Sol-Gel Synthesis of Carbon Xerogel-ZnO Composite for Detection of Catechol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Synthesis of CXZnO Composites
2.4. Preparation of Biosensors
3. Results and Discussion
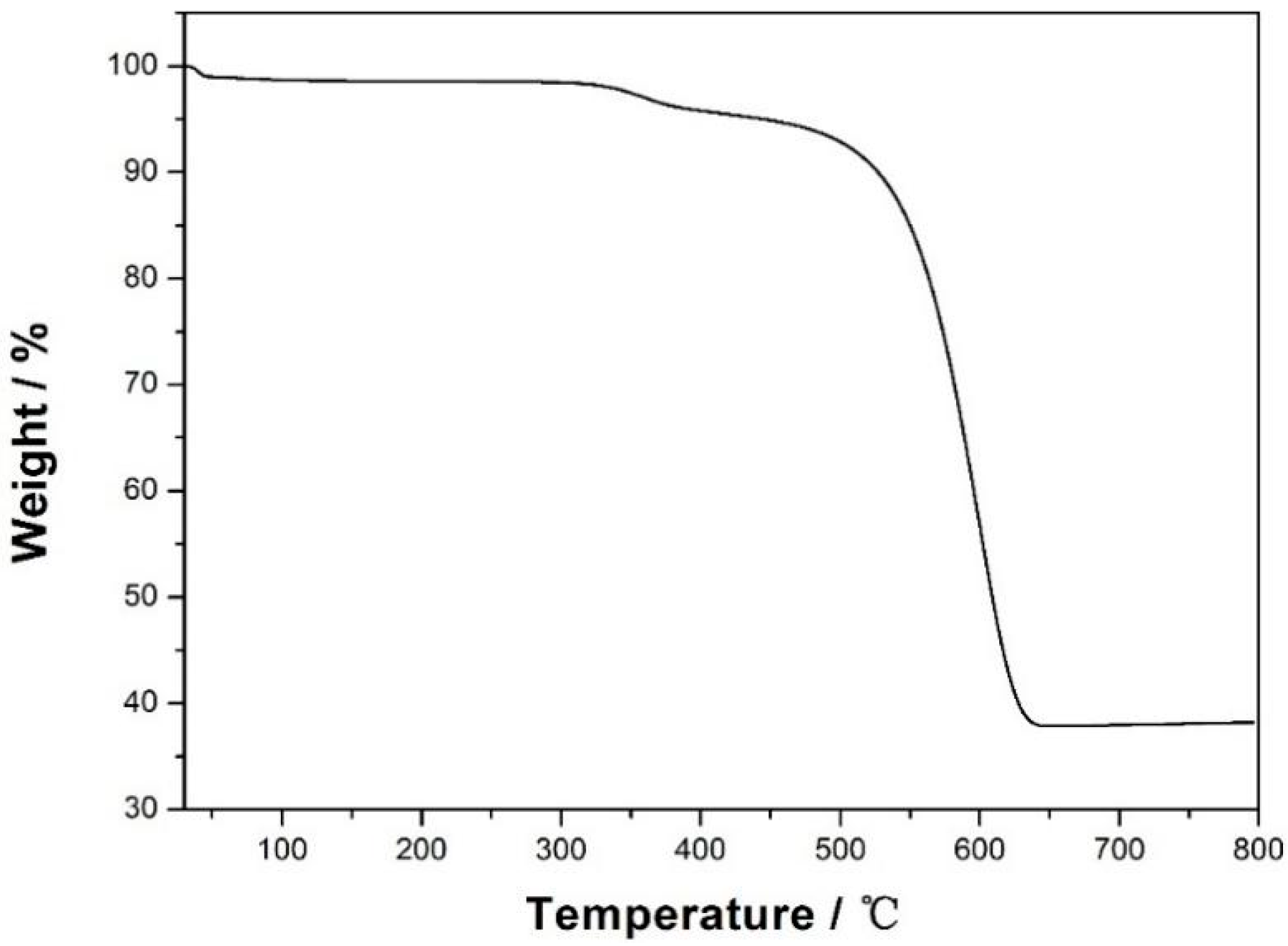
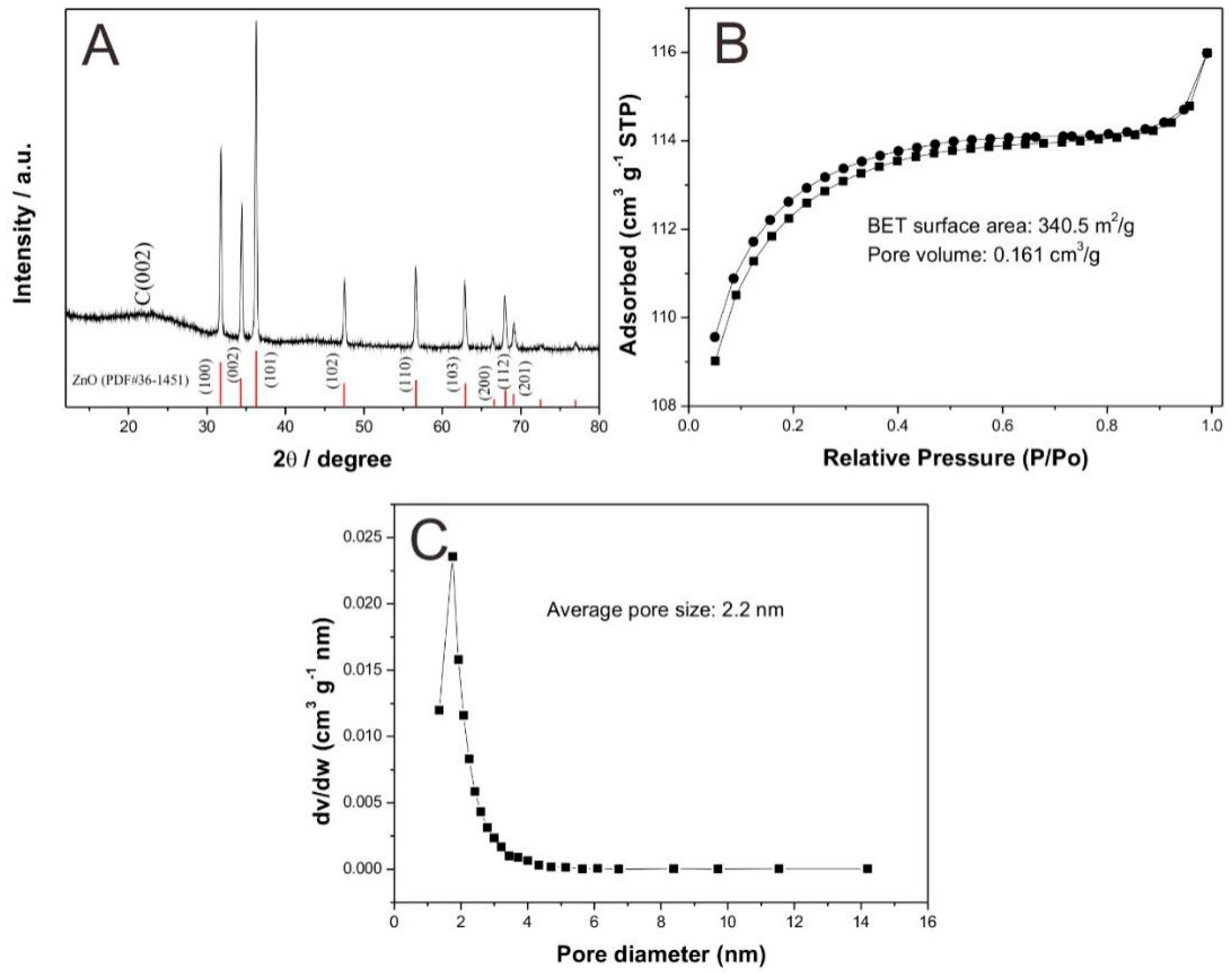
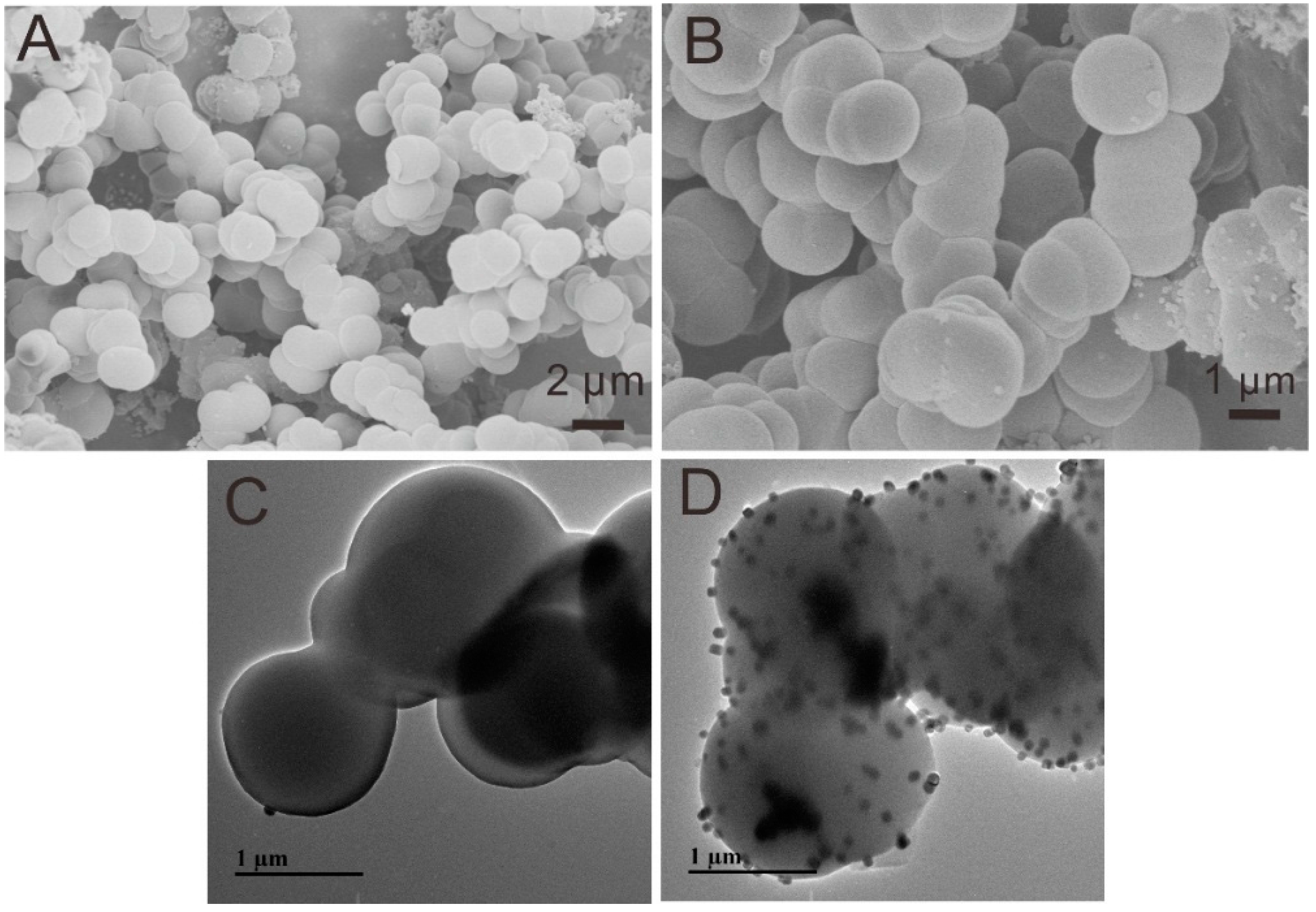
3.1. Characterization of CXZnO Composites
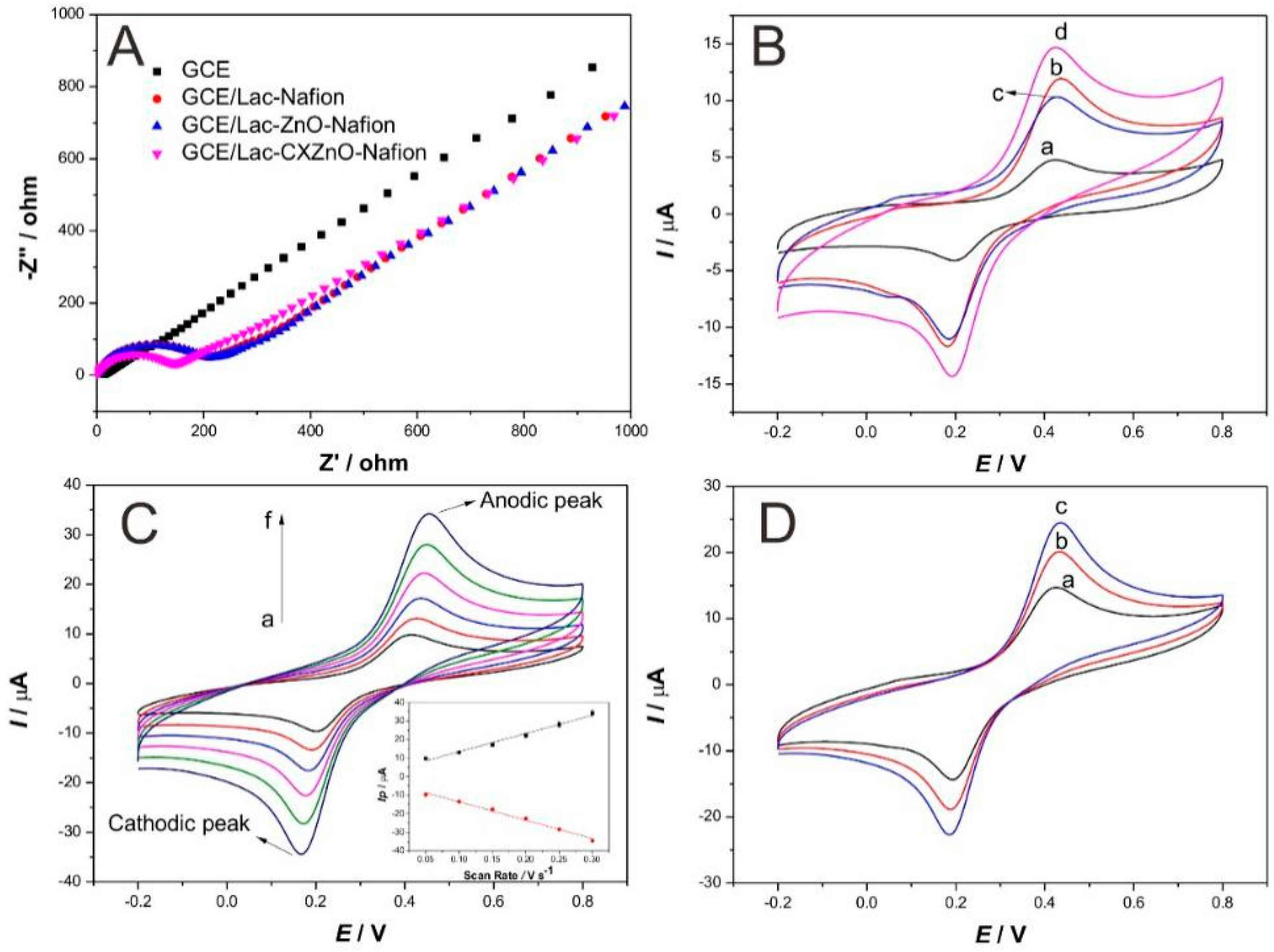
3.2. Electrochemical Studies of Modified Electrodes
Lac(deoxy) + O2 + 4H+ → Lac(oxy) + 2H2O
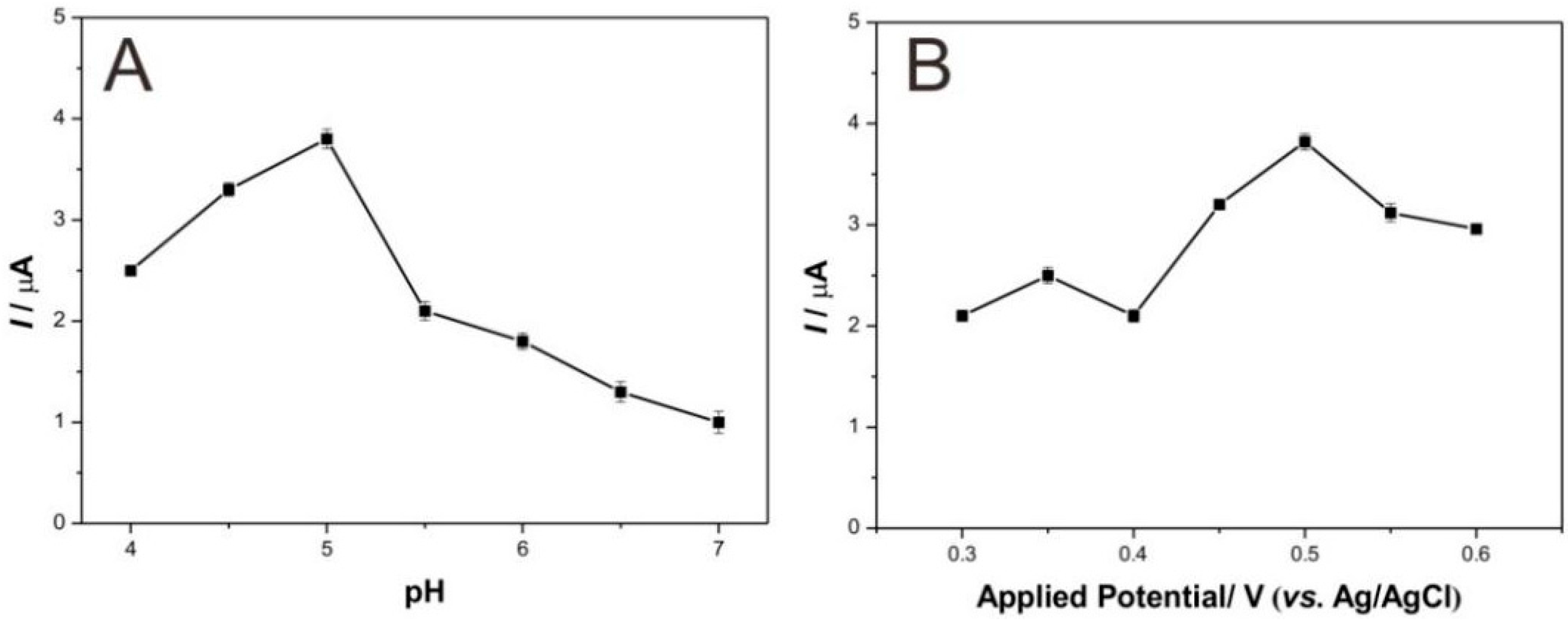
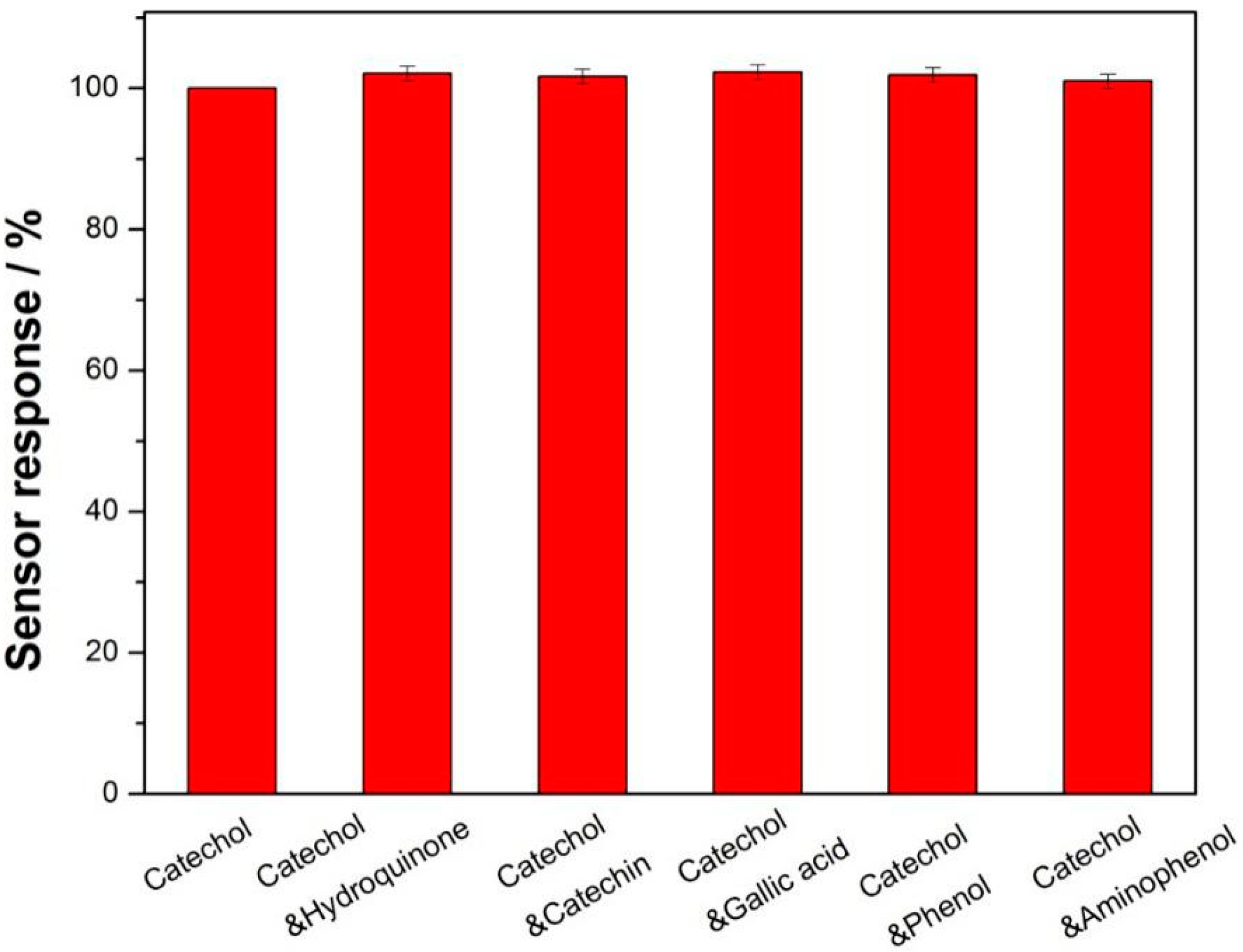
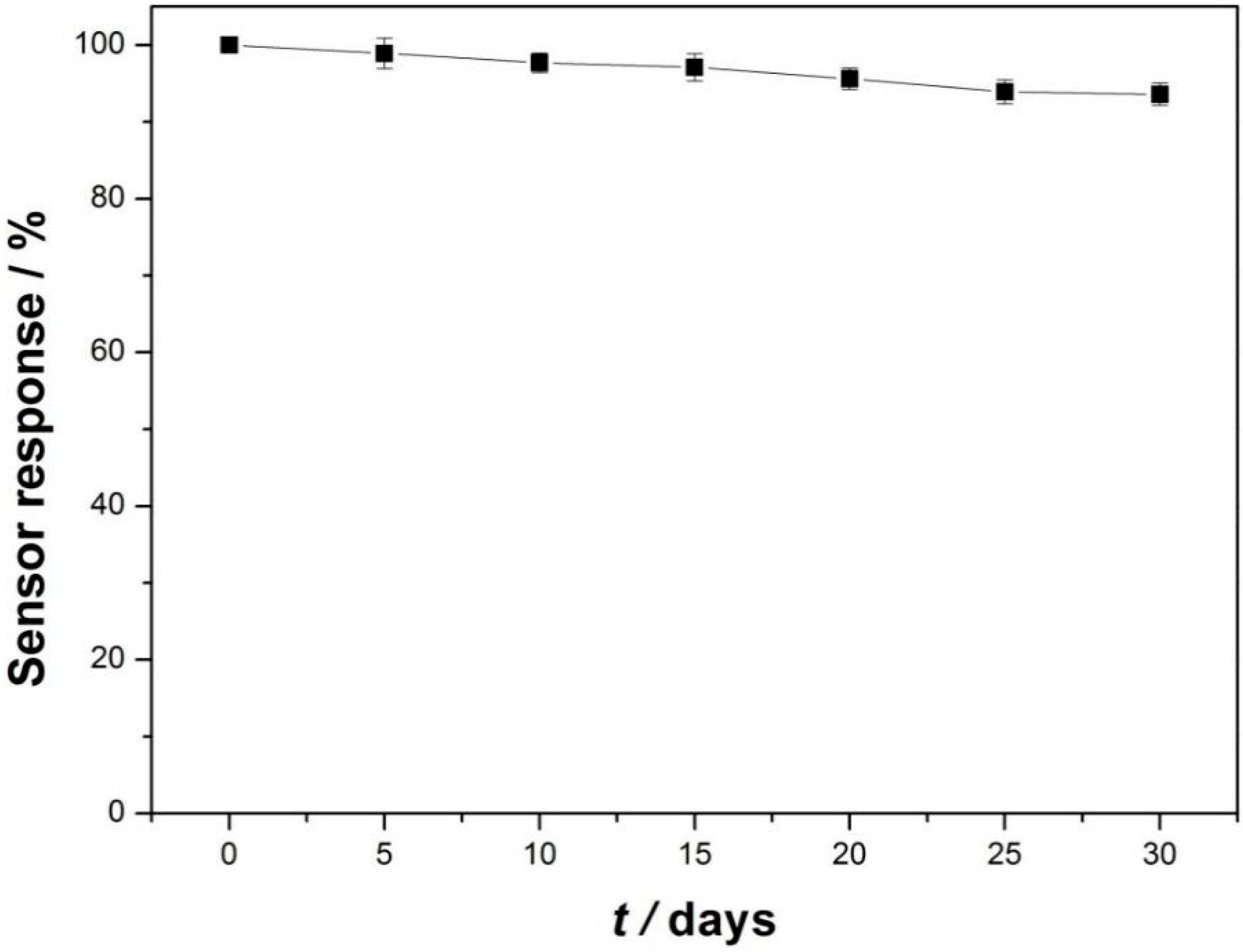
3.3. Analytical Performance for Detecting Catechol
3.4. Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cornell, B.; Braach-Maksvytis, V.; King, L.; Osman, P.; Raguse, B.; Wieczorek, L.; Pace, R. A biosensor that uses ion-channel switches. Nature 1997, 387, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Z.; Anzai, J. Recent Progress in Lectin-Based Biosensors. Materials 2015, 8, 8590–8607. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Elhag, S.; Alsalhi, M.S.; Nur, O.; Willander, M. Effect of urea on the morphology of Co3O4 nanostructures and their application for potentiometric glucose biosensor. Electroanalysis 2014, 26, 1773–1781. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Hua, Z.; Qin, Q.; Bai, X.; Wang, C.; Huang, X. β-Cyclodextrin inclusion complex as the immobilization matrix for laccase in the fabrication of a biosensor for dopamine determination. Sens. Actuators B 2015, 220, 1169–1177. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Vasilescu, I.; Radoi, A.; Litescu, S.C.; Radu, G.L. Disposable biosensor based on platinum nanoparticles-reduced graphene oxide-laccase biocomposite for the determination of total polyphenolic content. Talanta 2013, 110, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lanzellotto, C.; Favero, G.; Antonelli, M.L.; Tortolini, C.; Cannistraro, S.; Coppari, E.; Mazzei, F. Nanostructured enzymatic biosensor based on fullerene and gold nanoparticles: Preparation, characterization and analytical applications. Biosens. Bioelectron. 2013, 55, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, L.; Pang, Z.; Ding, L.; Wang, Q.; Ke, H.; Huang, F.; Wei, Q. Novel phenolic biosensor based on a magnetic polydopamine-laccase-nickel nanoparticle loaded carbon nanofiber composite. ACS Appl. Mater. Interfaces 2014, 6, 5144–5151. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Yang, C.; Gu, B.X.; Fang, S.J. Nanostructured ZnO for biosensing applications. Chin. Sci. Bull. 2013, 58, 2563–2566. [Google Scholar] [CrossRef]
- Gupta, V. ZnO based third generation biosensor. Thin Solid Films 2010, 519, 1141–1144. [Google Scholar] [CrossRef]
- Degen, A.; Kosec, M. Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. J. Eur. Ceram. Soc. 2000, 20, 667–673. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, E.; Kim, B.S.; Kim, S.G.; Lee, B.J.; Kim, M.S.; Jung, J.C. Activated carbon aerogel as electrode material for coin-type EDLC cell in organic electrolyte. Curr. Appl. Phys. 2014, 14, 603–607. [Google Scholar] [CrossRef]
- Tang, L.; Ding, X.; Zhao, X.; Wang, Z.; Zhou, B. Preparation of zinc oxide particles by using layered basic zinc acetate as a precursor. J. Alloy. Compd. 2012, 544, 67–72. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Xu, X.; Lu, P.; Zhou, Y.; Zhao, Z.; Guo, M. Laccase immobilized on methylene blue modified mesoporous silica MCM-41/PVA. Mater. Sci. Eng. C 2009, 29, 2160–2164. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Chen, J.; Cai, Y.; Zhang, Y.; Yang, G.; Liu, Y.; Zhang, C.; Tang, W. Mesoporous carbon nitride based biosensor for highly sensitive and selective analysis of phenol and catechol in compost bioremediation. Biosens. Bioelectron. 2014, 61, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Liu, L.H.; Bai, X.; Shi, H.C. A reduced graphene oxide based biosensor for high-sensitive detection of phenols in water samples. Sens. Actuators B 2013, 181, 661–667. [Google Scholar] [CrossRef]
- Shimomura, T.; Itoh, T.; Sumiya, T.; Hanaoka, T.A.; Mizukami, F.; Ono, M. Amperometric detection of phenolic compounds with enzyme immobilized in mesoporous silica prepared by electrophoretic deposition. Sens. Actuators B 2011, 153, 361–368. [Google Scholar] [CrossRef]
- Xu, X.; Guo, M.; Lu, P.; Wang, R. Development of amperometric laccase biosensor through immobilizing enzyme in copper-containing ordered mesoporous carbon (Cu-OMC)/chitosan matrix. Mater. Sci. Eng. C 2010, 30, 722–729. [Google Scholar] [CrossRef]


| Electrodes | Detection Limit (µM) | Linear Range (µM) | Sensitivity (µA/mM) | Ref. |
|---|---|---|---|---|
| MB-MCM-41/PVA/lac | 0.331 | 4–87.98 | – | [19] |
| GCE/MCN/Tyr | 0.01 | 0.05–12.5 | – | [20] |
| Lac/AP- rGOs/Chit/GCE | 7 | 15–700 | 15.79 | [21] |
| Lac-FSM7.0-GC | 2 | 2–100 | – | [22] |
| Cu-OMC/Lac/CS/Au | 0.67 | 0.67–13.8 | 104 | [23] |
| GCE/Lac-CXZnO-Nafion | 2.17 | 6.91–453 | 31.2 | This work |
| Sample | Cadded (µM) | Cfound (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| a | 100.00 | 103.26 | 103.26 | 2.52 |
| – | 100.00 | 98.93 | 98.93 | – |
| – | 100.00 | 97.31 | 97.31 | – |
| – | 100.00 | 102.47 | 102.47 | – |
| – | 100.00 | 101.95 | 101.95 | – |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zang, J.; Zhang, J.; Ao, K.; Wang, Q.; Dong, Q.; Wei, Q. Sol-Gel Synthesis of Carbon Xerogel-ZnO Composite for Detection of Catechol. Materials 2016, 9, 282. https://doi.org/10.3390/ma9040282
Li D, Zang J, Zhang J, Ao K, Wang Q, Dong Q, Wei Q. Sol-Gel Synthesis of Carbon Xerogel-ZnO Composite for Detection of Catechol. Materials. 2016; 9(4):282. https://doi.org/10.3390/ma9040282
Chicago/Turabian StyleLi, Dawei, Jun Zang, Jin Zhang, Kelong Ao, Qingqing Wang, Quanfeng Dong, and Qufu Wei. 2016. "Sol-Gel Synthesis of Carbon Xerogel-ZnO Composite for Detection of Catechol" Materials 9, no. 4: 282. https://doi.org/10.3390/ma9040282





