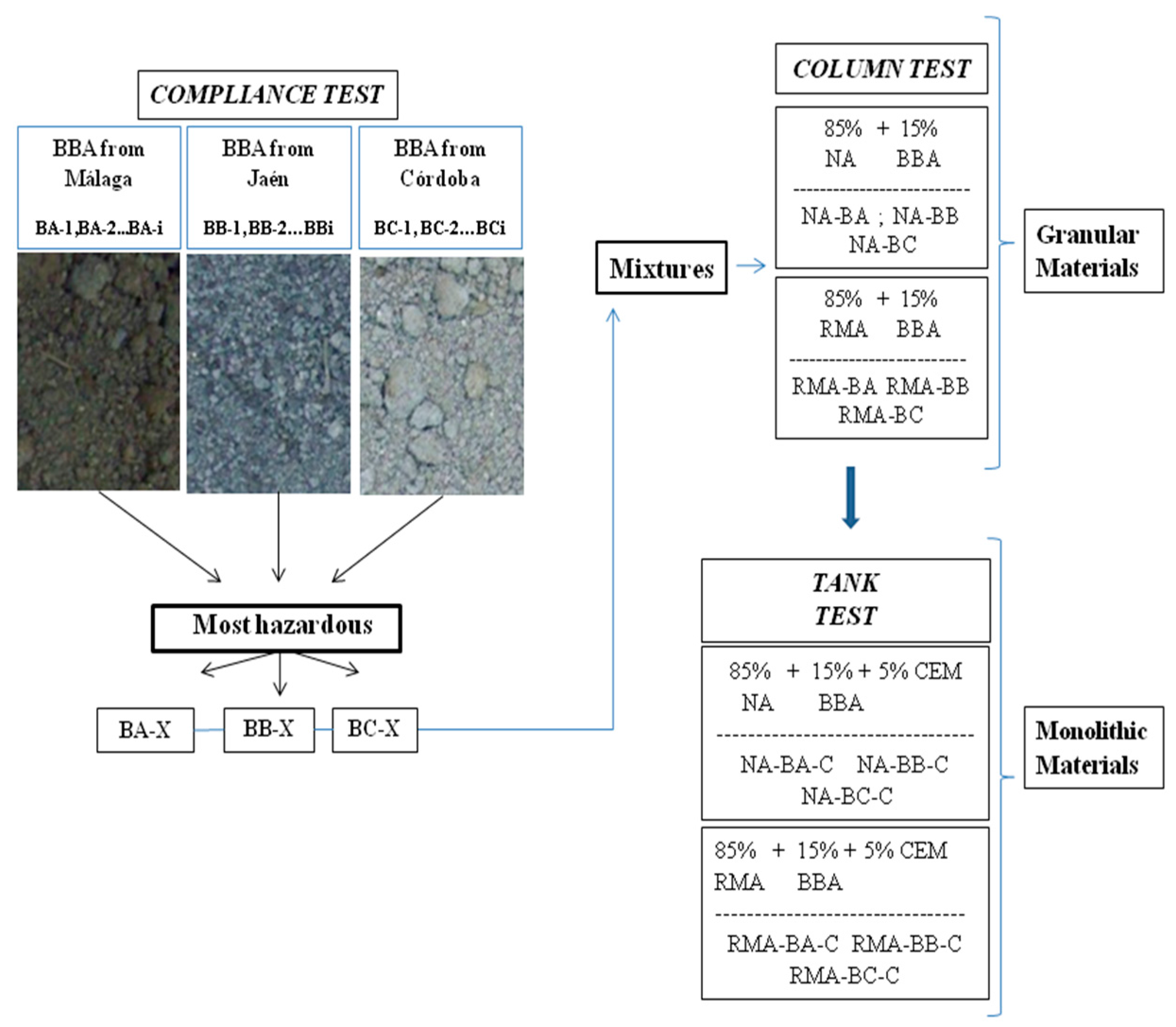
4.1. Classification of Materials as a Function of Their Pollutant Potential
Leachate concentrations according to the compliance test are shown in
Table 7,
Table 8,
Table 9 and
Table 10. Thirty-two materials (30 BBAs, a natural aggregate (NA) and a recycled aggregate (RMA)) have been classified according to the limit values regulated by the Landfill Directive 2003/33/EC. Green represents inert materials, inert value limits that are exceeded are given in bold and yellow, while non-hazardous limits that are exceeded are underlined and in red.
As and Hg are identified as the most conflictive elements. The concentration of As present was at hazardous levels in 43.3% of the 30 samples, and at non-hazardous and inert levels in the other 56.7%. Hg was detected at hazardous concentration levels in 20% of the samples and at non-hazardous and inert levels in the other 80%.
Other relevant elements are Cr, Ni, Cu, Se and Mo (exceeding the inert limit values in most cases).
Based on the results of the compliance test, due to the high potential contaminants of BBAs, they are unable to be applied in civil engineering as isolated materials. For that reason, the motivation of the present study is to analyze how to reduce the pollutant potential of those products to apply them as secondary materials in construction works.
That is why this study proceeded to analyze mixtures of BBA with other materials to evaluate if reducing the volume of BBA in the sample reduced the contaminant load.
Regarding the dosage of the mixtures, prior studies were revised. Thus, previous works [
35] have proven that mixtures composed of 10%–15% BBA with other aggregates present an appropriate physical and mechanical behavior for use as materials in embankments or road layers [
13].
To establish the comparison of results between a recycled material and natural aggregate (which should provide better environmental conditions), two groups of samples were prepared in the laboratory: 85% NA with 15% BBA, and 85% RMA with 15% BBA.
Due to the intended application of the materials in civil engineering works (located outdoors and subjected to environmental phenomena), it is necessary that the laboratory study for leaching characterization simulates closely the effect of rain episodes percolating through the granular material and takes place in engineering applications in which this type of material has proven to be suitable and feasible [
44,
45,
46].
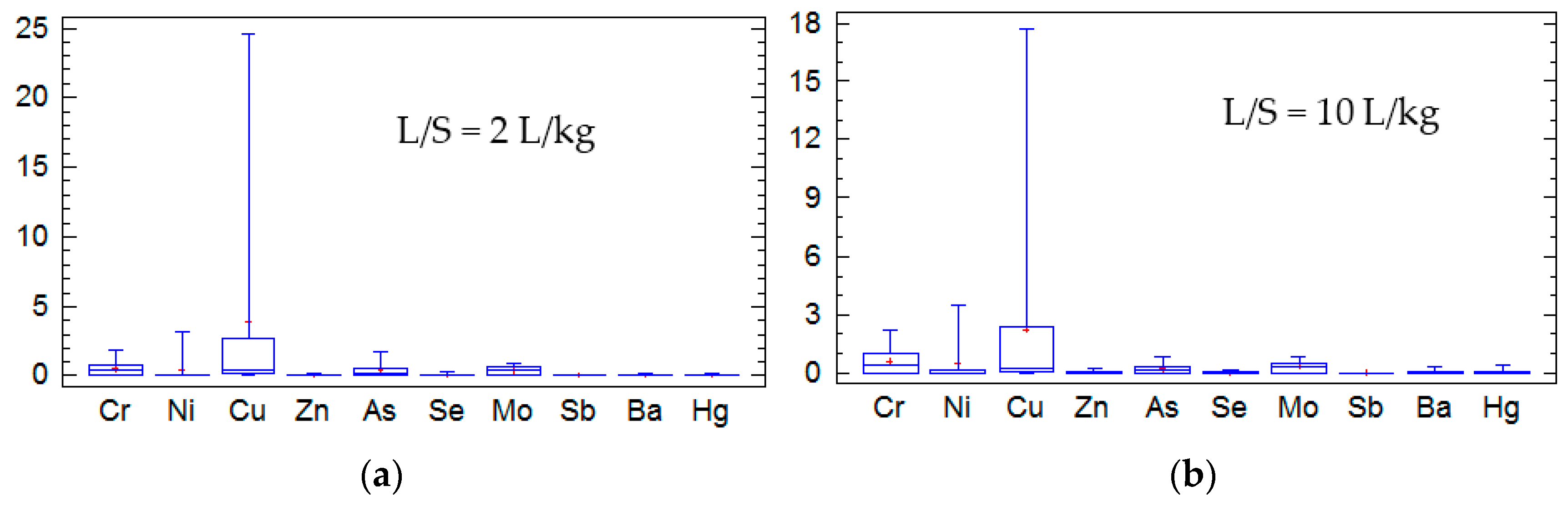
Figure 2 shows the results of a statistical analysis performed on data from UNE EN 12457-3 [
19] procedure for all of the tested BBA. The analysis is focused on the heavy metals regulated by the UE Landfill Directive. However, Cd and Pb were not included in the analysis because their detected amounts were negligible. The results of the test are shown by means of whisker plots. The first quartile indicates the lowest 25% of the data set, the median separates the lower and upper 50% of the data set, and the lowest 75% represent the fourth quartile. The data from the statistical analysis are summarized in
Table 11.
Table 11 shows standard deviations for the most of the studied metals. Only higher standard deviations were observed in the Cu values for L/S = 2 L/kg and L/S = 10 L/kg.
4.2. Data from the Percolation Test
The analysis performed according to the Dutch percolation test, which is itself based on the standard NEN 7343:1994 [
36]., was focused on the heavy metals that have been identified as more conflictive according to the obtained results of the prior section: compliance test data (Cr, Ni, Cu, Se, Mo, As and Hg).
In addition, one representative sample was taken from each of the three combustion power plants to perform a representative study of the different BBAs produced in the region of Andalusia. Therefore, from each plant, the BBA with the highest contaminant load according to the data provided in
Table 7,
Table 8 and
Table 9 was chosen in each case: BA-10, BB-3, and BC-7.
To compare the reduction of the contaminant load between the samples composed of 100% BBA (BA-10, BB-3 and BC-7) and the mixtures with 15% BBA,
Figure 3,
Figure 4 and
Figure 5 show the cumulative release curves obtained by the percolation test, including the curves of the mixtures with aggregates (NA-BA, RMA-BA, NA-BB, RMA-BB, NA-BC and RMA-BC).
All graphics include the inert legal limit value for the percolation leaching test data imposed by the Landfill Directive at the L/S of 0.1 L/kg (IL-LS0.1, grey rhombus) and the non-hazardous limit (NHL-LS0.1, grey short line). Thus, the samples that have exceeded this limit are marked, being the material (BBA or mixtures) classified as non-hazardous. According to the results, the release of As has exceeded the inert and non-hazardous limits in all samples, with the BBA and mixtures classified as non-hazardous materials according to the percolation data.
Regarding the elements Cr, Ni, Cu, Se and Mo, which were detected as more contaminant-filled by the compliance test in the BBA samples based on the column tests performed for the mixtures of BBA with aggregates, their pollutant potential is even higher and, in most of cases, the inert limit is obviously exceeded; the highest release was obtained for the three BBAs, BA-10, BB-3 and BC-7. The patterns described during the percolation test and, as a consequence, the cumulative percolation curves, were quite similar in both BBA and the mixtures. However, as expected, the cumulative releases of BA-10, BB-3 and BC-7 were the highest of the total data represented. Obviously, and according to the results illustrated in
Table 10, the mixtures of BBA with NA presented the lowest release levels, while the RMA mixtures showed higher pollutant levels.
The most noteworthy difference between both types of mixtures is observed in the release pattern of Cr: in all cases, the percolation curves of Cr in RMA mixtures were markedly higher than NA mixtures and BBA curves. That difference is caused by the high content of Cr in the ceramic particles present in recycled aggregates from construction and demolition waste (CDW) [
47].
After the discussion of the percolation data, the reduction of the pollution load of hazardous BBAs (BA-10, BB-3 and BC-7) following their combination with aggregates can be confirmed. This reduction implies the possibility of reuse as secondary materials in construction works rather than discarding them and depositing them in landfills (which favors a negative environmental impact and does not contribute to increasing the value of industrial by-products).
Accordingly, the environmental assessment has confirmed the feasibility of the combination of both materials for its use in civil works.
To expand the scope of the study within the framework of civil engineering, the following section analyses the pollution potential of BBA mixtures treated with cement. Previous studies have proven their viability [
44]. For that, the Dutch diffusion test was performed according to the standard EA NEN-7375:2004 [
37] for the monolithic samples prepared in the laboratory (six BBA mixtures of RMA and NA with 5% cement).
In order to evaluate the immobilization degree reached with the experimental procedure developed,
Table 12 shows the percentage of reduction of the release levels measured in the BBA samples (BA10, BB3 and BC7, representative of all the studied combustion plants) compared with the release levels obtained for the mixtures with the natural material NA and the recycled aggregate RMA. For establishing the comparison and calculation of the ratio, the last data (cumulative release at L/S of 10 L/kg) of the column test was considered.
The immobilization ratio is shown in
Table 12, calculated according to Equation (2):
where Bi: Release level of each element for biomass bottom ashes: BA10 for first plant; BB3 for second plant or BC7 for third plant; AGi: Release level of each element for mixtures of 85% of natural aggregate or recycled mixed aggregates + 15% of BBA: NA-BA; RMA-BA; NA-BB; RMA-BB; NA-BC; RMA-BC.
According to data, in general a reduction of the pollutant levels is observed for all mixtures and all elements. The highest immobilization is observed in the mixture of BA10 with the natural aggregate NA (BA10 & NA-BA). The lowest immobilization ratio was observed for the mixtures of NA and RMA with the BB3 (BB3 & NA-BB and BB3 & RMA-BB).
4.3. Data from the Diffusion Test
After verifying the reduction of the pollution load of the BBA after being mixed with RMA and NA, this investigation advanced a step further by analyzing the behavior of the stabilized/solidified mixtures. The tank test or diffusion process through the Dutch EA NEN 7375:2004 [
37] was performed for the monolithic cylindrical samples (Ø 10 cm × 12.5 cm) prepared in the laboratory with 5% cement.
This percentage was chosen because it is the most common for civil engineering materials used as road base and sub-base dosage [
48,
49]. The objective is to prove the reduction of the pollution load of granular materials after their treatment with cement. Thus, the environmental benefit will be shown in addition to checking that these materials can be applied as construction materials (which was previously demonstrated by other researchers).
Figure 6 shows the diffusion curves of the most conflictive elements detected in 6 monolithic samples cemented with CEM II/BL 32.5.
Figure 6 illustrates the diffusion release curves of the elements As, Cr, Se and Ni. Additionally, a slope of 1:0.5 (the grey line) is represented graphically to facilitate the identification of the mechanisms that govern the release. Previous researchers have proven that pure diffusion-controlled release implies a 1:0.5 slope [
50]. Additionally, other patterns can be observed in the graphic, such as the depletion of elements that describe a flat line during different periods of time.
The patterns of diffusion have been described according to
Figure 6. The element present at the highest levels in the tested BBA was As. It presented mixed behavior: in the first stages, until the fourth day, the release was very stable and there were hardly any significant differences with time. However, from the fourth day on, the diffusion curve of As was parallel to the slope 1:0.5 and, therefore, the governing mechanism was the diffusion of the element.
Cr presents a similar pattern to that of As, but Cr showed depletion in the last days of the test. It must be noted that for the monolithic samples (after the treatment with cement of the RMA and NA mixtures), no differences were observed between the release diffusion curves of both mixtures. This could imply/prove that the treatment with cement is causing the isolation of the material matrix, and that the expected higher Cr release in the mixture due to the presence of ceramic particles in RMA is not produced in the monolithic samples. This phenomenon occurred in the granular samples RMA-BA, RMA-BB, RMA-BC due to the absence of isolation of their internal matrix.
From the fourth day on, Se exhibited a curve parallel to the 1:0.5 slope; this behavior was more evident in the RMA mixtures. Finally, Ni presented a flat phase for most of the test, which demonstrated its low solubility. Only from day 19 was its release curve parallel to the diffusion slope.
4.4. Comparison between the Percolation and Diffusion Tests
The present section is focused on evaluating the reduction of the contaminant load of the more contaminated BBAs: BA-10, BB-3 and BC-7.
The data treatment was performed for
Section 4.3 and
Section 4.4 as follows. The standard EA NEN 7375:2004 [
37] contains, in Section “8. Calculation”, the formula for the calculation of the measured leaching per fraction:
where
Ei* is the measured leaching of a component in fraction
i in mg/m
2.
Ci is the concentration of the component in fraction
i in µg/L.
V is the volume of the eluate in L.
A is the surface area of the test piece in m
2.
f is a conversion factor: 1000 µg/mg.
The measured cumulative leach
in each period (
n = 1 to
N) was calculated by:
where
is the measured cumulative leaching of a component for period
n consisting of fractions
i = 1 to
n in mg/m
2.
Ei* is the measured leaching of a component in fraction
i in mg/m
2.
N is the number of periods equal to the amount of specified replenishment time (
N = 8).
According to Equation (3), the cumulative curves were obtained and represented in
Figure 5. To compare the data of the percolation test with the diffusion curves, Equation (4) was applied. Thus, concentrations expressed in µg/L were transformed into mg/m
2, and the data are presented in
Figure 6.
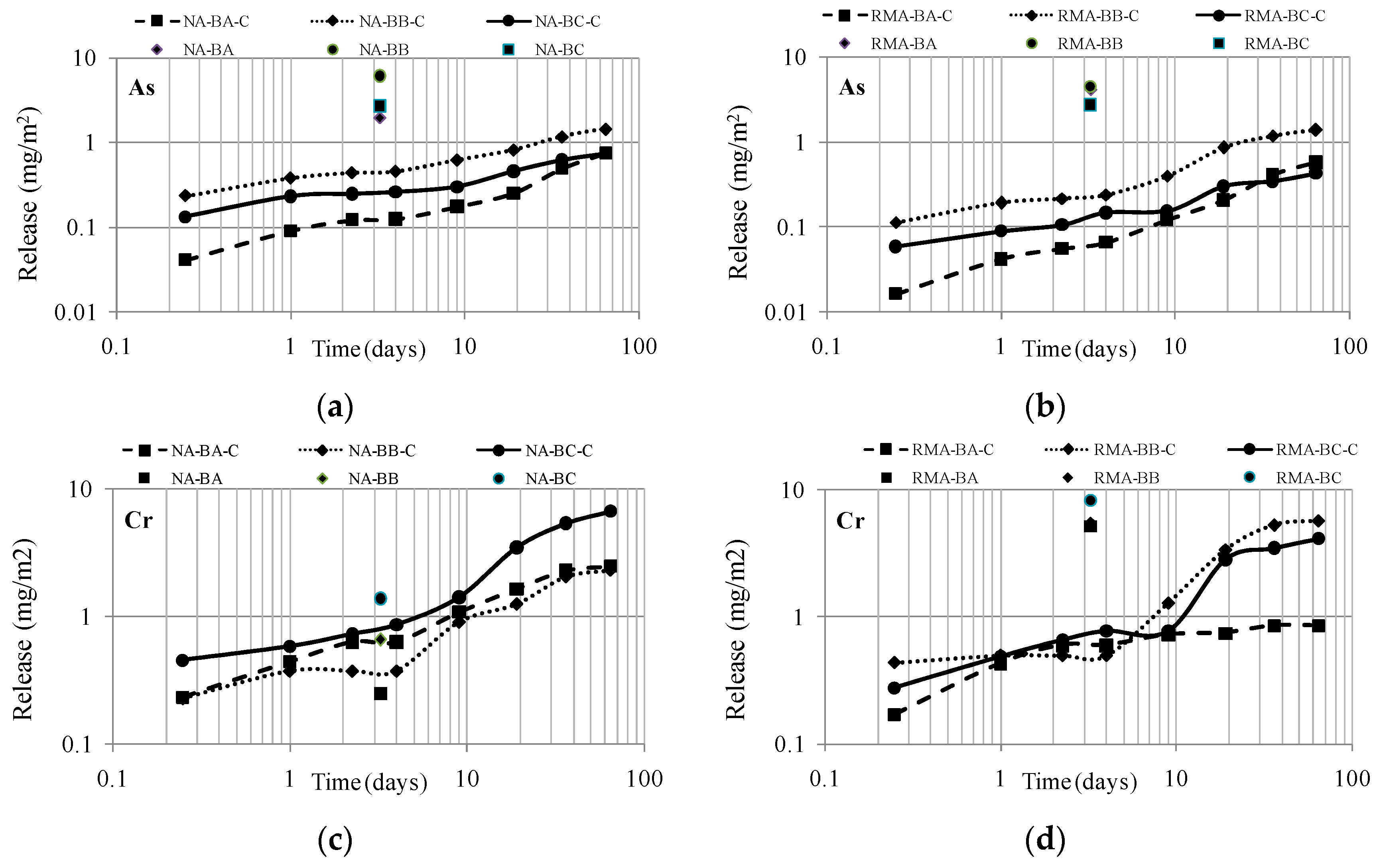
To compare the results of both tests, the leachate value of the percolation test at an L/S of 2 L/kg is assumed. This value was transformed into the unit time to superimpose the data on the diffusion curves (
Figure 7).
The L/S ratio depends directly on the volume (expressed in cm
3) and the dry mass (d.m.) of the sample (kg d.m.) because the process described by the standard EA NEN 7375:2004 [
37] requires a constant water flow of 0.3 mL/min. Starting from these variables, it was decided to adopt the data at L/S = 2 L/kg (being the commonly used test leaching compliance) to superimpose the diffusion and percolation data. According to the percolation test procedure, this L/S ratio is reached after 77.78 h, which is 3.25 days, as observed in the x-axis of the graphs of
Figure 7.
As a function of the results obtained, only the two more conflicting elements, from the environmental point of view, As and Cr, have been chosen to be studied in depth.
Regarding the release of As in leachates, identical diffusion curves are obtained in both mixtures. Thus, no differences are observed between the mixtures with the natural aggregate, NA, and the recycled one, RMA. Comparison highlights the significant decrease in As released by the monoliths (NA-BA-C, NA-BB-C and NA-BC-C) compared to the high value of metal released from the samples in their granular form (NA-BA, NA-BB and NA-BC). The data are consistent with previous research studies [
51].
Regarding leaching behavior of Cr, a different response was observed depending on the type of aggregate used for the mixture elaboration. The fundamental difference lies in the composition of the leachate of NA and RMA (see
Table 10). While the natural aggregate released only 0.001 mg/kg and 0.012 mg/kg (at an L/S of 2 and 10, respectively), the recycled construction aggregate released 0.265 mg/kg and 0.374 mg/kg, respectively.
Therefore, when preparing mixtures to be tested by the percolation leaching method, the contaminant load is conditioned by the aggregate used. Thus, when the data of the tank and column are overlapped in
Figure 7, it is observed that, in mixtures of BBA and RMA, the reduction of pollution potential after the S/S treatment with cement was remarkable compared to the poor reduction observed in the mixture of BBA and NA after the S/S treatment with cement. The results are logical and coherent with expectations, as a comparison confirms that no contamination reduction occurs in materials that presented low Cr levels in the source material (NA, see
Table 10).
The effectiveness of the S/S treatment with cement for materials with high pollutant loads (BBA samples BA-10, BB-3 and BC-7, classified as hazardous materials) can be based on two observations: (1) The release of mixtures of the granular materials RMA-BA, RMA-BB and RMA-BC were much higher than those obtained by the monolithic RMA-BA-C, RMA-BB-C and RMA-BC-C; (2) The diffusion curves of the mixtures of BBA manufactured with aggregates with different pollutant loads presented similar diffusion curves and similar release levels after the S/S treatment.