Microparticles for Sustained Growth Factor Delivery in the Regeneration of Critically-Sized Segmental Tibial Bone Defects
Abstract
:1. Introduction
2. Results
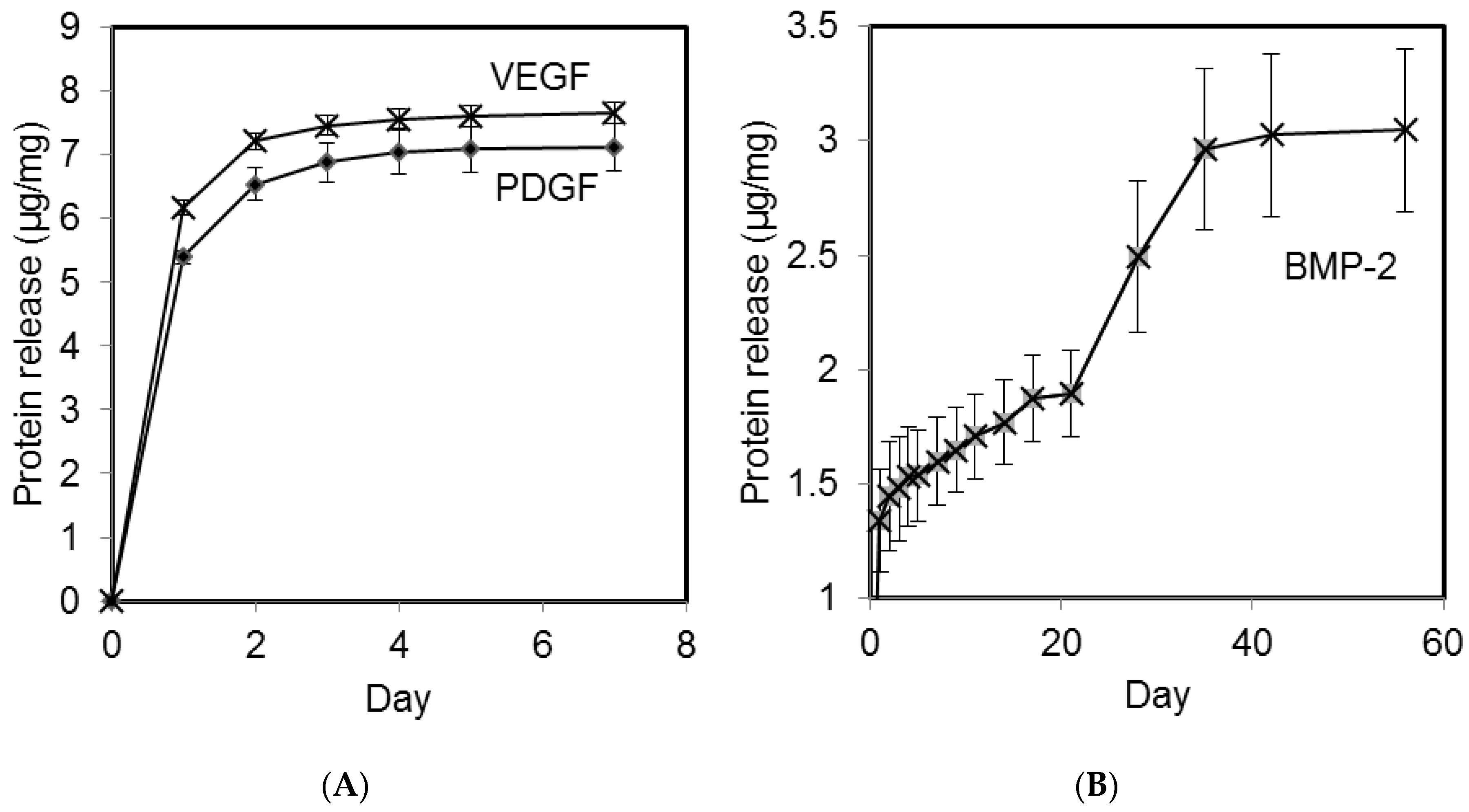
2.1. Growth Factor Release in vitro
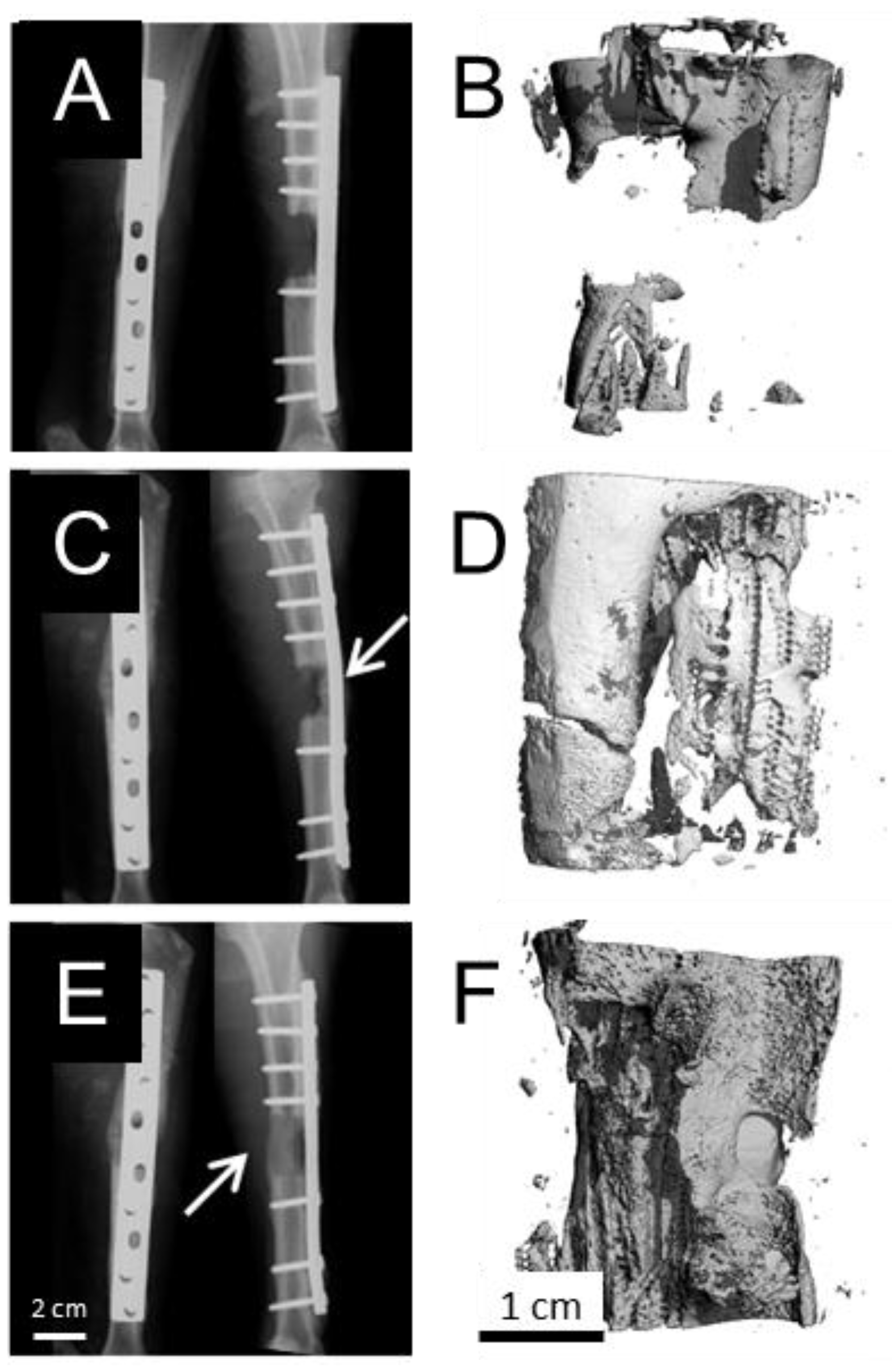
2.2. Explant Analysis
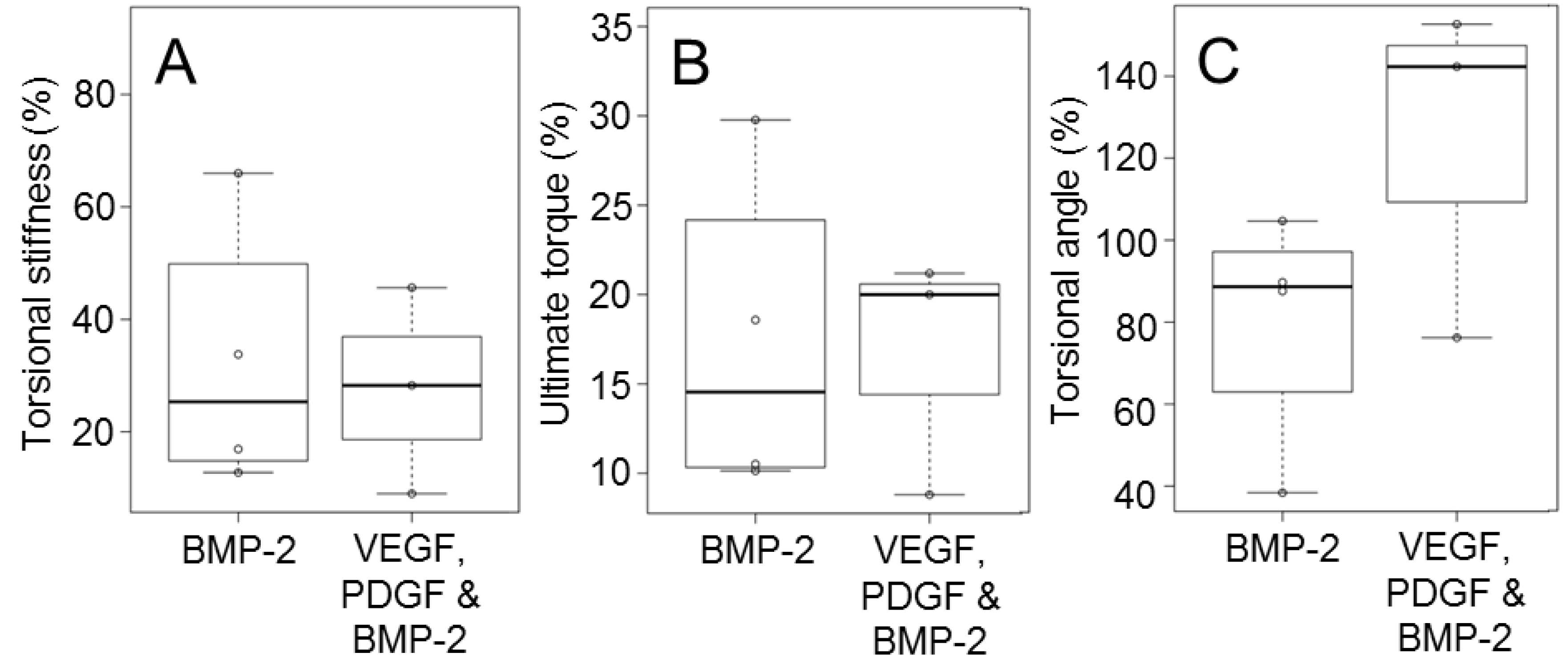
2.3. Biomechanical Testing
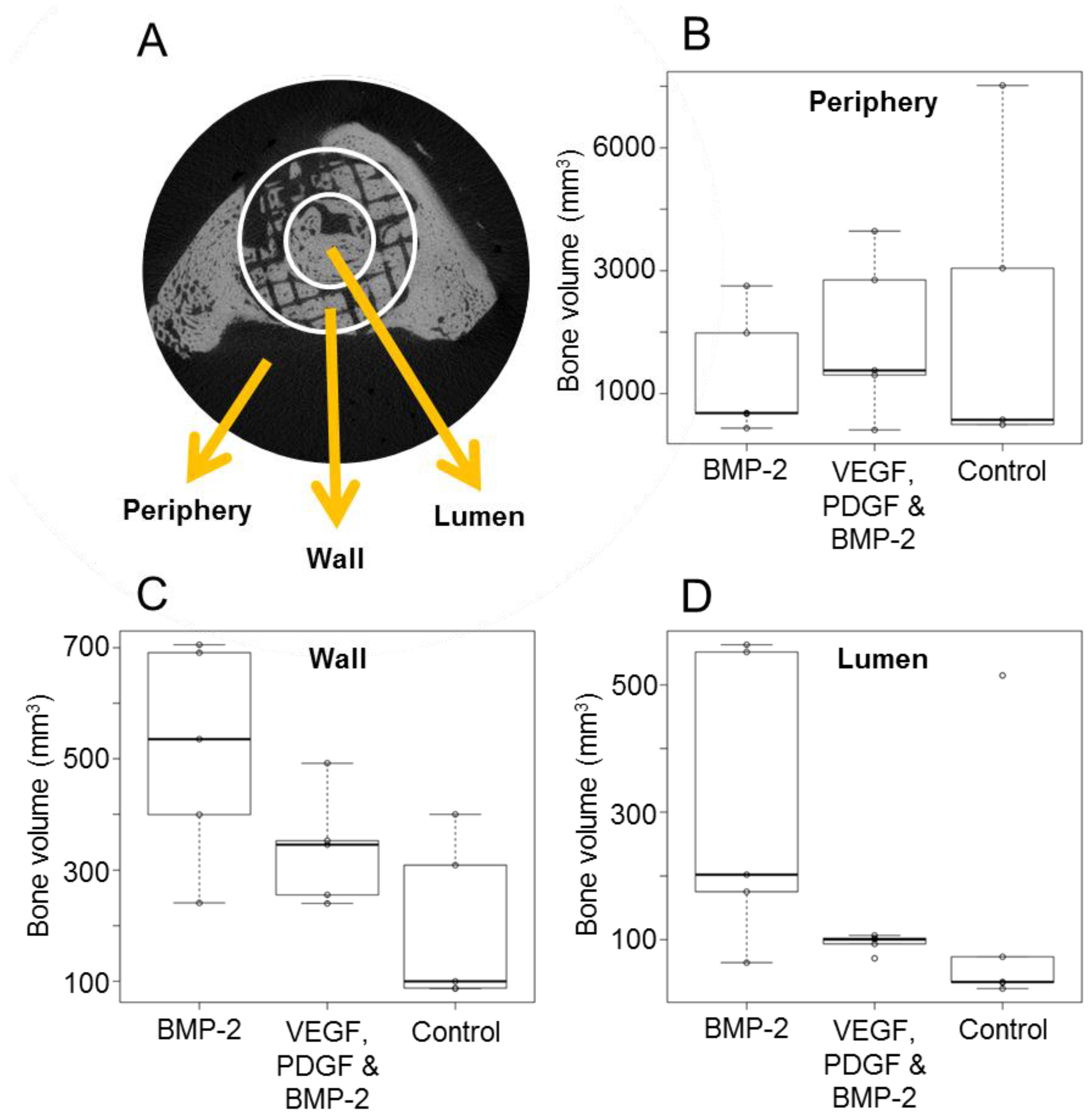
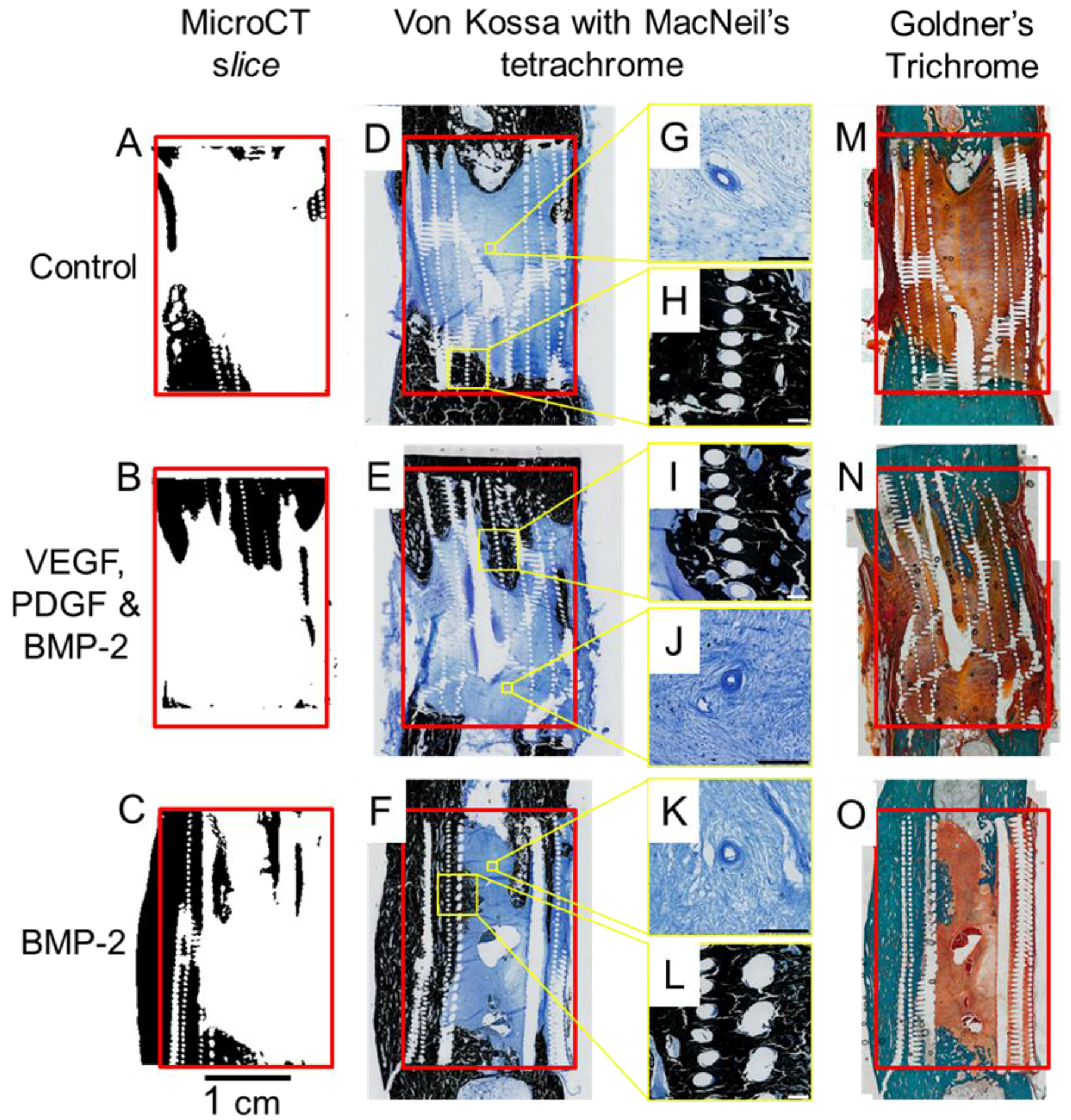
2.4. Micro Computed Tomography
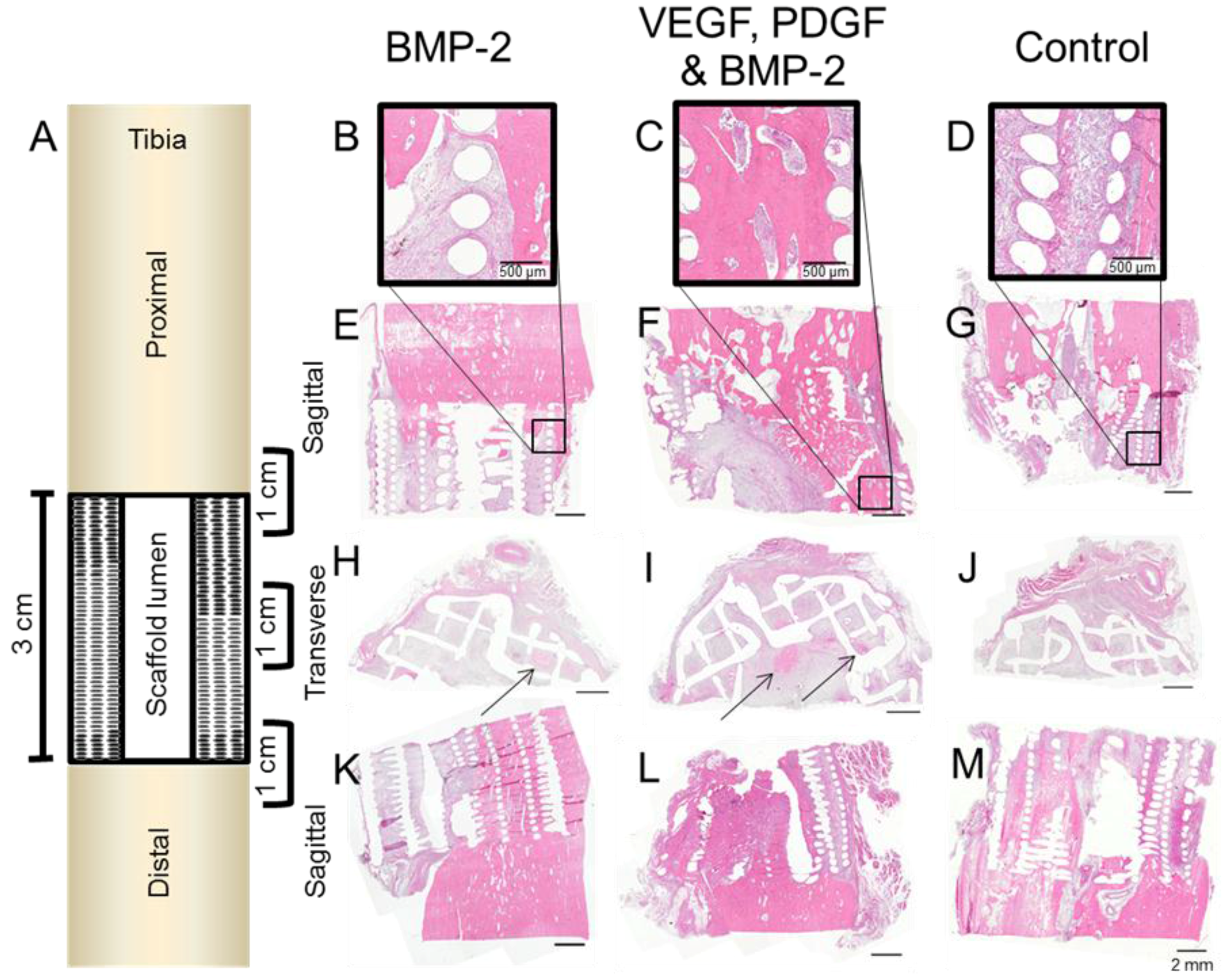
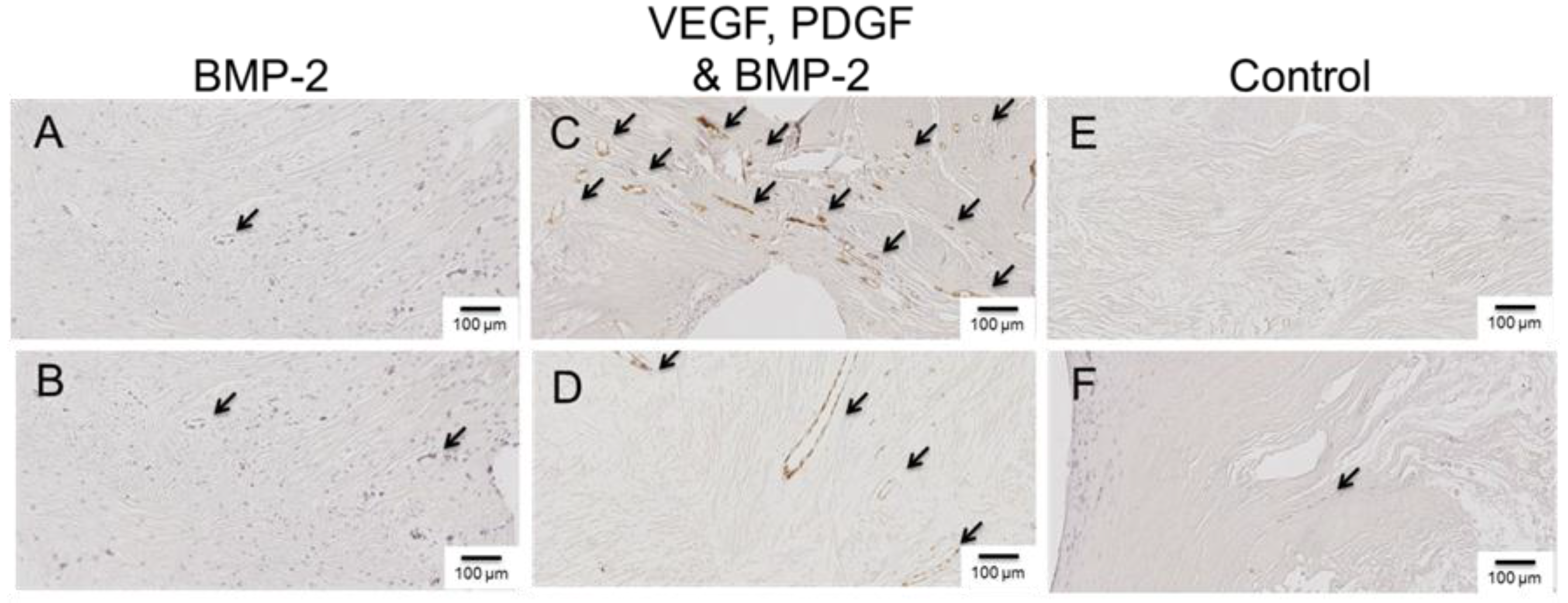
2.5. Histology
3. Discussion
4. Materials and Methods
4.1. Triblock Copolymer Fabrication
4.2. Microparticle Fabrication
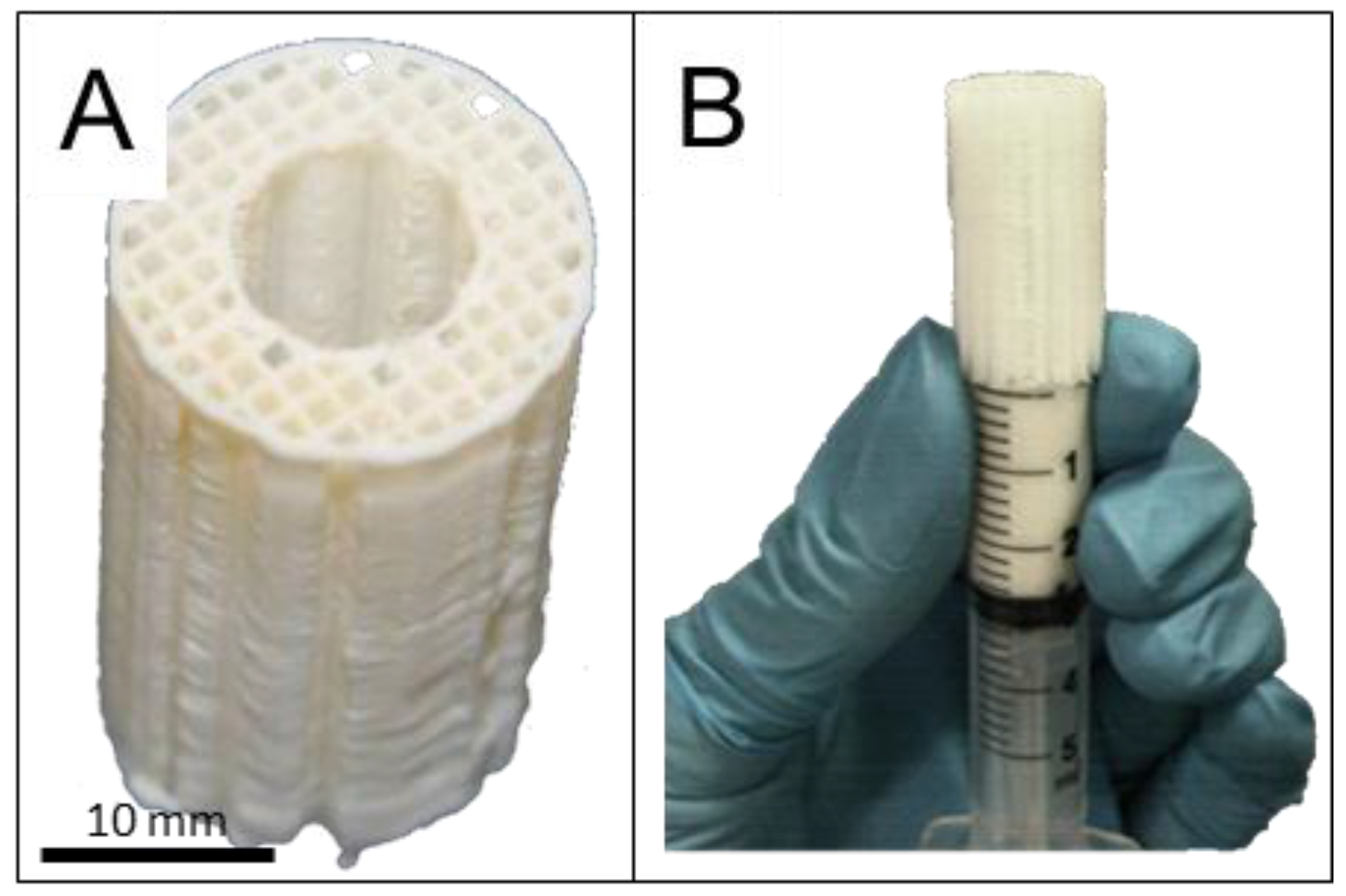
4.3. Scaffold Design and Fabrication
4.4. Loading of Microparticles into the Scaffold
4.5. Implantation
4.6. Clinical Monitoring
4.7. Mechanical Testing
4.8. Micro Computed Tomography
4.9. Histological Sample Preparation
4.10. Von Kossa Staining of Mineralised Sections
4.11. Goldner’s Trichrome Staining of Mineralised Sections
4.12. Haematoxylin and Eosin Staining of Demineralised Sections
4.13. Immunohistochemistry of Demineralised Sections
4.14. Statistical analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid |
| BMP | Bone morphogenetic protein |
| CD | Cluster of differentiation |
| CT | Computed tomography |
| DCP | Dynamic compression plate |
| GF | Growth factor |
| IHC | Immunihistochemistry |
| OC | Osteocalcin |
| P | P-value (test statistic) |
| PBS | Phospahte buffered saline |
| PCL | Polycaprolactone |
| PDGF | Platelet derived growth factror |
| PEG | Polyethylene glycol |
| PLGA | Poly(D,L-lactide-co-glycolide) |
| PRP | Platelet rich plasma |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
| vWF | Von Willebrand factor |
References
- Sela, J.J.; Bab, I.A. Healing of bone fracture: General concepts. In Principles of bone regeneration; Springer: New York, NY, USA, 2012; pp. 1–8. [Google Scholar]
- Cipitria, A.; Reichert, J.C.; Epari, D.R.; Saifzadeh, S.; Berner, A.; Schell, H.; Mehta, M.; Schuetz, M.A.; Duda, G.N.; Hutmacher, D.W. Polycaprolactone scaffold and reduced rhbmp-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 2013, 34, 9960–9968. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Le, T.K.; Burge, R.; Mershon, J. Tibia shaft fractures: Costly burden of nonunions. BMC Musculoskel. Dis. 2013, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.; Bittar, R.G. Bmp-7 (op-1®) safety in anterior cervical fusion surgery. J. Clin. Neurosci. 2009, 16, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (infuse® bone graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Niezgoda, J.; Van Gils, C.; Frykberg, R.; Hodde, J. Randomized clinical trial comparing oasis wound matrix to regranex gel for diabetic ulcers. Adv Skin Wound Care 2005, 18, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.; Hoefnagels, N.; Bulstra, S. Osteogenic activity of op-1 bone morphogenetic protein (bmp-7) in a human fibular defect. J Bone Joint Surg. Br. 1999, 81, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Carreon, L.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R. Posterolateral lumbar spine fusion with infuse bone graft. Spine J. 2007, 7, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Poynton, A.R.; Lane, J.M. Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 2002, 27, S40. [Google Scholar] [CrossRef] [PubMed]
- Buttermann, G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008, 8, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Perri, B.; Cooper, M.; Lauryssen, C.; Anand, N. Adverse swelling associated with use of rh-bmp-2 in anterior cervical discectomy and fusion: A case study. Spine J. 2007, 7, 235–239. [Google Scholar] [CrossRef] [PubMed]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of bmp-7/op-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Ghanayem, A.J.; Weiner, B.K.; Rothman, D.J.; Bono, C.M. A challenge to integrity in spine publications: Years of living dangerously with the promotion of bone growth factors. Spine J. 2011, 11, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Lazarous, D.F.; Shou, M.; Scheinowitz, M.; Hodge, E.; Thirumurti, V.; Kitsiou, A.N.; Stiber, J.A.; Lobo, A.D.; Hunsberger, S.; Guetta, E.; et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation 1996, 94, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Pope, D.F.; Malpass, T.W.; Foster, D.M.; Ross, R. Platelet-derived growth factor in vivo: Levels, activity, and rate of clearance. Blood 1984, 64, 458–469. [Google Scholar] [PubMed]
- Jeon, O.; Song, S.J.; Kang, S.W.; Putnam, A.J.; Kim, B.S. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly (l-lactic-co-glycolic acid) scaffold. Biomaterials 2007, 28, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Dadsetan, M.; Runge, M.B.; Spinner, R.J.; Yaszemski, M.J.; Windebank, A.J.; Wang, H. Controlled release of vascular endothelial growth factor using poly-lactic-co-glycolic acid microspheres: In vitro characterization and application in polycaprolactone fumarate nerve conduits. Acta Biomater. 2012, 8, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential bmp-2/bmp-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. A 2010, 93, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kirby, G.T.S.; White, L.J.; Rahman, C.V.; Cox, H.C.; Qutachi, O.; Rose, F.R.A.J.; Hutmacher, D.W.; Shakesheff, K.M.; Woodruff, M.A. Plga-based microparticles for the sustained release of bmp-2. Polymers 2011, 3, 571–586. [Google Scholar] [CrossRef] [Green Version]
- White, L.J.; Kirby, G.T.S.; Cox, H.C.; Qodratnama, R.; Qutachi, O.; Rose, F.R.A.J.; Shakesheff, K.M. Acelerating protein release from microparticles for regenerative medicine applications. Mat. Sci. Eng. C 2013, 33, 2578–2583. [Google Scholar] [CrossRef] [PubMed]
- Marui, A.; Kanematsu, A.; Yamahara, K.; Doi, K.; Kushibiki, T.; Yamamoto, M.; Itoh, H.; Ikeda, T.; Tabata, Y.; Komeda, M. Simultaneous application of basic fibroblast growth factor and hepatocyte growth factor to enhance the blood vessels formation. J. Vasc. Surg. 2005, 41, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Mooney, D. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost. 2007, 5, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Saif, J.; Schwarz, T.M.; Chau, D.Y.; Henstock, J.; Sami, P.; Leicht, S.F.; Hermann, P.C.; Alcala, S.; Mulero, F.; Shakesheff, K.M. Combination of injectable multiple growth factor–releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscl. Throm. Vas. 2010, 30, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, C.; Jin, C.; Zhang, Z.; Kong, D.; Xu, W.; Xiu, Y. Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur. Urol. 2011, 59, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation rates of oral resorbable implants (polylactates and polyglycolates): Rate modification with changes in pla/pga copolymer ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Keles, H.; Naylor, A.; Clegg, F.; Sammon, C. Investigation of factors influencing the hydrolytic degradation of single plga microparticles. Polym. Degrad. Stabil. 2015, 119, 228–241. [Google Scholar] [CrossRef]
- Street, J.; Winter, D.; Wang, J.H.; Wakai, A.; McGuinness, A.; Redmond, H.P. Is human fracture hematoma inherently angiogenic? Clin. Orthop. Relat. R. 2000, 378, 224–237. [Google Scholar] [CrossRef]
- Carano, R.A.; Filvaroff, E.H. Angiogenesis and bone repair. Drug Discov. Today 2003, 8, 980–989. [Google Scholar] [CrossRef]
- Banfi, A.; von Degenfeld, G.; Gianni-Barrera, R.; Reginato, S.; Merchant, M.J.; McDonald, D.M.; Blau, H.M. Therapeutic angiogenesis due to balanced single-vector delivery of vegf and pdgf-bb. FASEB J. 2012, 26, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Crotts, G.; Park, T.G. Protein delivery from poly (lactic-co-glycolic acid) biodegradable microspheres: Release kinetics and stability issues. J. Microencapsul. 1998, 15, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Giteau, A.; Venier-Julienne, M.C.; Aubert-Pouessel, A.; Benoit, J.P. How to achieve sustained and complete protein release from plga-based microparticles? Int. J. Pharm. 2008, 350, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Kempen, D.H.R.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J.A. Effect of local sequential vegf and bmp-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 2009, 30, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Algenstaedt, N.; Joscheck, C.; Wolfram, L.; Schaefer, C.; Müller, I.; Böttcher, A.; Deuretzbacher, G.; Wiesner, L.; Leunig, M.; Algenstaedt, P. Sequential changes in vessel formation and micro-vascular function during bone repair. Acta Orthop. 2006, 77, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. Vegf couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [PubMed]
- Karvinen, H.; Pasanen, E.; Rissanen, T.; Korpisalo, P.; Vähäkangas, E.; Jazwa, A.; Giacca, M.; Ylä-Herttuala, S. Long-term vegf-a expression promotes aberrant angiogenesis and fibrosis in skeletal muscle. Gene Ther. 2011, 18, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Usas, A.; Olshanski, A.; Ho, A.M.; Gearhart, B.; Cooper, G.M.; Huard, J. Vegf improves, whereas sflt1 inhibits, bmp2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner. Res. 2005, 20, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4, 141ra193–141ra193. [Google Scholar] [CrossRef] [PubMed]
- Berner, A.; Reichert, J.C.; Woodruff, M.A.; Saifzadeh, S.; Morris, A.J.; Epari, D.R.; Nerlich, M.; Schuetz, M.A.; Hutmacher, D.W. Autologous vs. Allogenic mesenchymal progenitor cells for the reconstruction of critical-sized segmental tibial bone defects in aged sheep. Acta Biomater. 2013, 9, 7874–7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, M.; Jortikka, L.; Halttunen, T.; Nevalainen, J.; Aho, A.; Marttinen, A.; Lindholm, T. Measurement of total and local bone morphogenetic protein concentration in bone tumours. Int. Orthop. 1997, 21, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef] [PubMed]
- Karal-Yilmaz, O.; Serhatli, M.; Baysal, K.; Baysal, B.M. Preparation and in vitro characterization of vascular endothelial growth factor (vegf)-loaded poly (d, l-lactic-co-glycolic acid) microspheres using a double emulsion/solvent evaporation technique. J. Microencapsul. 2011, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zentner, G.M.; Rathi, R.; Shih, C.; McRea, J.C.; Seo, M.H.; Oh, H.; Rhee, B.G.; Mestecky, J.; Moldoveanu, Z.; Morgan, M.; et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control. Release 2001, 72, 203–215. [Google Scholar] [CrossRef]
- Hou, Q.; Chau, D.; Pratoomsoot, C.; Tighe, P.J.; Dua, H.S.; Shakesheff, K.M.; Rose, F.R.A.J. In situ gelling hydrogels incorporating microparticles as drug delivery carriers for regenerative medicine. J. Pharm. Sci. 2008, 97, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Dinucci, D.; Cometa, S.; Alderighi, M.; Bártolo, P.J.; Chiellini, F. Polycaprolactone scaffolds fabricated via bioextrusion for tissue engineering applications. Int. J. Biomat. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Artitua, E.; Andia, I.; Ardanza, B.; Nurden, P.; Nurden, A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thrombosis And Haemostasis-Stuttgart 2004, 91, 4–15. [Google Scholar]
- Weibrich, G.; Kleis, W.; Kunz-Kostomanolakis, M.; Loos, A.H.; Wagner, W. Correlation of platelet concentration in platelet-rich plasma to the extraction method, age, sex, and platelet count of the donor. Int. J. Oral. Max. Impl. 2000, 16, 693–699. [Google Scholar]
- Reichert, J.C.; Epari, D.R.; Wullschleger, M.E.; Saifzadeh, S.; Steck, R.; Lienau, J.; Sommerville, S.; Dickinson, I.C.; Schütz, M.A.; Duda, G.N.; et al. Establishment of a preclinical ovine model for tibial segmental bone defect repair by applying bone tissue engineering strategies. Tissue Eng. Part B Rev. 2010, 16, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Wullschleger, M.E.; Cipitria, A.; Lienau, J.; Cheng, T.K.; Schütz, M.A.; Duda, G.N.; Nöth, U.; Eulert, J.; Hutmacher, D.W. Custom-made composite scaffolds for segmental defect repair in long bones. Int. Orthop. 2011, 35, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.; Turner, A.S.; Wark, J.D. The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone 1995, 16, S277–S284. [Google Scholar] [CrossRef]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The bonferroni vs holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef] [PubMed]
- R Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/.
| Group | Growth Factor Loaded Microparticles |
|---|---|
| GF-free | Control microparticles (no growth factor) |
| Combination | VEGF (0.24 mg), PDGF (0.24 mg) and BMP-2 (1.12 mg) |
| BMP-2 | BMP-2 only (1.12 mg) |
| MNa | % Mole Lactidea | % Mole Glycolidea | MNb | MWb | PDIb |
|---|---|---|---|---|---|
| 1706-1500-1706 | 71 | 29 | 2442 | 4022 | 1.65 |
| PLGA Lactide:Glycolide Ratio | PLGA-PEG-PLGA % w/w | Growth Factor |
|---|---|---|
| 50:50 | 10 | BMP-2 |
| 50:50 | 20 | PDGF-BB |
| 85:15 | 30 | VEGF-165 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirby, G.T.S.; White, L.J.; Steck, R.; Berner, A.; Bogoevski, K.; Qutachi, O.; Jones, B.; Saifzadeh, S.; Hutmacher, D.W.; Shakesheff, K.M.; et al. Microparticles for Sustained Growth Factor Delivery in the Regeneration of Critically-Sized Segmental Tibial Bone Defects. Materials 2016, 9, 259. https://doi.org/10.3390/ma9040259
Kirby GTS, White LJ, Steck R, Berner A, Bogoevski K, Qutachi O, Jones B, Saifzadeh S, Hutmacher DW, Shakesheff KM, et al. Microparticles for Sustained Growth Factor Delivery in the Regeneration of Critically-Sized Segmental Tibial Bone Defects. Materials. 2016; 9(4):259. https://doi.org/10.3390/ma9040259
Chicago/Turabian StyleKirby, Giles T. S., Lisa J. White, Roland Steck, Arne Berner, Kristofor Bogoevski, Omar Qutachi, Brendan Jones, Siamak Saifzadeh, Dietmar W. Hutmacher, Kevin M. Shakesheff, and et al. 2016. "Microparticles for Sustained Growth Factor Delivery in the Regeneration of Critically-Sized Segmental Tibial Bone Defects" Materials 9, no. 4: 259. https://doi.org/10.3390/ma9040259







