Effect of Sn Content in a CuSnZn Metal Precursor on Formation of MoSe2 Film during Selenization in Se+SnSe Vapor
Abstract
:1. Introduction
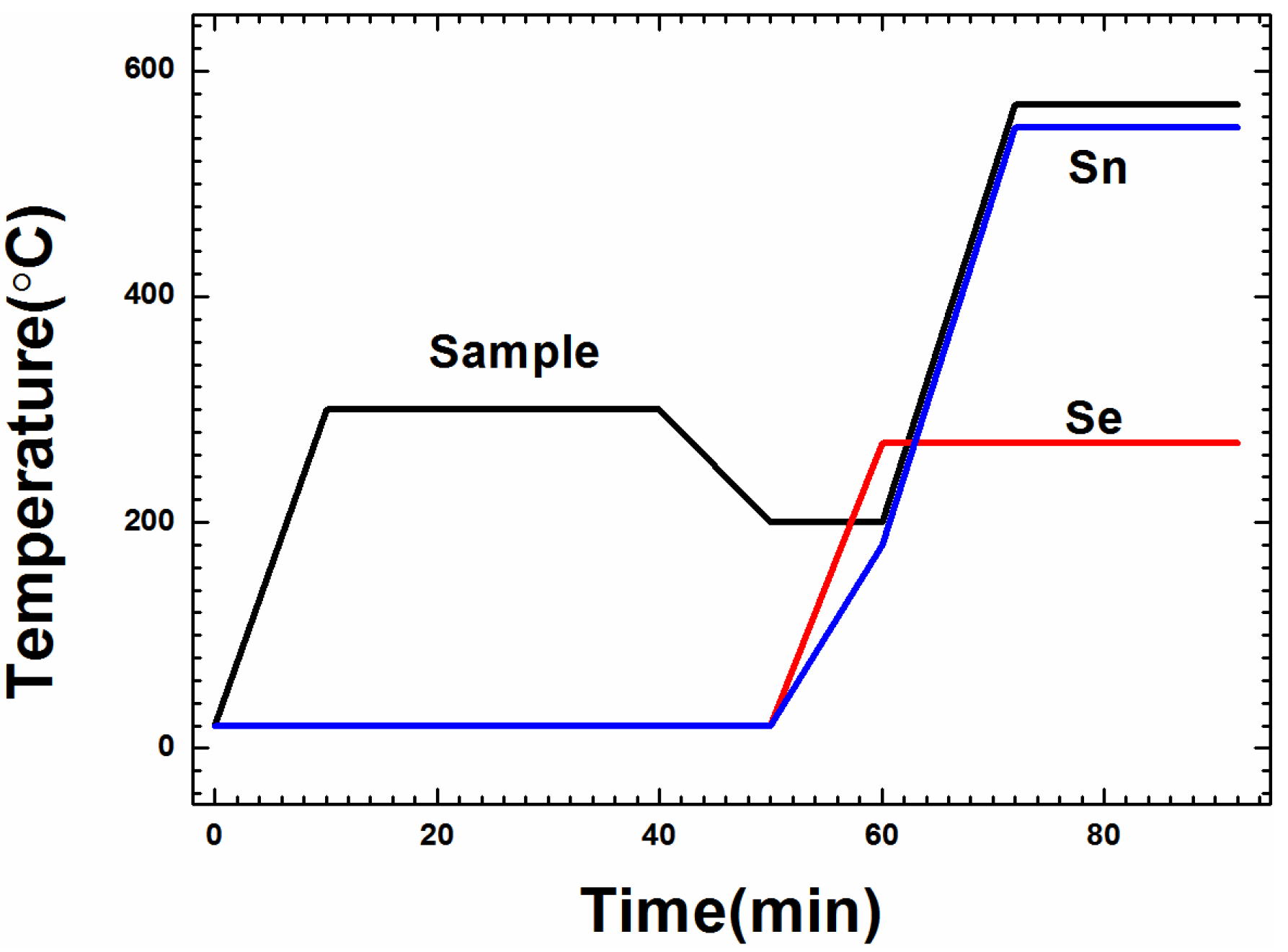
2. Experimental Section
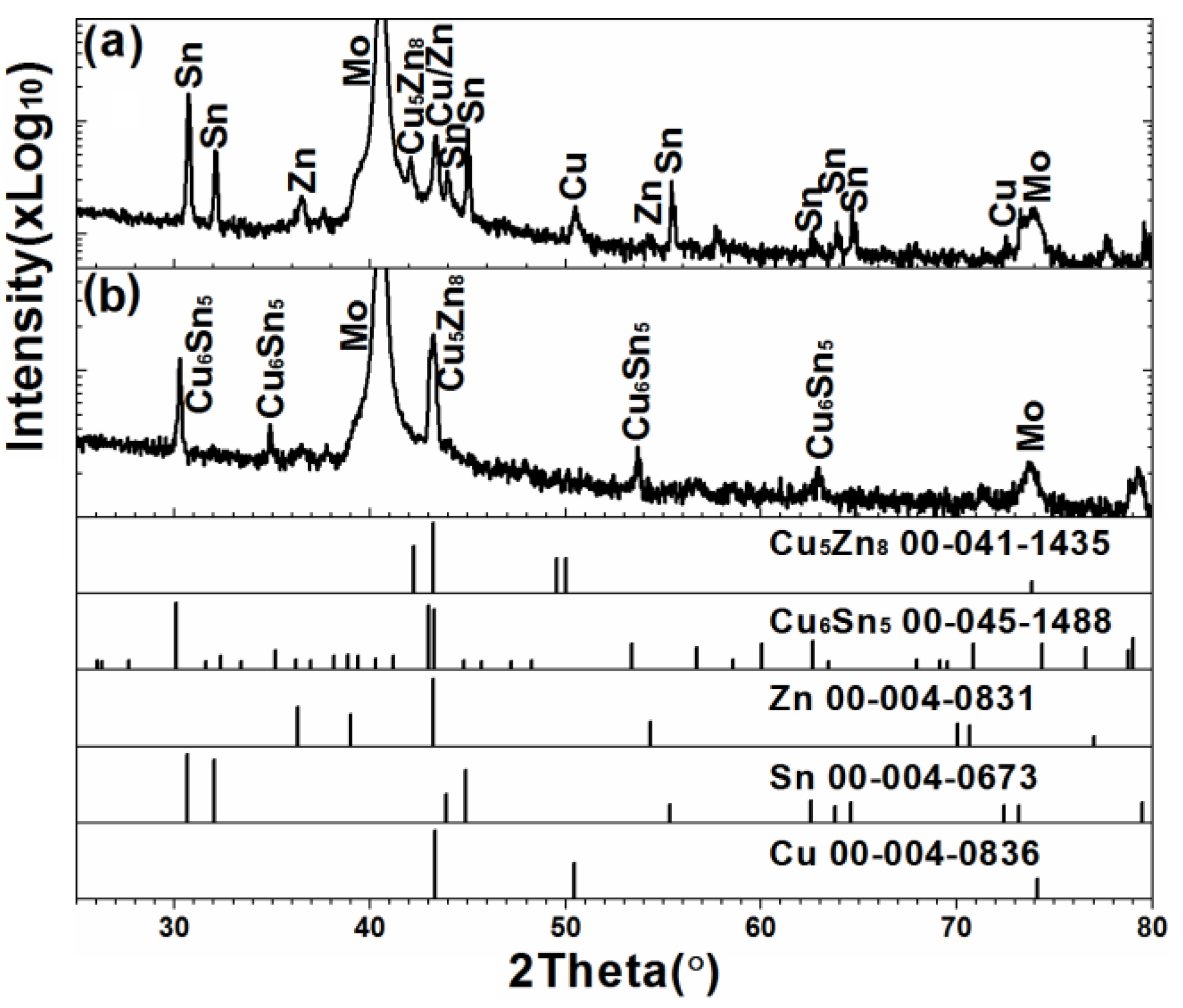
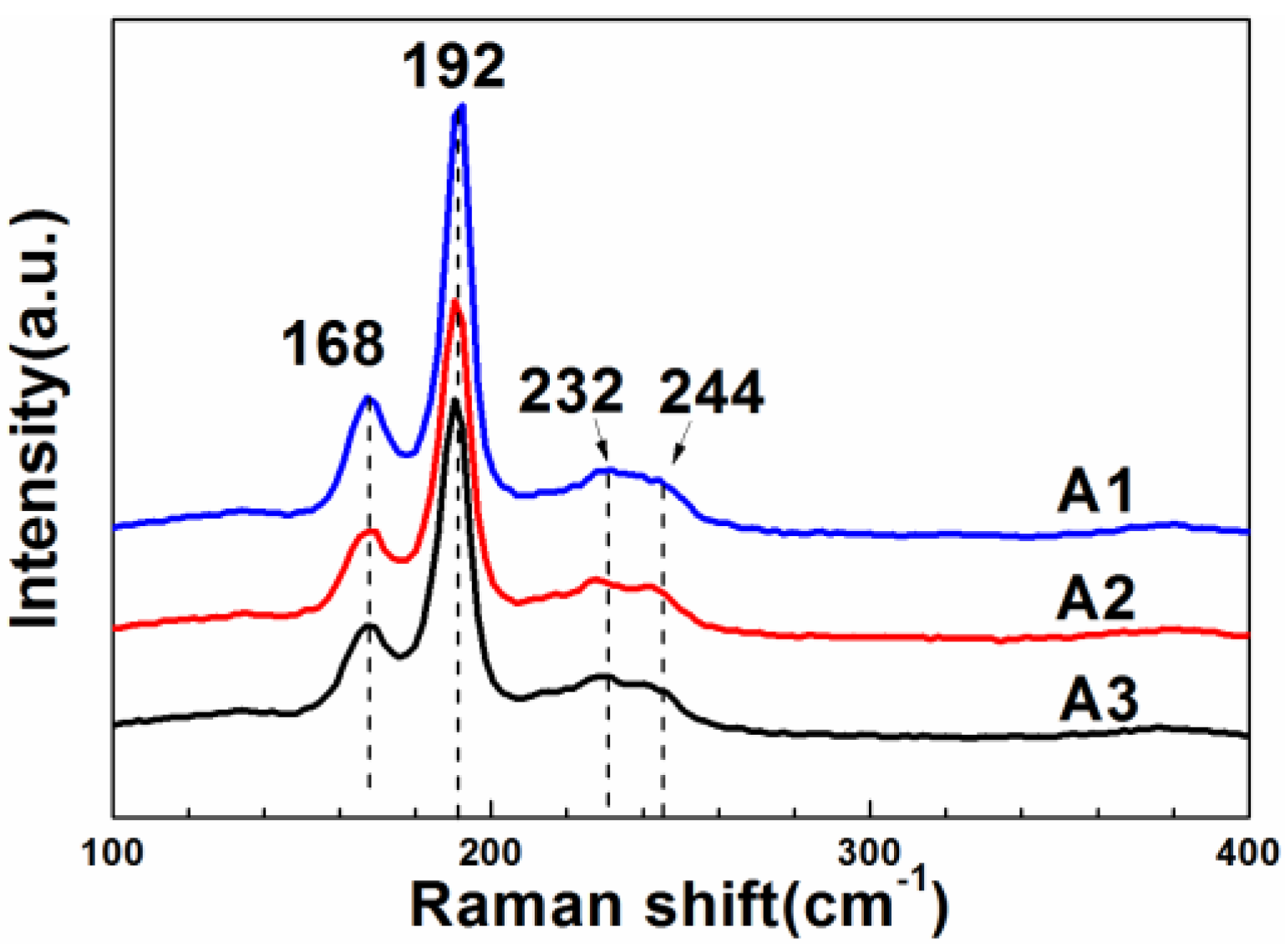
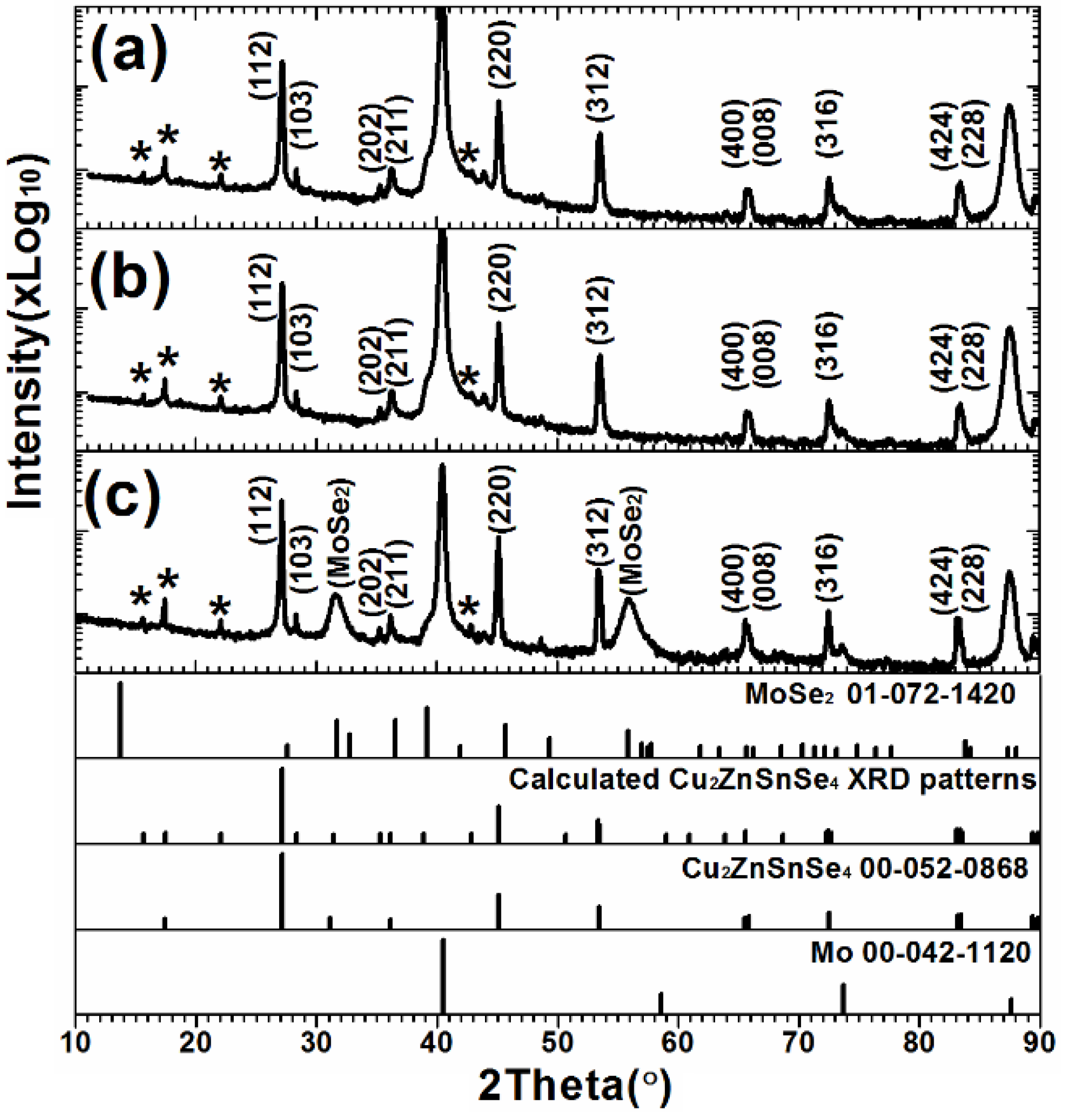
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ki, W.; Hillhouse, H.W. Earth-abundant element photovoltaics directly from soluble precursors with high yield using a non-toxic solvent. Adv. Energy Mater. 2011, 1, 732–735. [Google Scholar] [CrossRef]
- Polizzotti, A.; Repins, I.L.; Noufi, R.; Wei, S.-H.; Mitzi, D.B. The state and future prospects of kesterite photovoltaics. Energy Environ. Sci. 2013, 6, 3171–3182. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Gunawan, O.; Todorov, T.K.; Wang, K.; Guha, S. The path towards a high-performance solution-processed kesterite solar cell. Sol. Energy Mater. Sol. Cells 2011, 95, 1421–1436. [Google Scholar] [CrossRef]
- Lee, Y.S.; Gershon, T.; Gunawan, O.; Todorov, T.K.; Gokmen, T.; Virgus, Y.; Guha, S. Cu2ZnSnSe4 thin-film solar cells by thermal co-evaporation with 11.6% efficiency and improved minority carrier diffusion length. Adv. Energy Mater. 2014, 5. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Haass, S.G.; Diethelm, M.; Werner, M.; Bissig, B.; Romanyuk, Y.E.; Tiwari, A.N. 11.2% efficient solution processed kesterite solar cell with a low voltage deficit. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Collord, A.D.; Hillhouse, H.W. The effect of nanocrystal reaction time on Cu2ZnSn(S,Se)4 solar cells from nanocrystal inks. Sol. Energy Mater. Sol. Cells 2015, 141, 383–390. [Google Scholar] [CrossRef]
- Todorov, T.K.; Tang, J.; Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Mitzi, D.B. Beyond 11% efficiency: Characteristics of state-of-the-art Cu2ZnSn(S,Se)4 solar cells. Adv. Energy Mater. 2013, 3, 34–38. [Google Scholar] [CrossRef]
- Winkler, M.T.; Wang, W.; Gunawan, O.; Hovel, H.J.; Todorov, T.K.; Mitzi, D.B. Optical designs that improve the efficiency of Cu2ZnSn(S,Se)4 solar cells. Energy Environ. Sci. 2013, 7, 1029–1036. [Google Scholar] [CrossRef]
- Shin, B.; Gunawan, O.; Zhu, Y.; Bojarczuk, N.A.; Chey, S.J.; Guha, S. Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovol. Res. Appl. 2013, 21, 72–76. [Google Scholar] [CrossRef]
- Vauche, L.; Risch, L.; Sánchez, Y.; Dimitrievska, M.; Pasquinelli, M.; Goislard de Monsabert, T.; Grand, P.-P.; Jaime-Ferrer, S.; Saucedo, E. 8.2% pure selenide kesterite thin-film solar cells from large-area electrodeposited precursors. Prog. Photovol. Res. Appl. 2016, 24, 38–51. [Google Scholar] [CrossRef]
- Ahmed, S.; Reuter, K.B.; Gunawan, O.; Guo, L.; Romankiw, L.T.; Deligianni, H. A high efficiency electrodeposited Cu2ZnSnS4 solar cell. Adv. Energy Mater. 2012, 2, 253–259. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, Y.; Gunawan, O.; Gokmen, T.; Deline, V.R.; Ahmed, S.; Romankiw, L.T.; Deligianni, H. Electrodeposited Cu2ZnSnSe4 thin film solar cell with 7% power conversion efficiency. Prog. Photovol. Res. Appl. 2014, 22, 58–68. [Google Scholar] [CrossRef]
- Jiang, F.; Ikeda, S.; Harada, T.; Matsumura, M. Pure sulfide Cu2ZnSnS4 thin film solar cells fabricated by preheating an electrodeposited metallic stack. Adv. Energy Mater. 2013, 4. [Google Scholar] [CrossRef]
- Shin, B.; Bojarczuk, N.A.; Guha, S. On the kinetics of MoSe2 interfacial layer formation in chalcogen-based thin film solar cells with a molybdenum back contact. Appl. Phys. Lett. 2013, 102, 091907. [Google Scholar] [CrossRef]
- Weber, A.; Mainz, R.; Schock, H.W. On the Sn loss from thin films of the material system Cu-Zn-Sn-S in high vacuum. J. Appl. Phys. 2010, 107, 013516. [Google Scholar] [CrossRef] [Green Version]
- Scragg, J.J.; Ericson, T.; Kubart, T.; Edoff, M.; Platzer-Björkman, C. Chemical insights into the instability of Cu2ZnSnS4 films during annealing. Chem. Mater. 2011, 23, 4625–4633. [Google Scholar] [CrossRef]
- Redinger, A.; Berg, D.M.; Dale, P.J.; Siebentritt, S. The consequences of kesterite equilibria for efficient solar cells. J. Am. Chem. Soc. 2011, 133, 3320–3323. [Google Scholar] [CrossRef] [PubMed]
- Ennaoui, A.; Lux-Steiner, M.; Weber, A.; Abou-Ras, D.; Kotschau, I.; Schock, H.W.; Schurr, R.; Holzing, A.; Jost, S.; Hock, R.; et al. Cu2ZnSnS4 thin film solar cells from electroplated precursors: Novel low-cost perspective. Thin. Solid Films 2009, 517, 2511–2514. [Google Scholar] [CrossRef]
- Scragg, J.J.; Watjen, J.T.; Edoff, M.; Ericson, T.; Kubart, T.; Platzer-Bjorkman, C. A detrimental reaction at the molybdenum back contact in Cu2ZnSn(S,Se)4 thin-film solar cells. J. Am. Chem. Soc. 2012, 134, 19330–19333. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Walsh, A.; Gong, X.G.; Wei, S.H. Classification of lattice defects in the kesterite Cu2ZnSnS4 and Cu2ZnSnSe4 earth-abundant solar cell absorbers. Adv. Mater. 2013, 25, 1522–1539. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; He, Q.; Zhou, Z.; Sun, G.; Sun, Y.; Chang, L.-B. CZTSe solar cells prepared by electrodeposition of Cu/Sn/Zn stack layer followed by selenization at low Se pressure. Nanoscale Res. Lett. 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Fontané, X.; Izquierdo-Roca, V.; Espíndola-Rodríguez, M.; López-Marino, S.; Placidi, M.; Calvo-Barrio, L.; Pérez-Rodríguez, A.; Saucedo, E. On the formation mechanisms of Zn-rich Cu2ZnSnS4 films prepared by sulfurization of metallic stacks. Sol. Energy Mater. Sol. Cells 2013, 112, 97–105. [Google Scholar] [CrossRef]
- Beck, M.E.; Swartzlander-Guest, A.; Matson, R.; Keane, J.; Noufi, R. CuIn(Ga)Se2-based devices via a novel absorber formation process. Sol. Energy Mater. Sol. Cells 2000, 64, 135–165. [Google Scholar] [CrossRef]
- Yaws, C.L. Handbook of Vapor Pressure: Volume 4: Inorganic Compounds and Elements; Gulf Professional Publishing: Houston, TX, USA, 1995; Volume 4. [Google Scholar]
- Scragg, J.J.; Dale, P.J.; Colombara, D.; Peter, L.M. Thermodynamic aspects of the synthesis of thin-film materials for solar cells. ChemPhysChem 2012, 13, 3035–3046. [Google Scholar] [CrossRef] [PubMed]
- Dudchak, I.V.; Piskach, L.V. Phase equilibria in the Cu2SnSe3-SnSe2-ZnSe system. J. Alloy. Compd. 2003, 351, 145–150. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. The Cu-Se (copper-selenium) system. Bull. Alloy. Phase Diagrams 1981, 2, 305–315. [Google Scholar] [CrossRef]
- Fairbrother, A.; Fontané, X.; Izquierdo-Roca, V.; Placidi, M.; Sylla, D.; Espindola-Rodriguez, M.; López-Mariño, S.; Pulgarín, F.A.; Vigil-Galán, O.; Pérez-Rodríguez, A.; et al. Secondary phase formation in Zn-rich Cu2ZnSnSe4-based solar cells annealed in low pressure and temperature conditions. Prog. Photovol. Res. Appl. 2014, 22, 479–487. [Google Scholar] [CrossRef]
- Hsu, W.-C.; Repins, I.; Beall, C.; DeHart, C.; Teeter, G.; To, B.; Yang, Y.; Noufi, R. The effect of Zn excess on kesterite solar cells. Sol. Energy Mater. Sol. Cells 2013, 113, 160–164. [Google Scholar] [CrossRef]
- Wang, K.; Gunawan, O.; Todorov, T.; Shin, B.; Chey, S.J.; Bojarczuk, N.A.; Mitzi, D.; Guha, S. Thermally evaporated Cu2ZnSnS4 solar cells. Appl. Phys. Lett. 2010, 97, 143508. [Google Scholar] [CrossRef]
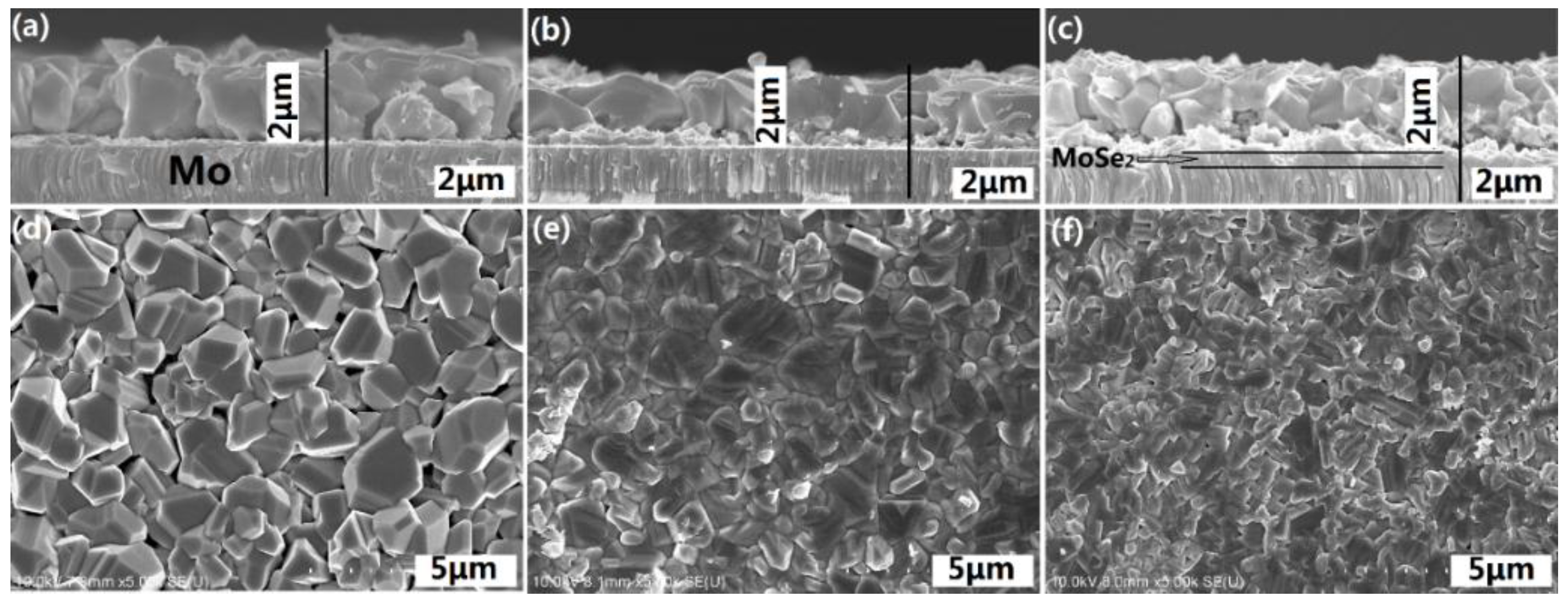
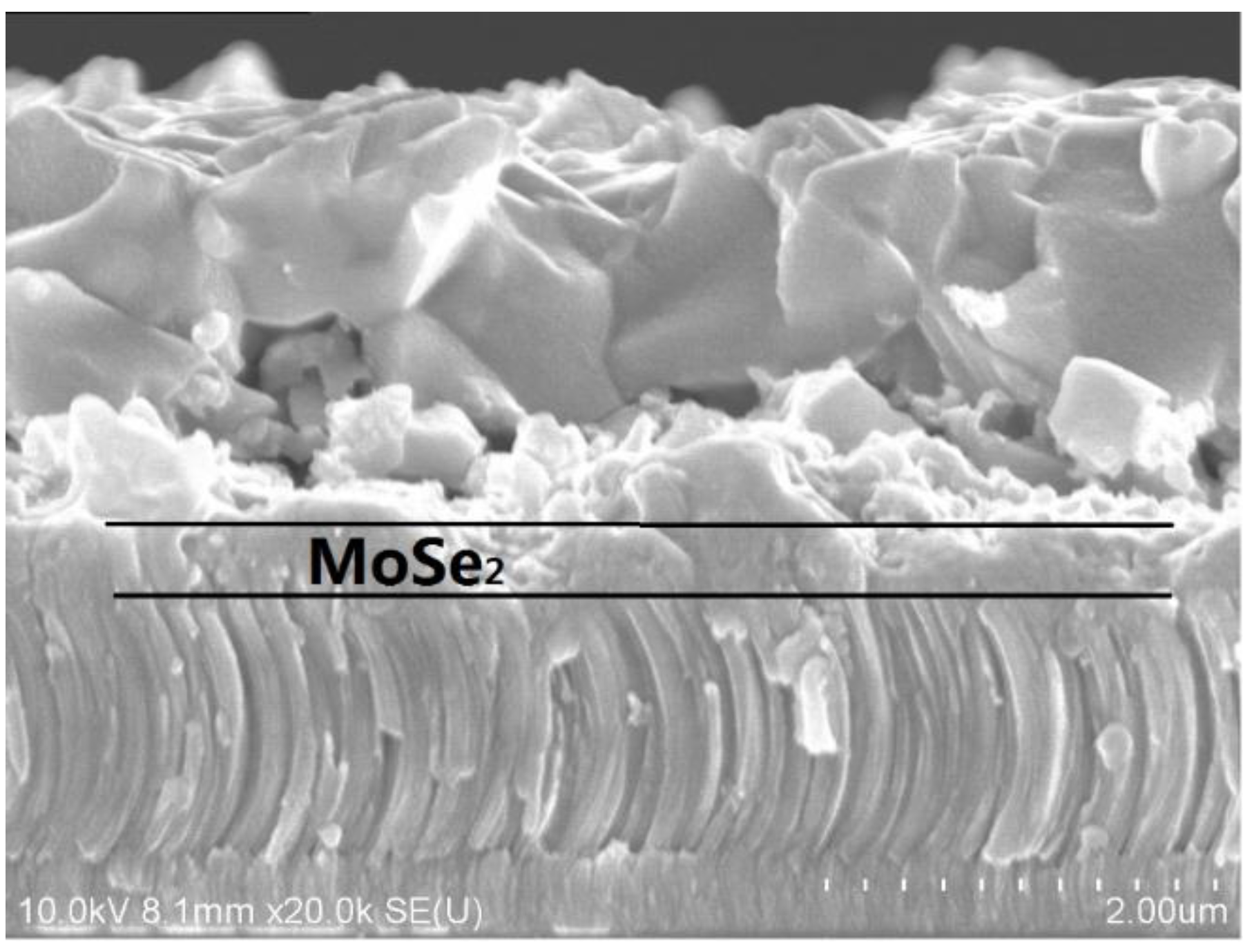
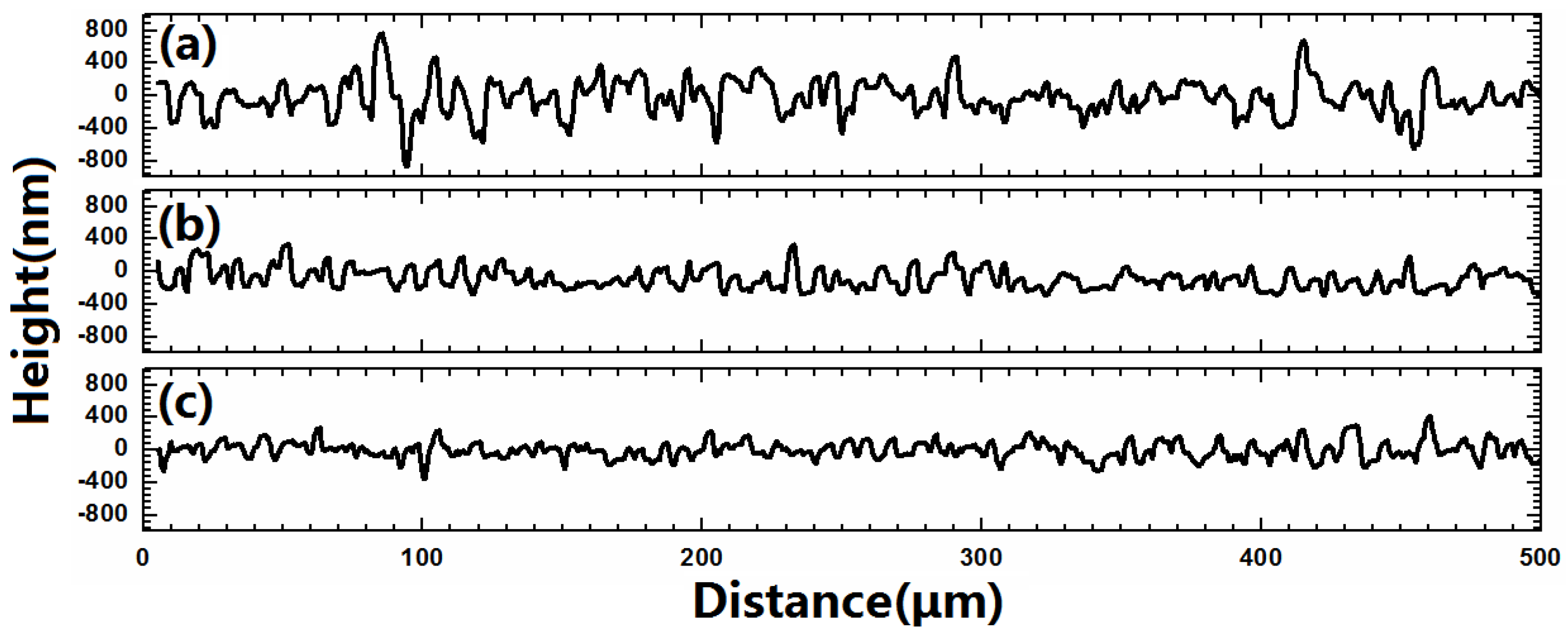
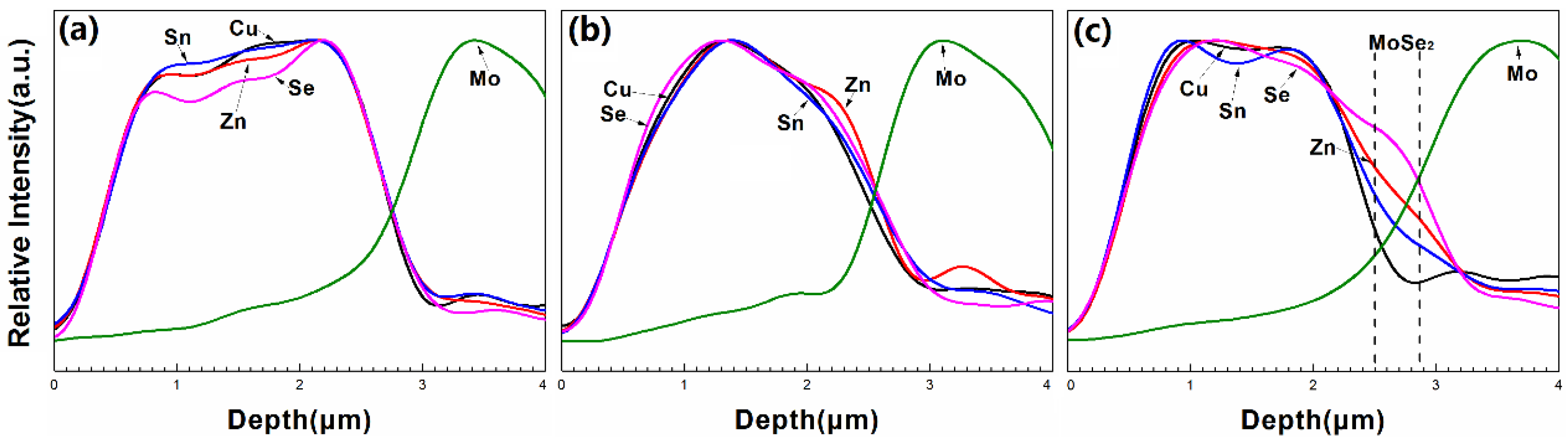


| Sample | Cu (%) | Zn (%) | Sn (%) | Zn/Cu | Sn/Cu | Cu/(Zn+Sn) | Zn/Sn |
|---|---|---|---|---|---|---|---|
| A1 | 51.28 | 35.9 | 12.82 | 0.70 | 0.25 | 1.05 | 2.80 |
| A2 | 45.45 | 31.81 | 22.74 | 0.70 | 0.50 | 0.83 | 1.40 |
| A3 | 40.82 | 28.57 | 30.61 | 0.70 | 0.75 | 0.69 | 0.93 |
| Sample | Cu (%) | Zn (%) | Sn (%) | Se(%) | Zn/Cu | Sn/Cu | Cu/(Zn+Sn) | Zn/Sn |
|---|---|---|---|---|---|---|---|---|
| A1 | 21.38 | 14.75 | 13.24 | 50.63 | 0.69 | 0.62 | 0.76 | 1.11 |
| A2 | 21.02 | 14.51 | 13.19 | 51.28 | 0.69 | 0.63 | 0.76 | 1.10 |
| A3 | 19.86 | 13.74 | 12.98 | 53.42 | 0.69 | 0.65 | 0.74 | 1.06 |
| Sample | Eff (%) | Voc (mV) | Jsc (mA·cm−2) | FF | Rs (Ω) | Rsh (Ω·cm2) |
|---|---|---|---|---|---|---|
| A1 | 4.30 | 316 | 30.59 | 0.445 | 3.1 | 49 |
| A2 | 7.94 | 394 | 34.51 | 0.584 | 2.6 | 265 |
| A3 | 7.10 | 376 | 33.92 | 0.557 | 2.72 | 112 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; Sun, G.; He, Q.; Zhou, Z.; Sun, Y.; Chang, L.-B. Effect of Sn Content in a CuSnZn Metal Precursor on Formation of MoSe2 Film during Selenization in Se+SnSe Vapor. Materials 2016, 9, 241. https://doi.org/10.3390/ma9040241
Yao L, Ao J, Jeng M-J, Bi J, Gao S, Sun G, He Q, Zhou Z, Sun Y, Chang L-B. Effect of Sn Content in a CuSnZn Metal Precursor on Formation of MoSe2 Film during Selenization in Se+SnSe Vapor. Materials. 2016; 9(4):241. https://doi.org/10.3390/ma9040241
Chicago/Turabian StyleYao, Liyong, Jianping Ao, Ming-Jer Jeng, Jinlian Bi, Shoushuai Gao, Guozhong Sun, Qing He, Zhiqiang Zhou, Yun Sun, and Liann-Be Chang. 2016. "Effect of Sn Content in a CuSnZn Metal Precursor on Formation of MoSe2 Film during Selenization in Se+SnSe Vapor" Materials 9, no. 4: 241. https://doi.org/10.3390/ma9040241






