High Pressure Synthesis of p-Type CeyFe4−xCoxSb12 Skutterudites
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Slack, G.A. New Materials and Performance Limits for Thermoeletric Cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Rull-Bravo, M.; Moure, A.; Fernandez, J.F.; Martin-Gonzalez, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Pei, Y.Z.; Yang, J.; Chen, L.D.; Zhang, W.; Salvador, J.R.; Yang, J. Improving thermoelectric performance of caged compounds through light-element filling. Appl. Phys. Lett. 2009, 95, 042101. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Salvador, J.R.; Chi, M.; Cho, J.Y.; Wang, H.; Bai, S.; Yang, J.; Zhang, W.; Chen, L. Multiple-Filled Skutterudites: High Thermoelectric Figure of Merit through Separately Optimizing Electrical and Thermal Transports. J. Am. Chem. Soc. 2011, 133, 7837–7846. [Google Scholar] [CrossRef] [PubMed]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Peranio, N.; Bauer, E.; Zehetbauer, M.; Eibl, O. n-Type skutterudites (R, Ba, Yb)yCo4Sb12 (R = Sr, La, Mm, DD, SrMm, SrDD) approaching ZT ≈ 2.0. Acta Mater. 2014, 63, 30–43. [Google Scholar] [CrossRef]
- Liu, R.; Yang, J.; Chen, X.; Shi, X.; Chen, L.; Uher, C. p-Type skutterudites RxMyFe3CoSb12 (R, M = Ba, Ce, Nd, and Yb): Effectiveness of double-filling for the lattice thermal conductivity reduction. Intermetallics 2011, 19, 1747–1751. [Google Scholar] [CrossRef]
- Yang, J.; Meisner, G.P.; Rawn, C.J.; Wang, H.; Chakoumakos, B.C.; Martin, J.; Nolas, G.S.; Pedersen, B.L.; Stalick, J.K. Low temperature transport and structural properties of misch-metal-filled skutterudites. J. Appl. Phys. 2007, 102, 083702. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Bauer, E.; Zehetbauer, M. A new generation of p-type didymium skutterudites with high ZT. Intermetallics 2011, 19, 546–555. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Heinrich, P.; Bauer, E.; Kumar, P.; Peranio, N.; Eibl, O.; Horky, J.; Zehetbauer, M.; Rogl, P. New bulk p-type skutterudites DD0.7Fe2.7Co1.3Sb12−xXx (X = Ge, Sn) reaching ZT > 1.3. Acta Mater. 2015, 91, 227–238. [Google Scholar] [CrossRef]
- Qiu, P.F.; Yang, J.; Liu, R.H.; Shi, X.; Huang, X.Y.; Snyder, G.J.; Zhang, W.; Chen, L.D. High-temperature electrical and thermal transport properties of fully filled skutterudites RFe4Sb12 (R = Ca, Sr, Ba, La, Ce, Pr, Nd, Eu, and Yb). J. Appl. Phys. 2011, 109, 063713. [Google Scholar] [CrossRef]
- Dong, Y.; Puneet, P.; Tritt, T.M.; Nolas, G.S. High-temperature thermoelectric properties of p-type skutterudites Ba0.15YbxCo3FeSb12 and YbyCo3FeSb9As3. J. Mater. Sci. 2015, 50, 34–39. [Google Scholar] [CrossRef]
- Caillat, T.; Kulleck, J.; Borshchevsky, A.; Fleurial, J.P. Preparation and thermoelectric properties of the skutterudite-related phase Ru0.5Pd0.5Sb3. J. Appl. Phys. 1996, 79, 8419–8426. [Google Scholar] [CrossRef]
- Anno, H.; Matsubara, K.; Notohara, Y.; Sakakibara, T.; Tashiro, H. Effects of doping on the transport properties of CoSb3. J. Appl. Phys. 1999, 86, 3780–3786. [Google Scholar] [CrossRef]
- Liu, W.S.; Zhang, B.P.; Li, J.F.; Zhang, H.L.; Zhao, L.D. Enhanced thermoelectric properties in CoSb3-xTex alloys prepared by mechanical alloying and spark plasma sintering. J. Appl. Phys. 2007, 102, 103717. [Google Scholar] [CrossRef]
- Su, X.L.; Li, H.; Wang, G.Y.; Chi, H.; Zhou, X.Y.; Tang, X.F.; Zhang, Q.J.; Uher, C. Structure and transport properties of double-doped CoSb2.75Ge0.25–xTex (x = 0.125–0.20) with in situ nanostructure. Chem. Mater. 2011, 23, 2948–2955. [Google Scholar] [CrossRef]
- Kim, I.H.; Ur, S.C. Electronic transport properties of Fe-doped CoSb3 prepared by encapsulated induction melting. Mater. Lett. 2007, 61, 2446–2450. [Google Scholar] [CrossRef]
- Katsuyama, S.; Shichijo, Y.; Ito, M.; Majima, K.; Nagai, H. Thermoelectric properties of the skutterudite Co1−xFexSb3 system. J. Appl. Phys. 1998, 84, 6708–6712. [Google Scholar] [CrossRef]
- Duan, B.; Zhai, P.; Xu, C.; Ding, S.; Li, P.; Zhang, Q. Thermoelectric performance of tellurium and sulfur double-substituted skutterudite materials. J. Mater. Sci. 2014, 49, 4445–4452. [Google Scholar] [CrossRef]
- Sales, B.C.; Mandrus, D.; Chakoumakos, B.C.; Keppens, V.; Thompson, J.R. Filled skutterudite antimonides: Electron crystals and phonon glasses. Phys. Rev. B 1997, 56, 15081–15089. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, P.; Chen, X.; Huang, X.; Chen, L. Composition optimization of p-type skutterudites CeyFexCo4−xSb12 and YbyFexCo4−xSb12. J. Mater. Res. 2011, 26, 1813–1819. [Google Scholar] [CrossRef]
- Tan, G.J.; Wang, S.Y.; Yan, Y.G.; Li, H.; Tang, X.F. Effects of Cobalt Substitution for Fe on the Thermoelectric Properties of p-Type CeFe4−xCoxSb12 Skutterudites. J. Electron. Mater. 2012, 41, 1147–1152. [Google Scholar] [CrossRef]
- Tan, G.J.; Wang, S.Y.; Tang, X.F. Thermoelectric Performance Optimization in p-Type CeyFe3CoSb12 Skutterudites. J. Electron. Mater. 2014, 43, 1712–1717. [Google Scholar] [CrossRef]
- Qiu, P.F.; Liu, R.H.; Yang, J.; Shi, X.; Huang, X.Y.; Zhang, W.; Chen, L.D.; Yang, J.; Singh, D.J. Thermoelectric properties of Ni-doped CeFe4Sb12 skutterudites. J. Appl. Phys. 2012, 111, 023705. [Google Scholar] [CrossRef]
- Yang, J.Q.; Zhang, L.; Liu, Y.D.; Chen, C.; Li, J.H.; Yu, D.L.; He, J.L.; Liu, Z.Y.; Tian, Y.J.; Xu, B. Investigation of skutterudite MgyCo4Sb12: High pressure synthesis and thermoelectric properties. J. Appl. Phys. 2013, 113, 113703. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, B.; Wang, L.M.; Yu, D.L.; Liu, Z.Y.; He, J.L.; Tian, Y.J. Great thermoelectric power factor enhancement of CoSb3 through the lightest metal element filling. Appl. Phys. Lett. 2011, 98, 072109. [Google Scholar] [CrossRef]
- Zhang, J.J.; Xu, B.; Wang, L.M.; Yu, D.L.; Yang, J.Q.; Yu, F.R.; Liu, Z.Y.; He, J.L.; Wen, B.; Tian, Y.J. High-pressure synthesis of phonon-glass electron-crystal featured thermoelectric LixCo4Sb12. Acta Mater. 2012, 60, 1246–1251. [Google Scholar] [CrossRef]
- Deng, L.; Wang, L.B.; Jia, X.P.; Ma, H.A.; Qin, J.M.; Wan, Y.C. Improvement of thermoelectric performance for Te-doped CoSb3 by higher synthesis pressure. J. Alloys Compd. 2014, 602, 117–121. [Google Scholar] [CrossRef]
- Deng, L.; Jia, X.P.; Su, T.C.; Jiang, Y.P.; Zheng, S.Z.; Guo, X.; Ma, H.A. The thermoelectric properties of Co4Sb12-xTex synthesized at different pressure. Mater. Lett. 2011, 65, 1057–1059. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.H.; Kang, Y.L.; Zhang, L.; Yu, D.L.; He, J.L.; Liu, Z.Y.; Tian, Y.J.; Xu, B. High pressure synthesis of Te-doped CoSb3 with enhanced thermoelectric performance. J. Mater. Sci. Mater. Electron. 2015, 26, 385–391. [Google Scholar] [CrossRef]
- Badding, J.V. High-pressure synthesis, characterization, and tuning of solid state materials. Annu Rev. Mater. Sci. 1998, 28, 631–658. [Google Scholar] [CrossRef]
- Schnelle, W.; Leithe-Jasper, A.; Rosner, H.; Cardoso-Gil, R.; Gumeniuk, R.; Trots, D.; Mydosh, J.; Grin, Y. Magnetic, thermal, and electronic properties of iron-antimony filled skutterudites MFe4Sb12 (M = Na, K, Ca, Sr, Ba, La, Yb). Phys. Rev. B 2008, 77, 094421. [Google Scholar] [CrossRef]
- Dyck, J.S.; Chen, W.; Uher, C.; Chen, L.; Tang, X.; Hirai, T. Thermoelectric properties of the n-type filled skutterudite Ba0.3Co4Sb12 doped with Ni. J. Appl. Phys. 2002, 91, 3698–3705. [Google Scholar] [CrossRef]
- Dimitrov, I.K.; Manley, M.E.; Shapiro, S.M.; Yang, J.; Zhang, W.; Chen, L.D.; Jie, Q.; Ehlers, G.; Podlesnyak, A.; Camacho, J.; Li, Q. Einstein modes in the phonon density of states of the single-filled skutterudite Yb0.2Co4Sb12. Phys. Rev. B 2010, 82, 174301. [Google Scholar] [CrossRef]
- Hermann, R.; Jin, R.; Schweika, W.; Grandjean, F.; Mandrus, D.; Sales, B.; Long, G. Einstein oscillators in thallium filled antimony skutterudites. Phys. Rev. Lett. 2003, 90, 135505. [Google Scholar] [CrossRef] [PubMed]
- Keppens, V.; Mandrus, D.; Sales, B.C.; Chakoumakos, B.C.; Dai, P.; Coldea, R.; Maple, M.B.; Gajewski, D.A.; Freeman, E.J.; Bennington, S. Localized vibrational modes in metallic solids. Nature 1998, 395, 876–878. [Google Scholar]
- Schnelle, W.; Leithe-Jasper, A.; Schmidt, M.; Rosner, H.; Borrmann, H.; Burkhardt, U.; Mydosh, J.; Grin, Y. Itinerant iron magnetism in filled skutterudites CaFe4Sb12 and YbFe4Sb12: Stable divalent state of ytterbium. Phys. Rev. B 2005, 72, 020402. [Google Scholar] [CrossRef]
- Cao, D.; Bridges, F.; Chesler, P.; Bushart, S.; Bauer, E.; Maple, M. Evidence for rattling behavior of the filler atom (L) in the filled skutterudites LT4X12 (L = Ce, Eu, Yb; T = Fe, Ru; X = P, Sb) from EXAFS studies. Phys. Rev. B 2004, 70, 094109. [Google Scholar] [CrossRef]
- Brazhkin, V.V. High-pressure synthesized materials: Treasures and hints. High Press. Res. 2007, 27, 333–351. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Bauer, E.; Kerber, M.B.; Zehetbauer, M.; Puchegger, S. Multifilled nanocrystalline p-type didymium-Skutterudites with ZT > 1.2. Intermetallics 2010, 18, 2435–2444. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Rogl, P.; Royanian, E.; Bauer, E.; Horky, J.; Setman, D.; Schafler, E.; Zehetbauer, M. Dependence of thermoelectric behaviour on severe plastic deformation parameters: A case study on p-type skutterudite DD0.60Fe3CoSb12. Acta Mater. 2013, 61, 6778–6789. [Google Scholar] [CrossRef]
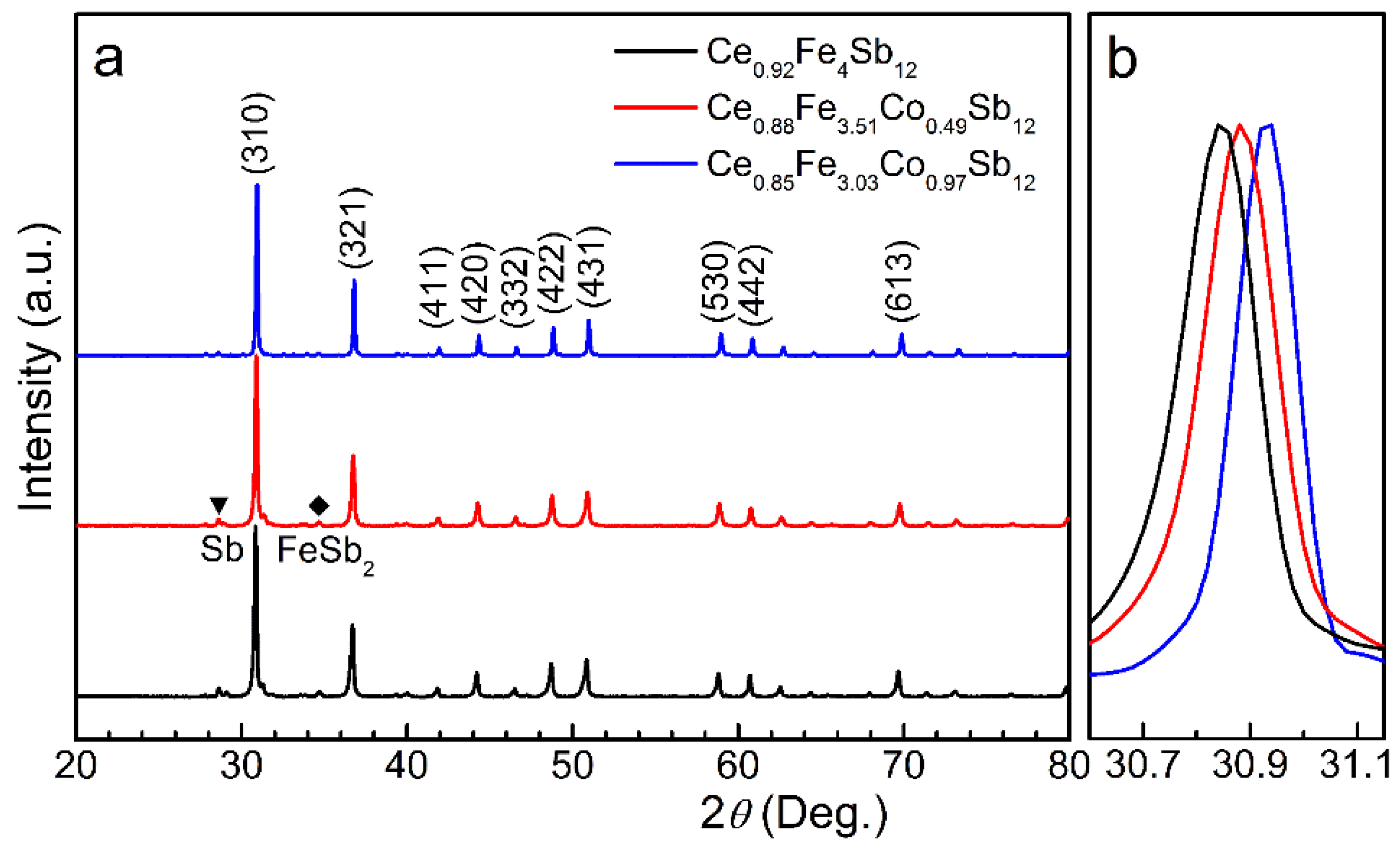

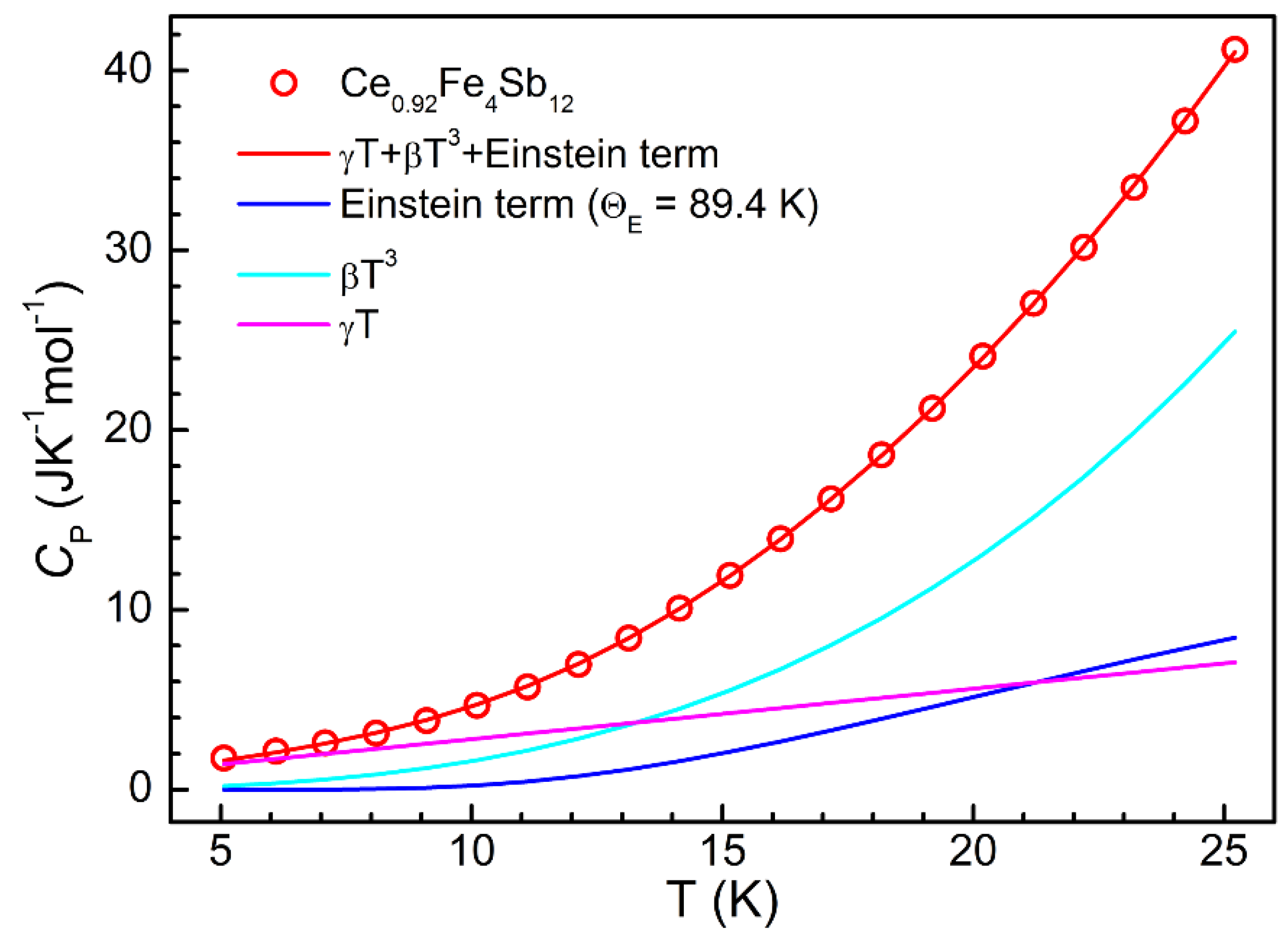

| x | y | a (nm) | S (μV K−1) | ρ (μΩ m) | p (1020 cm−3) | μ (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|
| 0 | 0.92 (2) | 0.9167 (3) | 76.2 | 5.0 | 21.5 | 5.8 |
| 0.49 (1) | 0.88 (2) | 0.9153 (3) | 84.3 | 6.9 | 17.1 | 5.3 |
| 0.97 (2) | 0.85 (2) | 0.9140 (3) | 99.4 | 14.3 | 9.5 | 4.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, X.; Zhang, Q.; Zhang, L.; Yu, D.; Xu, B.; Tian, Y. High Pressure Synthesis of p-Type CeyFe4−xCoxSb12 Skutterudites. Materials 2016, 9, 257. https://doi.org/10.3390/ma9040257
Liu Y, Li X, Zhang Q, Zhang L, Yu D, Xu B, Tian Y. High Pressure Synthesis of p-Type CeyFe4−xCoxSb12 Skutterudites. Materials. 2016; 9(4):257. https://doi.org/10.3390/ma9040257
Chicago/Turabian StyleLiu, Yadi, Xiaohui Li, Qian Zhang, Long Zhang, Dongli Yu, Bo Xu, and Yongjun Tian. 2016. "High Pressure Synthesis of p-Type CeyFe4−xCoxSb12 Skutterudites" Materials 9, no. 4: 257. https://doi.org/10.3390/ma9040257






