Polystyrene-co-Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography
Abstract
:1. Introduction
2. Results and Discussion
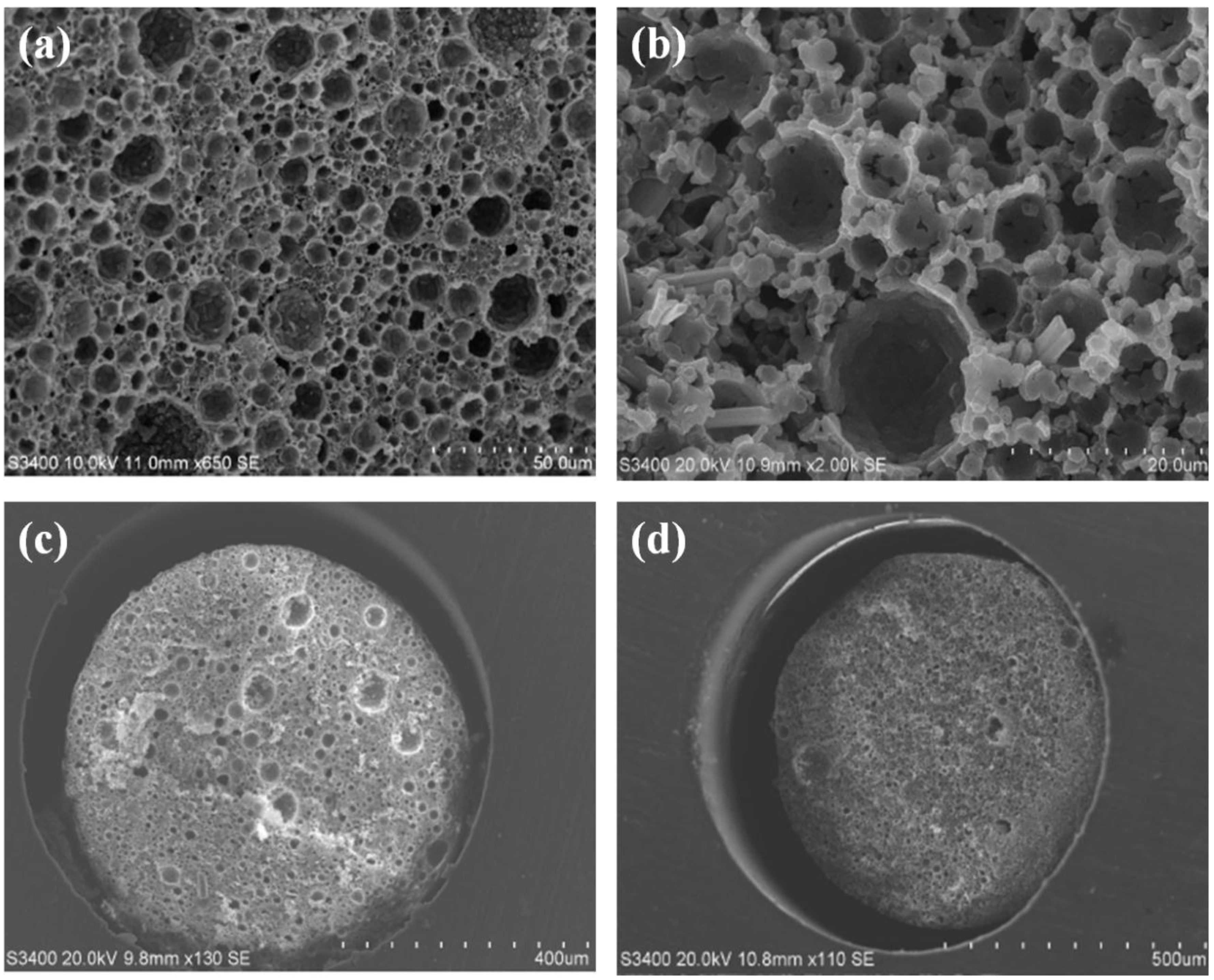
2.1. Polymerisation of Glycidyl Methacrylate PolyHIPES in Poly Etherether Ketone (PEEK) Tubing
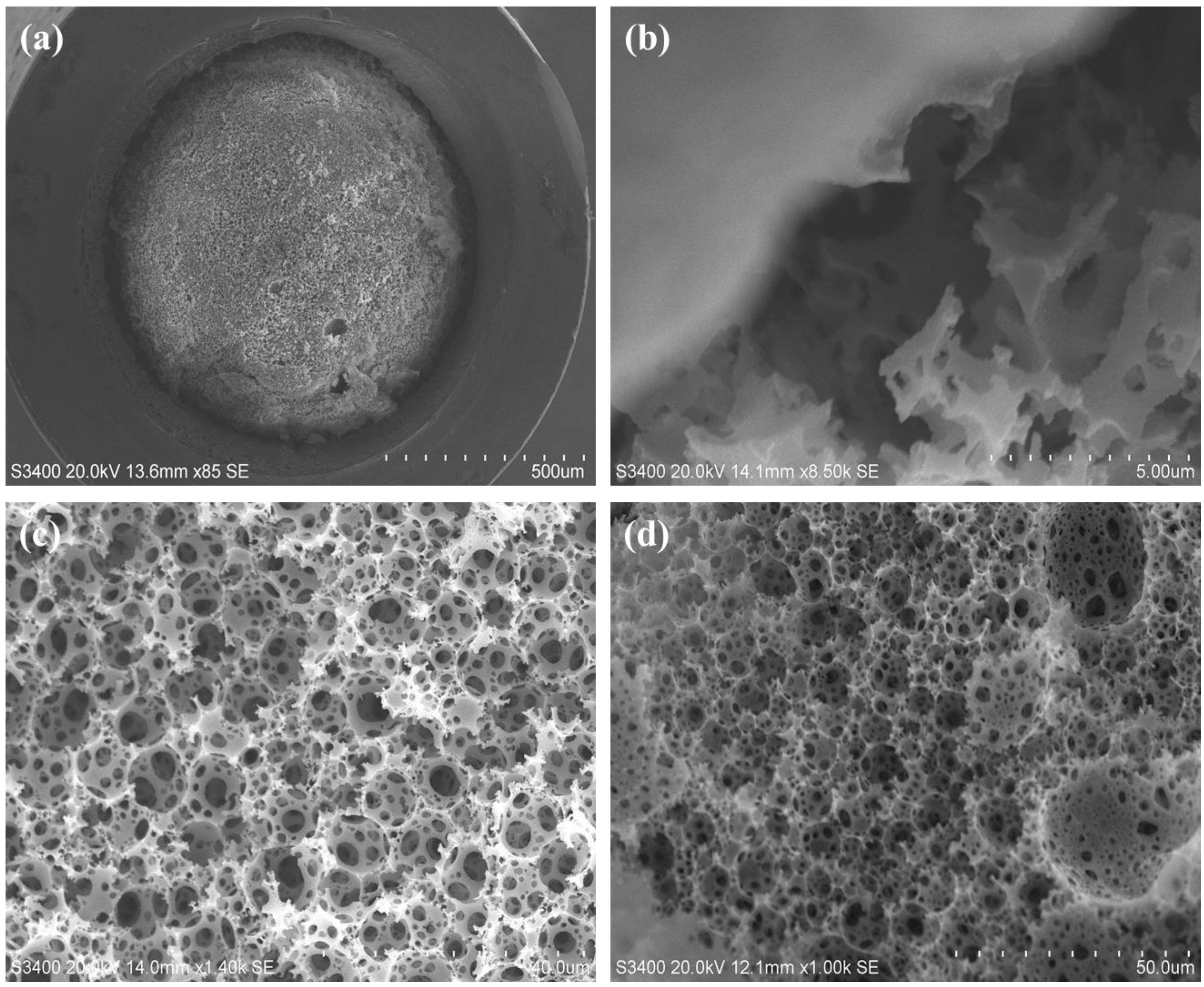
2.2. Polymerisation of Polystyrene-co-Divinylbenzene PolyHIPEs in Silcosteel Tubing
2.3. Backpressure and Permeability Studies for PS-co-DVB polyHIPEs
2.4. Chromatographic Characterisation of PS-co-DVB PolyHIPEs
3. Experimental
3.1. Instrumentation
3.2. Materials
3.2.1. Modification of PEEK with Methacryloyl Groups
3.2.2. Silanisation of Silcosteel Tubing
3.2.3. Fabrication of PS-co-DVB and GMA-co-EDMA PolyHIPEs
3.2.4. Physical Characterization of PS-co-DVB polyHIPEs in Batch and Column Formats
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vazquez, M.; Paull, B. Review on recent and advanced applications of monoliths and related porous polymer gels in micro-fluidic devices. Chim. Acta. 2010, 668, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Guiochon, G. Monolithic columns in high-performance liquid chromatograph. J. Chromatogr. A 2007, 1168, 101–168. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. There’s more than one way to skin a cat: A wide selection of techniques used for the preparation of porous polymer monoliths. J. Chromatogr. A 2010, 1217, 902–924. [Google Scholar]
- Urban, J. Current trends in the development of porous polymer monoliths for the separation of small molecules. J. Sep. Sci. 2016, 39, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Jandera, P. Recent advances in the design of organic polymer monoliths for reversed-phase and hydrophilic interaction chromatography separations of small molecules. Anal. Bioanal. Chem. 2013, 405, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Aggarwal, P.; Lawson, J.S.; Tolley, H.D.; Lee, M.L. Organic monoliths for high-performance reversed-phase liquid chromatography. J. Sep. Sci. 2013, 36, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Svec, F. Quest for organic polymer-based monolithic columns affording enhanced efficiency in high performance liquid chromatography separations of small molecules in isocratic mode. J. Chromatogr. A 2012, 1228, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Teasdale, I.; Bruggemann, O. Porous polymer monoliths for small molecule separations: advancements and limitations. Anal. Bioanal. Chem. 2011, 400, 2289–2304. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I. Porous polymer monoliths: Morphology, porous properties, polymer nanoscale gel structure and their impact on chromatographic performance. J. Chromatogr. A 2013, 1287, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Causon, T.J. Porous polymer monoliths: From their fundamental structure to analytical engineering applications. Trend. Anal. Chem. 2016, 75, 108–117. [Google Scholar] [CrossRef]
- Nischang, I. On the chromatographic efficiency of analytical scale column format porous polymer monoliths: Interplay of morphology and nanoscale gel porosity. J. Chromatogr. A 2012, 1236, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Causon, T.J.; Hilder, E.F.; Nischang, I. Impact of mobile phase composition on the performance of porous polymeric monoliths in the elution of small molecules. J. Chromatogr. A 2012, 1263, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Laher, M.; Causon, T.J.; Buchberger, W.; Hild, S.; Nischang, I. Assessing the nanoscale structure and mechanical properties of polymer monoliths used for chromatography. Anal. Chem. 2013, 85, 5645–5649. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Teasdale, I.; Bruggemann, O. Towards porous polymer monoliths for the efficient, retention-independent performance in the isocratic separation of small molecules by means of nano-liquid chromatography. J. Chromatogr. A 2010, 1217, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Trojer, L.; Bisjak, C.P.; Wieder, W.; Bonn, G.K. High capacity organic monoliths for the simultaneous application to biopolymer chromatography and the separation of small molecules. J. Chromatogr. A 2009, 1216, 6303–6309. [Google Scholar] [CrossRef] [PubMed]
- Greiderer, A.; Trojer, L.; Huck, C.W.; Bonn, G.K. Influence of the polymerisation time on the porous and chromatographic properties of monolithic poly(1,2-bis(p-vinylphenyl))ethane capillary columns. J. Chromatogr. A 2009, 1216, 7747–7754. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Brüggemann, O. On the separation of small molecules by means of nano-liquid chromatography with methacrylate-based macroporous polymer monoliths. J. Chromatogr. A 2010, 1217, 5389–5397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Ou, J.J.; Wei, Y.M.; Wang, H.W.; Liu, Z.S.; Chen, L.F.; Zou, H.F. A novel polymeric monolith prepared with multi-acrylate crosslinker for retention-independent efficient separation of small molecules in capillary liquid chromatography. Anal. Chim. Acta. 2015, 883, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Svec, F.; Frechet, J.M.J. Efficient separation of small molecules using a large surface area hypercrosslinked monolithic polymer capillary column. Anal. Chem. 2010, 82, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Svec, F.; Frechet, J.M.J. Hypercrosslinking: New approach to porous polymer monolithic capillary columns with large surface area for the highly efficient separation of small molecules. J. Chromatogr. A 2010, 1217, 8212–8221. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.S.; Liu, S.X.; Wang, H.Q.; Jia, Q. Recent advances of polymer monolithic columns functionalized with micro/nanomaterials: Synthesis and application. Chromatographia 2014, 77, 5–14. [Google Scholar] [CrossRef]
- Hu, W.W.; Hong, T.T.; Gao, X.; Ji, Y.B. Applications of nanoparticle-modified stationary phases in capillary electrochromatography. Trend. Anal. Chem. 2014, 61, 29–39. [Google Scholar] [CrossRef]
- Nesterenko, E.P.; Nesterenko, P.N.; Connolly, D.; He, X.Y.; Floris, P.; Duffy, E.; Paull, B. Nano-particle modified stationary phases for high-performance liquid chromatography. Analyst 2013, 138, 4229–4254. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Guo, Y.; Xiong, C.M.; Liu, S.J.; Liu, X.; Jiang, S.X. Nanoparticle-based monoliths for chromatographic separations. Analyst 2014, 139, 4103–4117. [Google Scholar] [CrossRef] [PubMed]
- Kimmins, S.D.; Cameron, N.R. Functional porous polymers by emulsion templating: Recent advances. Adv. Func. Mater. 2011, 21, 211–225. [Google Scholar] [CrossRef]
- Schramm, L.L. Emulsion, Foams and Suspensions: Fundamentals and Applications; Wiley: Weinheim, Germany, 2005; ISBN 978-3-527-30743-2. [Google Scholar]
- Choudhury, S.; Connolly, D.; White, B. Supermacroporous polyHIPE and cryogel monolithic materials as stationary phases in separation science: A review. Anal. Methods 2015, 7, 6967–6982. [Google Scholar] [CrossRef]
- Tunc, Y.; Golgelioglu, Ç.; Hasirci, N.; Ulubayram, K.; Tuncel, A. Acrylic-based high internal phase emulsion polymeric monolith for capillary electrochromatography. J. Chromatogr. A 2010, 1217, 1654–1659. [Google Scholar] [CrossRef] [PubMed]
- Tunc, Y.; Golgelioglu, Ç.; Tuncel, A.; Ulubayram, K. Polystyrene-based high internal phase emulsion polymer monolithic stationary phase for capillary electrochromatography. Separ. Sci. Technol. 2012, 47, 2444–2449. [Google Scholar]
- Hughes, J.M.; Budd, P.M.; Tiede, K.; Lewis, J. Polymerized high internal phase emulsion monoliths for the chromatographic separation of engineered nanoparticles. J. Appl. Polym. Sci. 2015, 132, 41229. [Google Scholar] [CrossRef]
- He, M.; Zeng, Y.; Sun, X.J.; Harrison, D.J. Confinement effects on the morphology of photopatterned porous polymer monoliths for capillary and microchip electrophoresis of proteins. Electrophoresis 2008, 29, 2980–2986. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Svec, F.; Frechet, J.M.J. Downscaling limits and confinement effects in the miniaturization of porous polymer monoliths in narrow bore capillaries. Anal. Chem. 2009, 81, 7390–7396. [Google Scholar] [CrossRef] [PubMed]
- Nischang, I.; Svec, F.; Frechet, J.M.J. Effect of capillary cross-section geometry and size on the separation of proteins in gradient mode using monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) columns. J. Chromatogr. A 2009, 1216, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Mullner, T.; Zankel, A.; Mayrhofer, C.; Reingruber, A.; Hoeltzel, H.; Lv, Y.; Svec, F.; Tallarek, U. Reconstruction and characterization of a polymer-based monolithic stationary phase using serial block-face scanning electron microscopy. Langmuir 2012, 28, 16733–16737. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Kobayashi, H.; Okubo, M.; Sabarudin, A.; Butsugan, M.; Umemura, T. Chemical anchoring of lauryl methacrylate-based reversed phase monolith to 1/16″ o.d. polyetheretherketone tubing. J. Chromatogr. A 2012, 1242, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, P.; Leber, N.; Stefanec, D.; Kontrec, S. Podgornik, Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation medi. J. Chromatogr. A 2005, 1065, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Hage, D. Affinity monolith chromatography. J. Sep. Sci. 2006, 29, 1686–1704. [Google Scholar] [CrossRef] [PubMed]
- Causon, T.J.; Nischang, I. Critical differences in chromatographic properties of silica- and polymer-based monoliths. J. Chromatogr. A 2014, 1358, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; Guiochon, G. Mass transport of small retained molecules in polymer-based monolithic columns. J. Chromatogr. A 2014, 1362, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Cheong, W.J. Organic monolith frits encased in polyether ether ketone tubing with improved durability for liquid chromatography. J. Sep. Sci. 2015, 38, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Hao, M.M.; Li, R.; Bai, L.G.; Yang, G.L. Preparation and characterization of poly(triallyl isocyanurate -co- trimethylolpropane triacrylate) monolith and its applications in the separation of small molecules by liquid chromatography. J. Chromatogr. A 2014, 1333, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nesterenko, E.P.; Nesterenko, P.N.; Connolly, D.; Lacroix, F.; Paull, B. Micro-bore titanium housed polymer monoliths for reversed-phase liquid chromatography of small molecules. J. Chromatogr. A 2010, 1217, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Sandron, S.; Heery, B.; Gupta, V.; Collins, D.A.; Nesterenko, E.P.; Nesterenko, P.N.; Talebi, M.; Beirne, S.; Thompson, F.; Wallace, G.G.; et al. 3D printed metal columns for capillary liquid chromatography. Analyst 2014, 139, 6343–6347. [Google Scholar] [CrossRef] [PubMed]
- Umemura, T.; Ueki, Y.; Tsunoda, K.; Katakai, A.; Tamada, M.; Haraguchi, H. Preparation and characterization of methacrylate-based semi-micro monoliths for high-throughput bioanalysis. Anal. Bioanal. Chem. 2006, 386, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Kobayashi, H.; Kojima, N.; Sabarudin, A.; Umemura, T. Preparation and characterization of lauryl methacrylate-based monolithic microbore column for reversed-phase liquid chromatography. J. Chromatogr. A. 2011, 1218, 5228–5234. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Cameron, N.R. Morphology and surface area of emulsion-derived (PolyHIPE) solid foams prepared with oil-phase soluble porogenic solvents: Span 80 as surfactant. Macromolecules 2004, 37, 3188–3201. [Google Scholar] [CrossRef]
- Cameron, N.R.; Sherrington, D.C.; Albiston, L.; Gregory, D.P. Study of the formation of the open-cellular morphology of poly(styrene/divinylbenzene) polyHIPE materials by cryo-SEM. Colloid Polym. Sci. 1996, 274, 592–595. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Dereli, B.; De Geest, B.G.; Du Prez, F.E. Complexity from simplicity: Unique polymer capsules, rods, monoliths, and liquid marbles prepared via HIPE in microfluidics. Part. & Part. Sys. Character. 2013, 30, 438–444. [Google Scholar]
- Peters, E.C.; Svec, F.; Frechet, J.M.J. Preparation of large-diameter “molded” porous polymer monoliths and the control of pore structure homogeneity. Chem. Mater. 1997, 9, 1898–1902. [Google Scholar] [CrossRef]
- Nischang, I.; Brueggemann, O.; Svec, F. Advances in the preparation of porous polymer monoliths in capillaries and microfluidic chips with focus on morphological aspects. Anal. Bioanal. Chem. 2010, 397, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Arrua, R.D.; Haddad, P.R.; Hilder, E.F. Monolithic cryopolymers with embedded nanoparticles. II. Capillary liquid chromatography of proteins using charged embedded nanoparticles. J. Chromatogr. A 2013, 1311, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Du, K.F.; Yang, D.; Sun, Y. Fabrication of high-permeability and high-capacity monolith for protein chromatography. J. Chromatogr. A 2007, 1163, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, J.; Wang, F.; Crommen, J.; Jiang, Z. Preparation of a β-cyclodextrin functionalized monolith via a novel and simple one-pot approach and application to enantioseparations. J. Chromatogr. A 2014, 1325, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Stol, R.; Poppe, H.; Kok, W.T. Effects of pore flow on the separation efficiency in capillary electrochromatography with porous particles. Anal. Chem. 2001, 73, 3332–3339. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.R.; Barbetta, A. The influence of porogen type on the porosity, surface area and morphology of poly(divinylbenzene) PolyHIPE foams. J. Mater. Chem. 2000, 10, 2466–2471. [Google Scholar] [CrossRef]

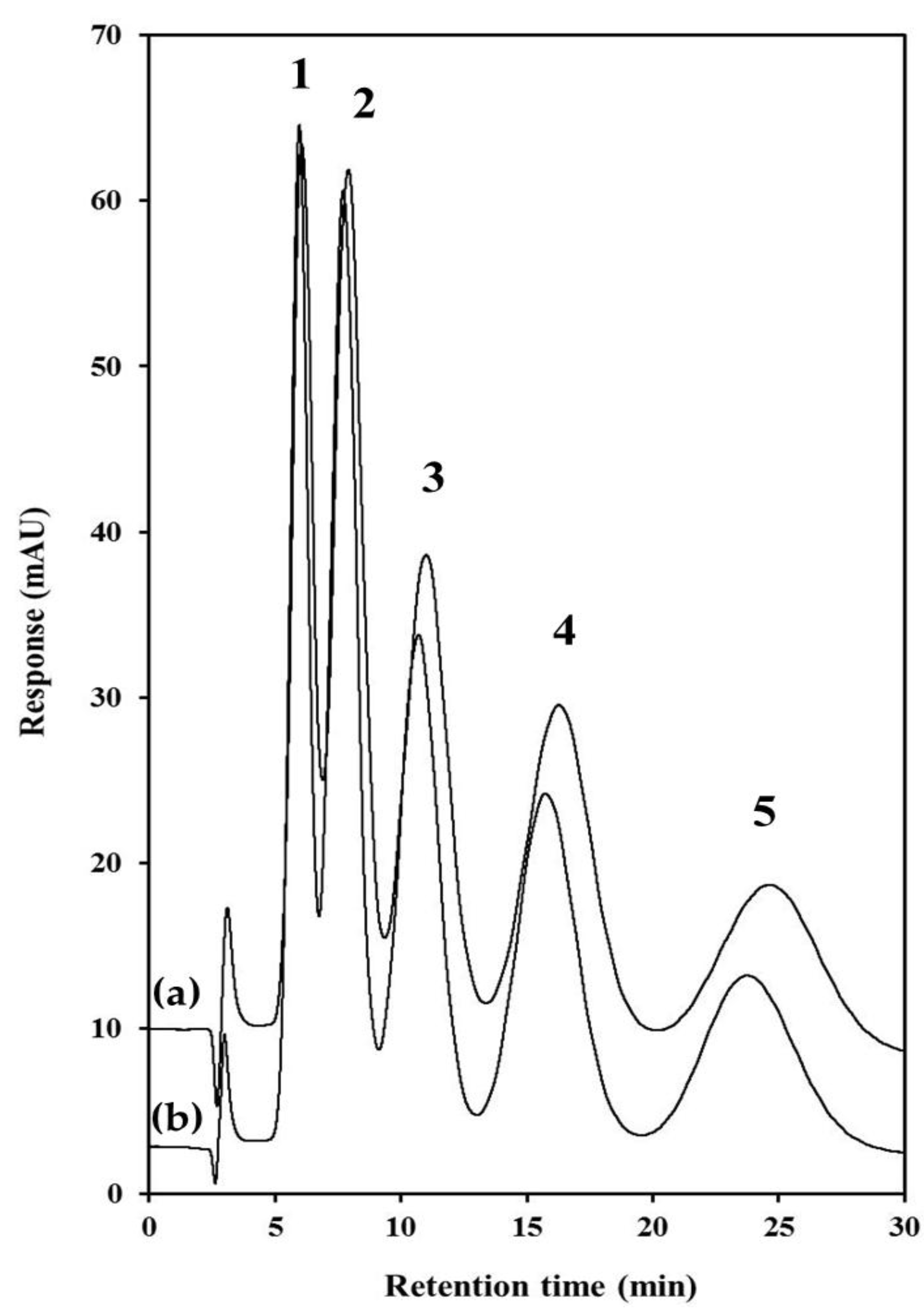
| Chromatographic Performance Indicator | Toluene | Ethylbenzene | Propylbenzene | Butylbenzene | Pentylbenzene |
|---|---|---|---|---|---|
| Retention factor (k) | 0.97 (1.03) | 1.56 (1.63) | 2.56 (2.65) | 4.25 (4.38) | 6.95 (7.13) |
| Selectivity factor (α) | 1.6 (1.6) | 1.6 (1.6) | 1.7 (1.7) | 1.6 1.6 | – – |
| Resolution (Rs) | – – | 0.96 (0.98) | 1.10 (1.12) | 1.21 (1.25) | 1.25 (1.27) |
| Efficiency (N/m) | 2470 (3087) | 2332 (2591) | 1808 (2008) | 1565 (1597) | 1810 (1907) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhury, S.; Fitzhenry, L.; White, B.; Connolly, D. Polystyrene-co-Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography. Materials 2016, 9, 212. https://doi.org/10.3390/ma9030212
Choudhury S, Fitzhenry L, White B, Connolly D. Polystyrene-co-Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography. Materials. 2016; 9(3):212. https://doi.org/10.3390/ma9030212
Chicago/Turabian StyleChoudhury, Sidratul, Laurence Fitzhenry, Blánaid White, and Damian Connolly. 2016. "Polystyrene-co-Divinylbenzene PolyHIPE Monoliths in 1.0 mm Column Formats for Liquid Chromatography" Materials 9, no. 3: 212. https://doi.org/10.3390/ma9030212





