Google Scholar] [CrossRef]
- Liu, M.D.; Carter, J.P. Virgin compression of structured soils. Géotechnique 1999, 49, 43–57. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Davies, P.G.; Thomas, B.P.; Merry, R.H.; Fotheringham, D.G.; Hicks, W.S. Properties and distribution of South Australian coastal acid sulphate soils and their environmental hazards. In Proceedings of the 5th International Acid Sulfate Soils Conference, Tweed Heads, NSW, Australia, 25–30 August 2002.
- Gens, A.; Nova, R. Conceptual bases for a constitutive model for bonded soils and weak rocks. Geotech. Eng. Hard Soils Soft Rocks 1993, 1, 485–494. [Google Scholar]
- Islam, S.; Haque, A.; Wilson, S.A.; Ranjith, P.G. Time-dependent strength and mineralogy of lime-GGBS treated naturally occurred ASS. J. Mater. Civ. Eng. 2016, 28. [Google Scholar] [CrossRef]Erratum for “Time-dependent strength and mineralogy of lime-GGBS treated naturally occurring acid sulfate soils”. J. Mater. Civ. Eng. 2015, 28. [CrossRef]
- Islam, S.; Haque, A.; Wilson, S.A.; Ranjith, P.G. Improvement of acid sulfate soils using lime-activated slag. Proc. ICE Ground Improv. 2013, 167, 235–248. [Google Scholar] [CrossRef]
- Islam, S.; Haque, A.; Wilson, S.A. Effects of curing environment on the strength and mineralogy of lime-GGBS treated acid sulphate soils. J. Mater. Civ. Eng. 2013, 26, 1003–1008. [Google Scholar] [CrossRef]
- AS1289. Method of Testing Soils for Engineering Purposes, 6.6.1: Soil Strength and Consolidation Tests-Determination of the One-Dimensional Consolidation Properties of a Soil-Standard Method; Standards Australia: Strathfield, NSW, Australia, 2000.
- Rieltveld, H.M. A profile refinement method for nuclear and magnetic structure. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Hill, R.J.; Howard, C.J. Quantitative phase analysis from neutron powder diffraction data using the Rietveld method. J. Appl. Cryst. 1987, 20, 467–474. [Google Scholar] [CrossRef]
- Bish, D.L.; Howard, S.A. Quantitative phase analysis using the Rietveld method. J. Appl. Cryst. 1988, 21, 86–91. [Google Scholar] [CrossRef]
- Bruker, A.X.S. General Profile and Structure Analysis Software for Powder Diffraction Data; Topaz Version 3.0; Bruker Corporation: Karlsruhe, Germany, 2004. [Google Scholar]
- Cheary, R.W.; Coelho, A.A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Cryst. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Kamruzzaman, A.; Chew, S.; Lee, F. Structuration and destructuration behavior of cement-treated Singapore marine clay. J. Geotech. Geoenviron. Eng. 2009, 135, 573–589. [Google Scholar] [CrossRef]
- Chowdhury, B. Mechanical Behaviour of Lime-Slag Treated Coode Island Silt. Ph.D. Thesis, Monash University, Clayton, Australia, 2013. [Google Scholar]
- Little, D.; Nair, S.; Herbert, B. Addressing sulfate-induced heave in lime treated soils. J. Geotech. Geoenviron. Eng. 2010, 136, 110–118. [Google Scholar] [CrossRef]




| Constituents | wt % | Index Properties | Value |
|---|---|---|---|
| Quartz | 24 | Liquid Limit | 82 |
| K-feldspar | 2 | Plasticity Index | 43 |
| Na/Ca-feldspar | 3 | pH | 7.9 |
| Mica/Illite | 10 | Cation Exchange Capacity: meq/100 g | 33.83 |
| Kaolinite | 23 | Specific Gravity | 2.64 |
| Smectite | 32 | Particle Sizes | |
| Sulphide | 4 | Clay (<0.002 mm) (%) | 3 |
| Anatase | 1 | Slit (0.002–0.6 mm) (%) | 35 |
| Halite | 1 | Sand (0.6–2 mm) (%) | 62 |
| Chemical Compositions (wt %) | Lime | Slag |
|---|---|---|
| SiO2 | 1–2 | 35–37 |
| Al2O3 | 0–2 | 13.5 |
| MgO | – | 5.9 |
| Mg(OH)2 | 0.5–1.5 | – |
| CaO | – | 41–43 |
| Ca(OH)2 | 85–95 | – |
| Fe2O3 | 0–0.7 | 0.3 |
| SO3 | – | 2.9 |
| MnO | – | 0.4 |
| Slag (%) | 5 | 10 | 15 | 20 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curing (Days) | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 |
| ASS | ||||||||||||||||
| Cc | 1.20 | 1.50 | – | 1.71 | – | 1.85 | 2.02 | 2.15 | 1.76 | 1.97 | 2.16 | 2.22 | 1.71 | 2.02 | 2.50 | 2.69 |
| Cr | 0.14 | 0.12 | – | 0.09 | – | 0.09 | 0.08 | 0.07 | 0.08 | 0.08 | 0.07 | 0.07 | 0.08 | 0.07 | 0.07 | 0.07 |
| ASS with 4 wt % Additional Pyrite | ||||||||||||||||
| Cc | 1.15 | 1.35 | 1.46 | 1.50 | 1.32 | 1.79 | 1.84 | 1.87 | 1.73 | 1.91 | 2.14 | 2.29 | 1.47 | 1.98 | 2.04 | 2.04 |
| Cr | 0.12 | 0.10 | 0.09 | 0.08 | 0.09 | 0.08 | 0.08 | 0.07 | 0.08 | 0.08 | 0.07 | 0.06 | 0.10 | 0.09 | 0.07 | 0.07 |
| Slag (%) | 5 | 10 | 15 | 20 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Curing (Days) | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 | 30 | 90 | 180 | 365 |
| Cci (max)-ASS | 1.2 | 1.5 | – | 1.3 | – | 1.8 | 2.0 | 1.9 | 1.9 | 2.0 | 2.1 | 2.22 | 1.7 | 2.1 | 2.3 | 2.2 |
| Cci (max)-ASS + 4% pyrite | 1.1 | 1.4 | 1.5 | 1.6 | 1.3 | 1.8 | 1.9 | 1.8 | 1.7 | 1.9 | 2.0 | 1.9 | 1.6 | 2.1 | 1.7 | 1.8 |
| Slag (%) | Amorphous Quantity (wt %) | |||
|---|---|---|---|---|
| Curing (Days) | ||||
| 30 | 90 | 180 | 365 | |
| ASS | ||||
| 5 (σ) | 31.9 (3.6) | 31.9 (3.6) | 30 (3.8) | 29 (3.4) |
| 10 (σ) | 35.9 (3.4) | 39.5 (3.4) | 34.8 (3.8) | 41.1 (3.2) |
| 15 (σ) | 34.2 (4.2) | 35.1 (3.2) | 35.6 (3.4) | 38 (3.4) |
| 20 (σ) | 30.9 (3.6) | 33.3 (3.4) | 34.3 (3.4) | 36.1 (3.4) |
| ASS + 4% pyrite | ||||
| 5 (2σ) | 32.5 (3.6) | 34.5 (3.4) | 35.4 (3.4) | 34.4 (3.6) |
| 10 (2σ) | 38.7 (3.2) | 37.4 (3.4) | 34.9 (3.8) | 35.8 (3.4) |
| 15 (2σ) | 34.1 (3.6) | 32.9 (3.4) | 34.3 (3.6) | 37.1 (3.4) |
| 20 (2σ) | 35.9 (3.6) | 31.9 (3.6) | 33.6 (3.8) | 37.9 (3.4) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, S.; Haque, A.; Bui, H.H. 1-D Compression Behaviour of Acid Sulphate Soils Treated with Alkali-Activated Slag. Materials 2016, 9, 289. https://doi.org/10.3390/ma9040289
Islam S, Haque A, Bui HH. 1-D Compression Behaviour of Acid Sulphate Soils Treated with Alkali-Activated Slag. Materials. 2016; 9(4):289. https://doi.org/10.3390/ma9040289
Chicago/Turabian StyleIslam, Shahidul, Asadul Haque, and Ha Hong Bui. 2016. "1-D Compression Behaviour of Acid Sulphate Soils Treated with Alkali-Activated Slag" Materials 9, no. 4: 289. https://doi.org/10.3390/ma9040289