Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair
Abstract
:1. Introduction
2. Results
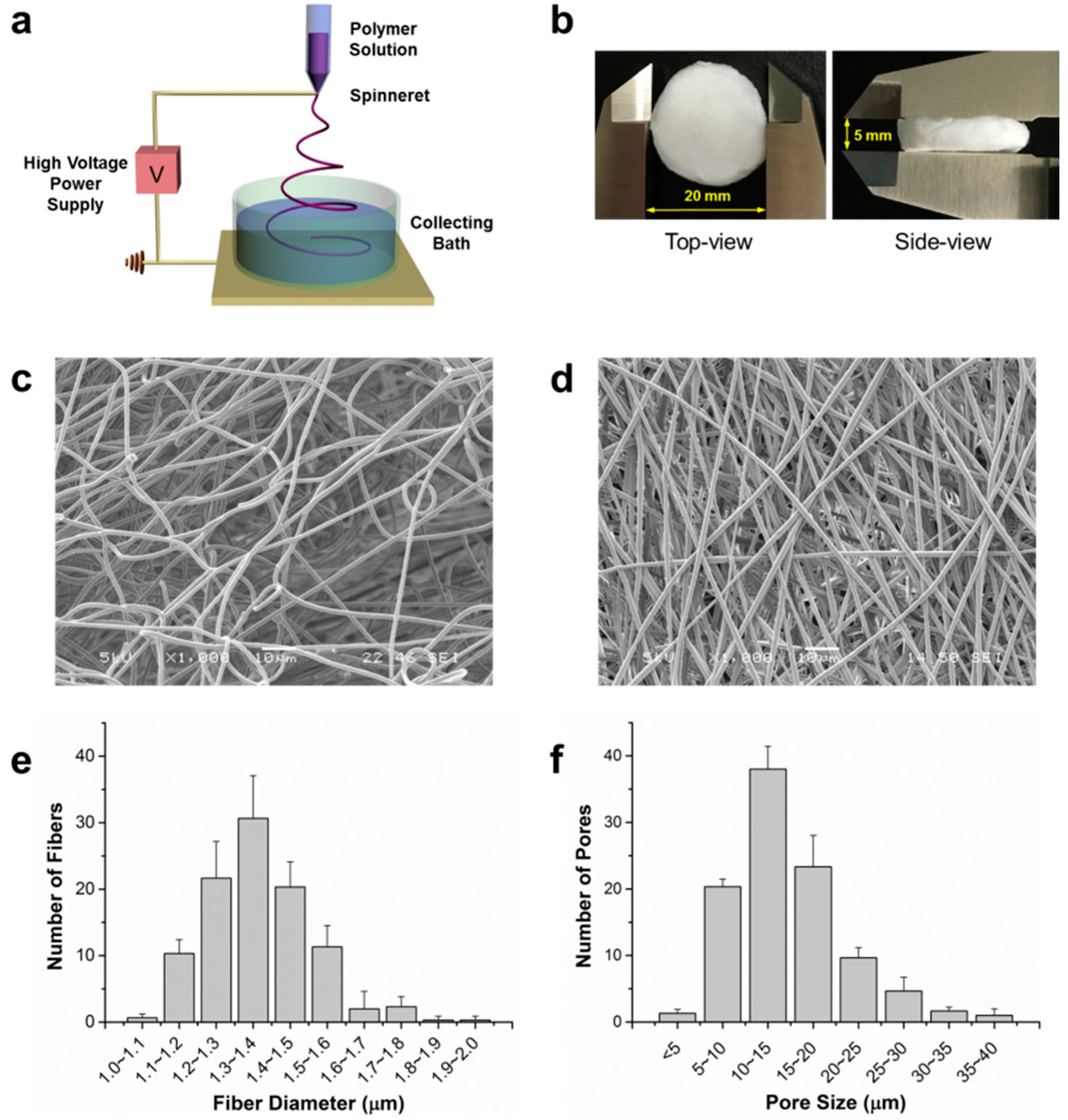
2.1. Characterization of 3D Fibrous Scaffolds
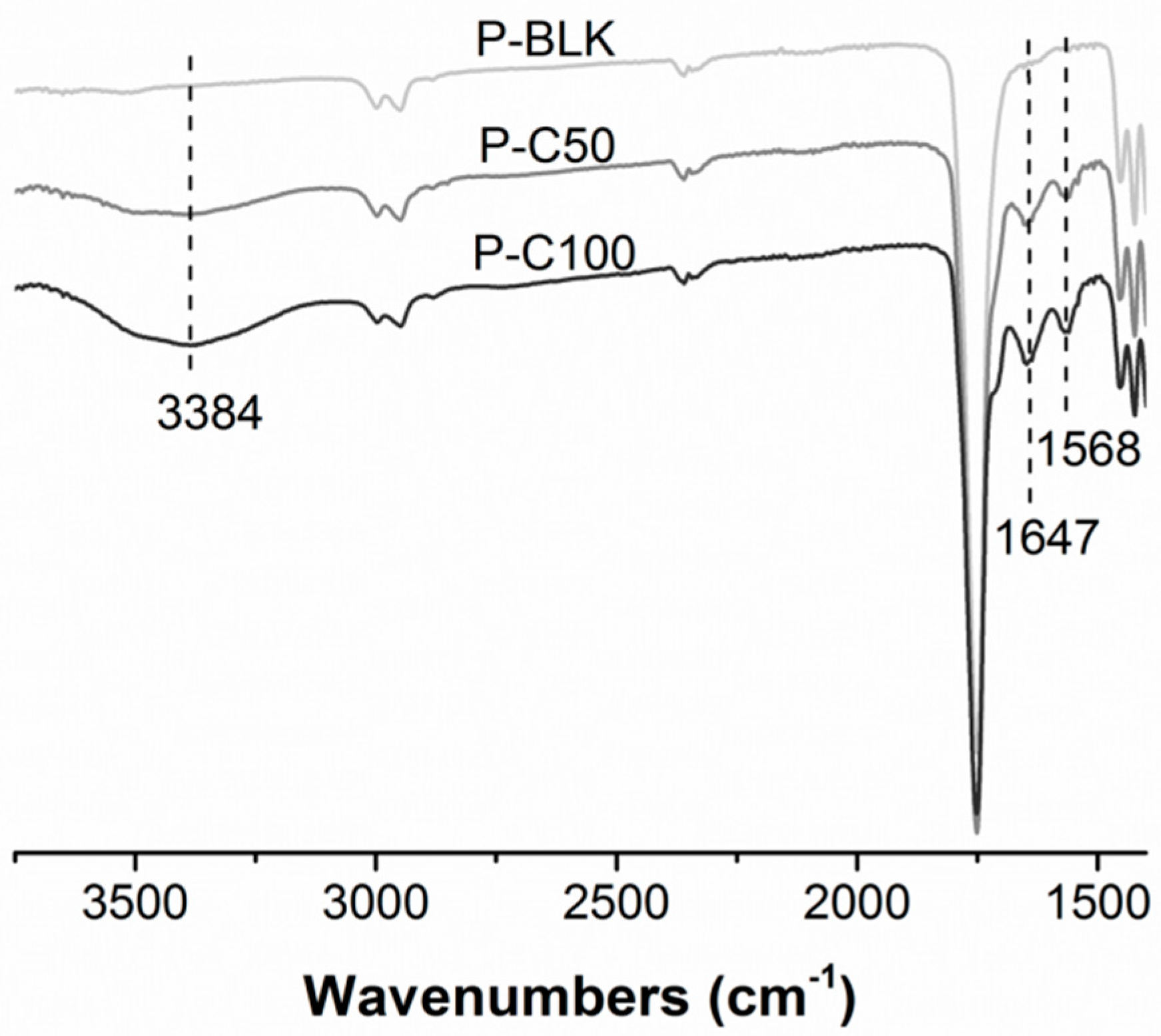
2.2. Surface Modification with Collagen Type I
2.3. Cellular Infiltration and Proliferation
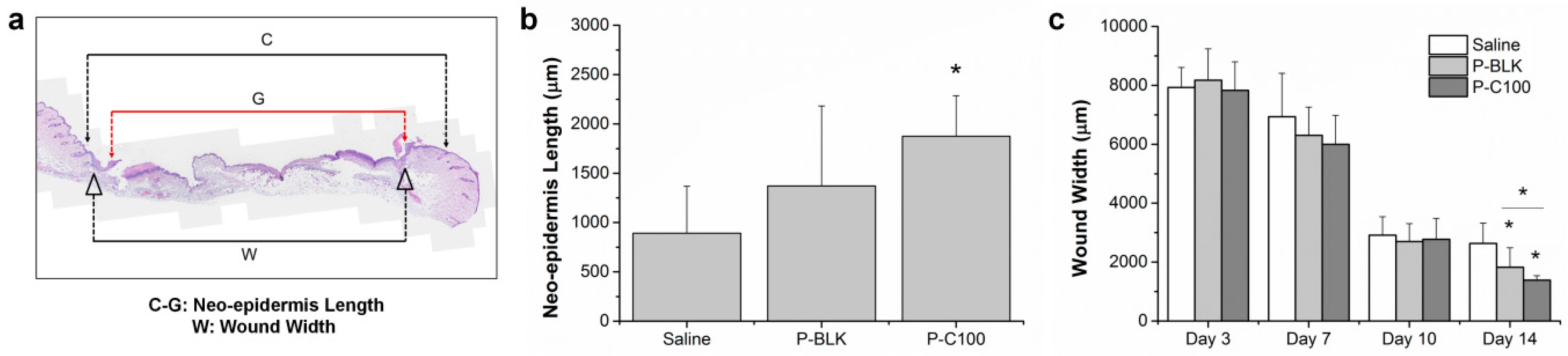
2.4. In Vivo Wound Healing
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Scaffolds Fabrication and Characterization
4.3. Cellular Infiltration and Proliferation
4.4. In Vivo Wound Healing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Embil, J.M.; Papp, K.; Sibbald, G.; Tousignant, J.; Smiell, J.M.; Wong, B.; Lau, C.Y.; Canadian Becaplermin Study Group. Recombinant human platelet-derived growth factor-bb (becaplermin) for healing chronic lower extremity diabetic ulcers: An open-label clinical evaluation of efficacy. Wound Repair Regen. 2000, 8, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Iqbal, S.A.; Baguneid, M.; Bayat, A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen. 2013, 21, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D. Bioengineered skin substitute considerations in the diabetic foot ulcer. Ann. Plast. Surg. 2014, 73, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Blakytny, R.; Jude, E.B. Altered molecular mechanisms of diabetic foot ulcers. Int. J. Low. Extremity Wounds 2009, 8, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xia, Y.N. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Ma, P.X. Nano-fibrous scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2004, 39, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78B, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, P.; Vasanthan, K.S.; Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Development of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) fibers for skin tissue engineering: Effects of topography, mechanical, and chemical stimuli. Biomacromolecules 2011, 12, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kwon, O.H.; Meng, W.; Kang, I.K. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, H.L.; Chen, W.L. Interaction of dermal fibroblasts with electrospun composite polymer scaffolds prepared from dextran and poly lactide-co-glycolide. Biomaterials 2006, 27, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kurpinski, K.; Quigley, R.; Gao, H.F.; Hsiao, B.S.; Poo, M.M.; Li, S. Bioactive nanofibers: Synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007, 7, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Gee, A.O.; Metter, R.B.; Nathan, A.S.; Marklein, R.A.; Burdick, J.A.; Mauck, R.L. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials 2008, 29, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Huang, Y.; Agarwal, S.; Lannutti, J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007, 13, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.; Martins, A.; Pinho, E.D.; Faria, S.; Reis, R.L.; Neves, N.M. Solving cell infiltration limitations of electrospun nanofiber meshes for tissue engineering applications. Nanomedicine 2010, 5, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules 2008, 9, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Jeong, S.I.; Bae, M.S.; Yang, D.H.; Heo, D.N.; Kim, C.H.; Alsberg, E.; Kwon, I.K. Highly porous electrospun nanofibers enhanced by ultrasonication for improved cellular infiltration. Tissue Eng. Part A 2011, 17, 2695–2702. [Google Scholar] [CrossRef] [PubMed]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.J.; Dean, D.R.; Jun, H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials 2011, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Hattori, S.; Yoshikawa, C.; Yasuda, Y.; Koyama, H.; Takato, T.; Kobayashi, H. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater. Lett. 2009, 63, 754–756. [Google Scholar] [CrossRef]
- Wen, F.; Chang, S.; Toh, Y.C.; Teoh, S.H.; Yu, H. Development of poly (lactic-co-glycolic acid)-collagen scaffolds for tissue engineering. Mater. Sci. Eng. C 2007, 27, 285–292. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, F.; Chen, H.; Pal, M.; Lai, Y.; Zhao, A.Z.; Tan, L.P. Micropatterning extracellular matrix proteins on electrospun fibrous substrate promote human mesenchymal stem cell differentiation toward neurogenic lineage. ACS Appl. Mater. Interfaces 2016, 8, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Lam, C.R.I.; Wen, F.; Chong, S.K.M.; Tan, N.S.; Chan, J.; Pal, M.; Tan, L.P. Novel method to improve vascularization of tissue engineered constructs with biodegradable fibers. Biofabrication 2016, 8, 015004. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Wong, Y.S.; Wen, F.; Ng, K.W.; Ng, G.K.L.; Venkatraman, S.S.; Boey, F.Y.C.; Tan, L.P. Human mesenchymal stem-cell behaviour on direct laser micropatterned electrospun scaffolds with hierarchical structures. Macromol. Biosci. 2013, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; Nukavarapu, S.P.; James, R.; Nair, L.S.; Laurencin, C.T. Electrospun poly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials 2008, 29, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (plga) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.W.; Mao, Z.W.; Gao, C.Y. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf. B Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Olsen, D.R.; Fazio, M.J. Extracellular matrix of the skin: 50 years of progress. J. Investig. Dermatol. 1989, 92, 61S–77S. [Google Scholar] [PubMed]
- Wang, S.; Taraballi, F.; Tan, L.P.; Ng, K.W. Human keratin hydrogels support fibroblast attachment and proliferation in vitro. Cell Tissue Res. 2012, 347, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Tambe, N.; Di, J.; Zhang, Z.; Bernacki, S.; El-Shafei, A.; King, M.W. Novel genipin-collagen immobilization of polylactic acid (pla) fibers for use as tissue engineering scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.B.; Gao, C.Y.; Liu, X.Y.; Shen, J.C. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Surface treatments of polymers for biocompatibility. Annu. Rev. Mater. Sci. 1996, 26, 365–394. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly(epsilon-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.R.; Bhattarai, N.; Yi, H.K.; Hwang, P.H.; Cha, D.I.; Kim, H.Y. Novel biodegradable electrospun membrane: Scaffold for tissue engineering. Biomaterials 2004, 25, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef] [PubMed]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Roh, H.W.; Lee, W.K.; Ryu, G.H. Evaluation of functions and tissue compatibility of poly (d,l-lactic-co-glycolic acid) seeded with human dermal fibroblasts. J. Biomater. Sci. Polym. Ed. 2006, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Ge, J.F.; Tredget, E.E.; Wu, Y.J. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat. Protocols 2013, 8, 302–309. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Peng, Y.; Wu, S.; Tan, L.P. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials 2016, 9, 272. https://doi.org/10.3390/ma9040272
Chen H, Peng Y, Wu S, Tan LP. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials. 2016; 9(4):272. https://doi.org/10.3390/ma9040272
Chicago/Turabian StyleChen, Huizhi, Yan Peng, Shucheng Wu, and Lay Poh Tan. 2016. "Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair" Materials 9, no. 4: 272. https://doi.org/10.3390/ma9040272




