Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Material and Pretreatment
2.2. Weight Loss Test
2.3. Characterization of the Corrosion Scale
3. Results
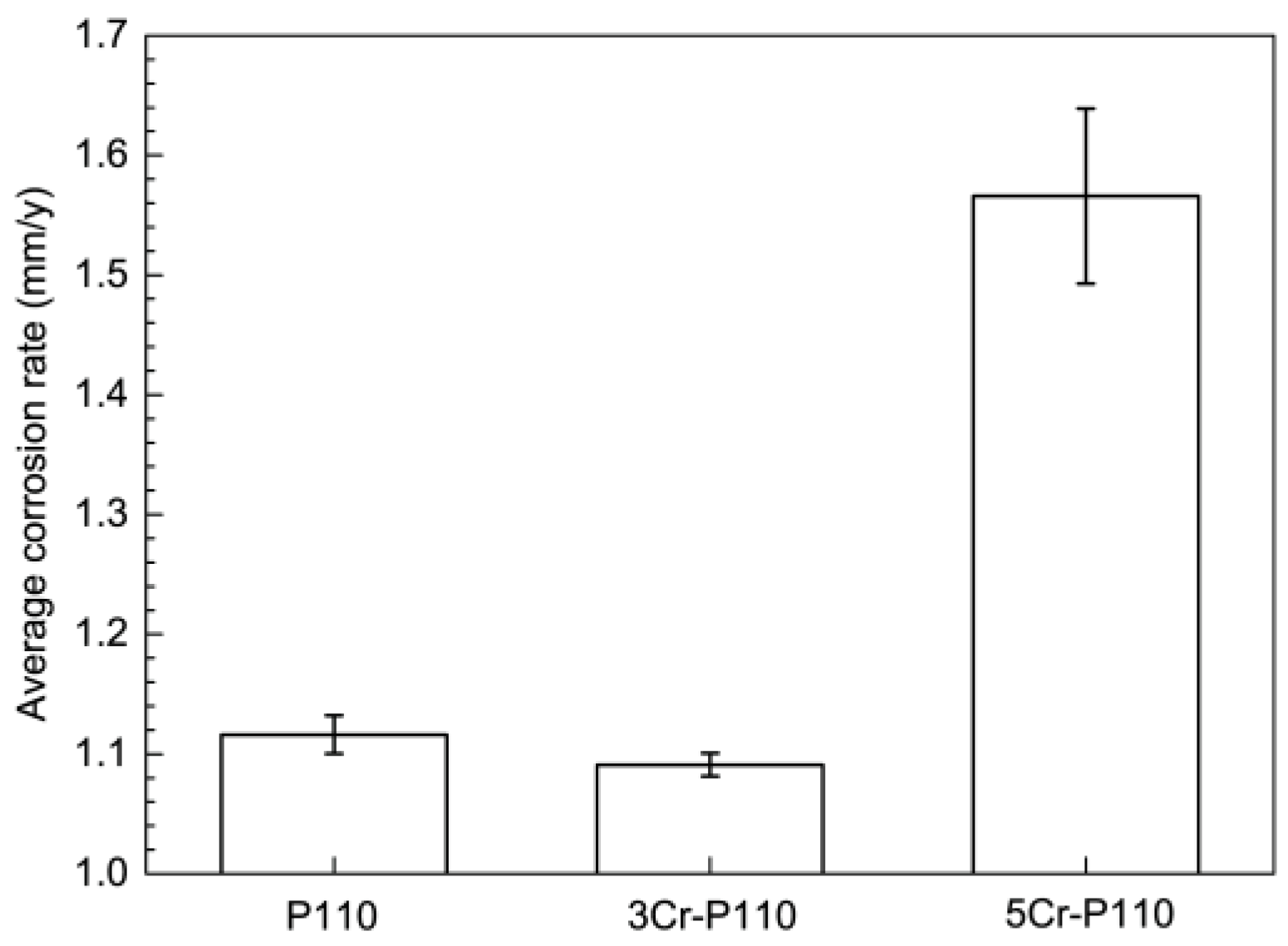
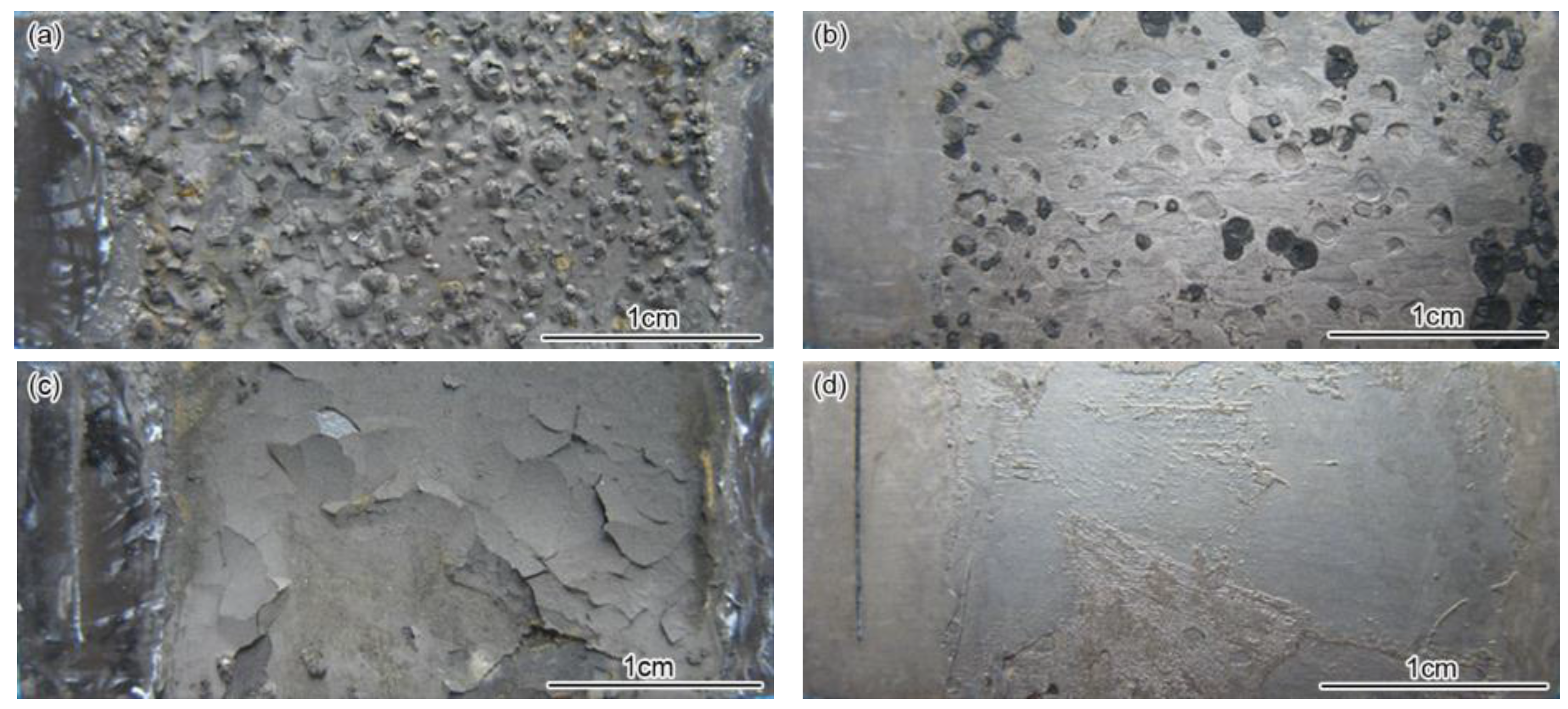
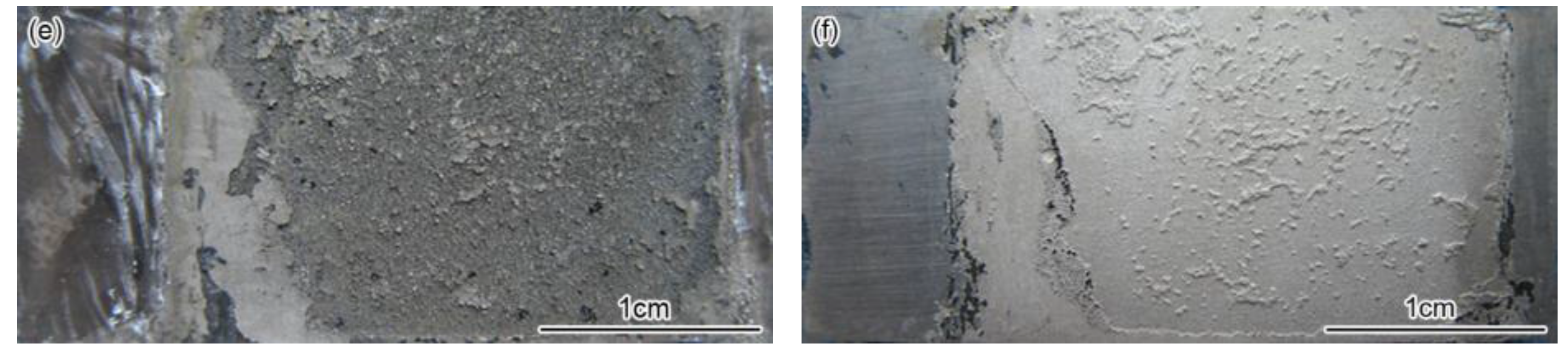
3.1. Corrosion Rate and Corrosion Form
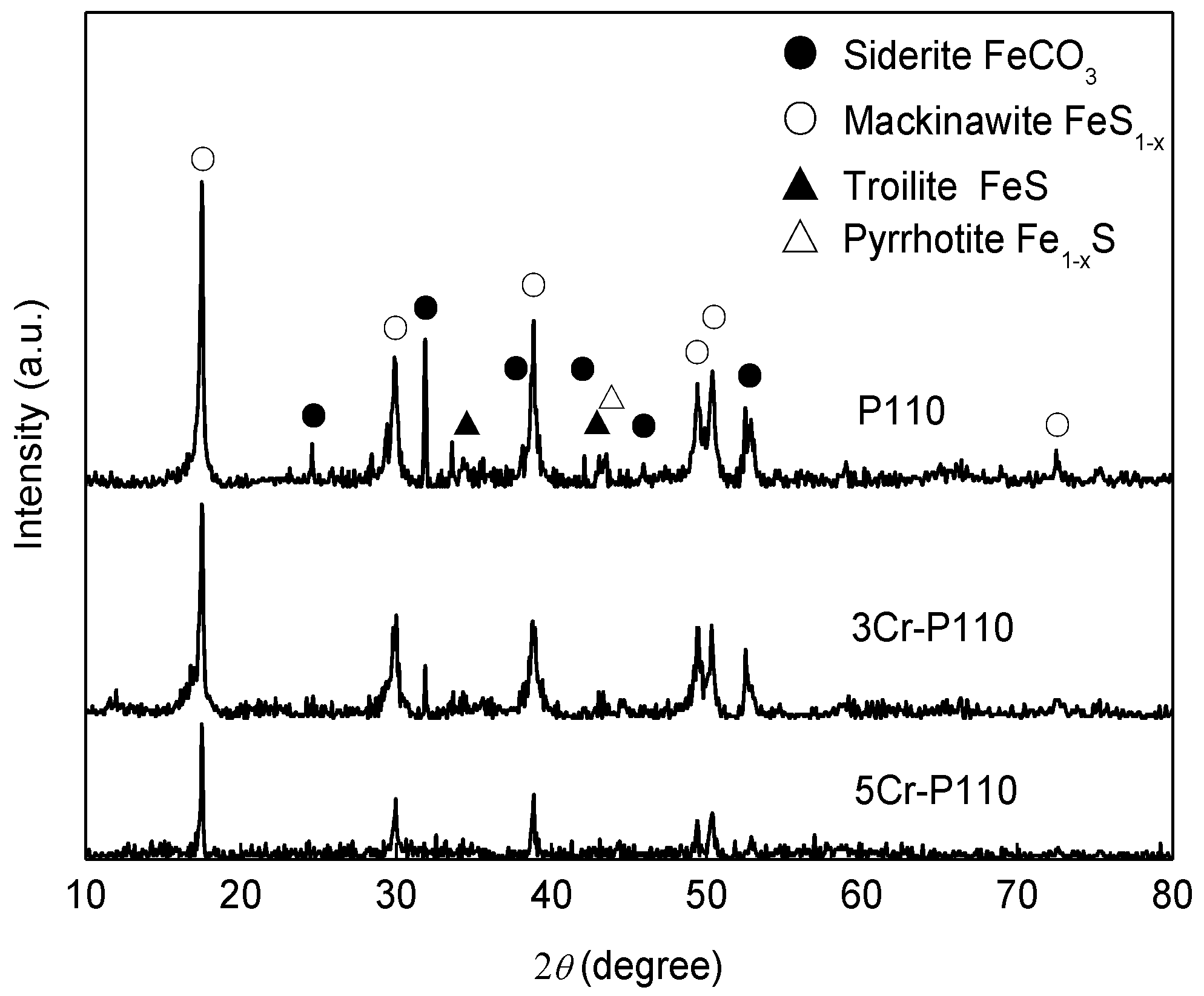
3.2. The Composition and Elements Distribution of Corrosion Film
3.3. The Effect of Cr Content on Formation of Corrosion Scale and Its Relation to Corrosion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kermani, M.B.; Morshed, A. Carbon dioxide corrosion in oil and gas production—A compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nesic, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochim. Acta 2011, 56, 1752–1760. [Google Scholar] [CrossRef]
- Sun, W.; Nesic, S.; Papavinasam, S. Kinetics of corrosion layer formation. Part 2—Iron sulfide and mixed iron sulfide/carbonate layers in carbon dioxide/hydrogen sulfide corrosion. Corrosion 2008, 64, 586–599. [Google Scholar] [CrossRef]
- López, D.A.; Pérez, T.; Simison, S.N. The influence of microstructure and chemical composition of carbon and low alloy steels in CO2 corrosion. Mater. Des. 2003, 24, 561–575. [Google Scholar] [CrossRef]
- Kermani, M.B.; Gonzales, J.C.; Turconi, G.L.; Edmonds, D.; Dicken, G.; Scoppio, L. Development of superior corrosion resistance 3% Cr steels for downhole applications. In CORROSION 2003; NACE International: Houston, TA, USA, 2003. [Google Scholar]
- Wu, Q.L.; Zhang, Z.H.; Dong, X.M.; Yang, J.Q. Corrosion behavior of low-alloy steel containing 1% chromium in CO2 environments. Corros. Sci. 2013, 75, 400–408. [Google Scholar] [CrossRef]
- Kermani, M.B.; Gonzales, J.C.; Linne, C.; Dougan, M.; Cochrane, R. Development of low carbon Cr-Mo steels with exceptional corrosion resistance for oilfield applications. In CORROSION 2001; NACE International: Houston, TA, USA, 2001. [Google Scholar]
- Kermani, M.B.; Gonzales, J.C.; Turconi, G.L.; Perez, T.; Morales, C. In-field corrosion performance of 3% Cr steels in sweet and sour downhole production and water injection. In CORROSION 2004; NACE International: Houston, TA, USA, 2004. [Google Scholar]
- Guo, S.Q.; Xu, L.N.; Zhang, L.; Chang, W.; Lu, M.X. Corrosion of alloy steels containing 2% chromium in CO2 environments. Corros. Sci. 2012, 63, 246–258. [Google Scholar] [CrossRef]
- Ueda, M. Effect of alloying elements and microstructure on stability of corrosion product in CO2 and/or H2S environments. Chem. Eng. Oil Gas 2005, 34, 43–52. (In Chinese) [Google Scholar]
- Takabe, H.; Ueda, M. The formation behavior of corrosion protective films of low Cr bearing steels in CO2 environments. In CORROSION 2001; NACE International: Houston, TA, USA, 2001. [Google Scholar]
- Sun, J.B.; Liu, W.; Chang, W.; Zhang, Z.H.; Li, Z.T.; Yu, T.; Lu, M.X. Characteristics and formation mechanism of corrosion scales on low-chromium X65 steels in CO2 environment. Acta Metall Sin 2009, 45, 84–90. (In Chinese) [Google Scholar]
- Chen, C.F.; Lu, M.X.; Sun, D.B.; Zhang, Z.H.; Chang, W. Effect of chromium on the pitting resistance of oil tube steel in a carbon dioxide corrosion system. Corrosion 2005, 61, 594–601. [Google Scholar] [CrossRef]
- Pigliacampo, L.; Gonzales, J.C.; Turconi, G.L.; Perez, T.; Morales, C.; Kermani, M.B. Window of application and operational track record of low carbon 3Cr steel tubular. In CORROSION 2006; NACE International: Houston, TA, USA, 2006. [Google Scholar]
- Perdomo, J.J.; Morales, J.L.; Viloria, A.; Lusinchi, A.J. CO2 and H2S corrosion of API 5L-B and 5L-X52 grade steels. In CORROSION 2000; NACE International: Houston, TA, USA, 2000. [Google Scholar]
- Omar, I.H.; Gunaltun, Y.M.; Kvarekval, J.; Dugstad, A. H2S corrosion of carbon steel under simulated kashagan field conditions. In CORROSION 2005; NACE International: Houston, TA, USA, 2005. [Google Scholar]
- Sun, W.; Nesic, S. A mechanistic model of uniform hydrogen sulfide/carbon dioxide corrosion of mild steel. Corrosion 2009, 65, 291–307. [Google Scholar] [CrossRef]
- Abayarathna, D.; Naraghi, A.; Obeyesekere, N. Inhibition of corrosion of carbon steel in the presence of CO2, H2S and S. In CORROSION 2003; NACE International: Houston, TA, USA, 2003. [Google Scholar]
- Smith, S.N.; Joosten, M.W. Corrosion of carbon steel by H2S in CO2 containing oilfield environments. In CORROSION 2006; NACE International: Houston, TA, USA, 2006. [Google Scholar]
- Sun, W.; Nesic, S. A mechanistic model of H2S corrosion of mild steel. In CORROSION 2007; NACE International: Houston, TA, USA, 2007. [Google Scholar]
- Valdes, A.; Case, R.; Ramirez, M.; Ruiz, A. The effect of small amounts of H2S on CO2 corrosion of a carbon steel. In CORROSION 98; NACE International: Houston, TA, USA, 1998. [Google Scholar]
- Ren, C.Q.; Liu, D.X.; Bai, Z.Q.; Li, T.H. Corrosion behavior of oil tube steel in simulant solution with hydrogen sulfide and carbon dioxide. Mater. Chem. Phys. 2005, 93, 305–309. [Google Scholar] [CrossRef]
- Li, D.P.; Zhang, L.; Yang, J.W.; Lu, M.X.; Ding, J.H.; Liu, M.L. Effect of H2S concentration on the corrosion behavior of pipeline steel under the coexistence of H2S and CO2. Int. J. Min. Met. Mater. 2014, 21, 388–394. [Google Scholar] [CrossRef]
- Kvarekval, J.; Nyborg, R.; Seiersten, M. Corrosion product films on carbon steel in semi-sour CO2/H2S environments. In CORROSION 2002; NACE International: Houston, TA, USA, 2002. [Google Scholar]
- Pots, B.F.M.; John, R.C.; Rippon, I.J.; Thomas, M.J.J.S.; Kapusta, S.D.; Girgis, M.M.; Whitham, T. Improvements on de waard-milliams corrosion prediction and applications to corrosion management. In CORROSION 2002; NACE International: Houston, TA, USA, 2002. [Google Scholar]
- Srinivasan, S.; Kane, R.D. Prediction of corrosivity of CO2/H2S production environments. In CORROSION 96; NACE International: Houston, USA, 1996. [Google Scholar]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Lopez, D.A.; Schreiner, W.H.; de Sanchez, S.R.; Simison, S.N. The influence of inhibitors molecular structure and steel microstructure on corrosion layers in CO2 corrosion. Appl. Surf. Sci. 2004, 236, 77–97. [Google Scholar] [CrossRef]
- Lee, K.L.J.; Nesic, S. The effect of trace amount of H2S on CO2 corrosion investigated by using the EIS technique. In CORROSION 2005; NACE International: Houston, TA, USA, 2005. [Google Scholar]
- Xiang, Y.; Wang, Z.; Xu, C.; Zhou, C.C.; Li, Z.; Ni, W.D. Impact of SO2 concentration on the corrosion rate of X70 steel and iron in water-saturated supercritical CO2 mixed with SO2. J. Supercrit. Fluids 2011, 58, 286–294. [Google Scholar] [CrossRef]
- Mullet, M.; Boursiquot, S.; Abdelmoula, M.; Genin, J.M.; Ehrhardt, J.J. Surface chemistry and structural properties of mackinawite prepared by reaction of sulfide ions with metallic iron. Geochim. Cosmochim. Acta 2002, 66, 829–836. [Google Scholar] [CrossRef]
- Desimoni, E.; Malitesia, C.; Zambonin, P.G.; Riviere, J.C. An X-ray photoelectron spectroscopic study of some chromium-oxygen systems. Surf. Interface Anal. 1988, 13, 173–179. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K. The X-ray photo-electron spectra of several oxides of iron and chromium. Corros. Sci. 1977, 17, 559–570. [Google Scholar] [CrossRef]
- Crolet, J.L.; Thevenot, N.; Nesic, S. The role of conductive corrosion products in the protectiveness of corrosion layers. Corrosion 1998, 54, 194–203. [Google Scholar] [CrossRef]
- Dugstad, A. Fundamental aspects of CO2 metal loss corrosion—Part I: Mechanism. In CORROSION 2006; NACE International: Houston, TA, USA, 2006. [Google Scholar]
- Schmitt, G.; Hörstemeier, M. Fundamental aspects of CO2 metal loss corrosion—Part II: Influence of different parameters on CO2 corrosion mechanisms. In CORROSION 2006; NACE International: Houston, TA, USA, 2006. [Google Scholar]
- Gulbrandsen, E.; Nesic, S.; Stangeland, A.; Burchardt, T.; Sundfær, B.; Hesjevik, S.M.; Skjerve, S. Effect of precorrosion on the performance of inhibitors for CO2 corrosion of carbon steel. In CORROSION 98; NACE International: Houston, TA, USA, 1998. [Google Scholar]
- Schmitt, G. Fundamental Aspects of CO2 Corrosion. In Advances in CO2 Corrosion; NACE International: Houston, TA, USA, 1984; pp. 10–19. [Google Scholar]
- Xie, Y.; Xu, L.N.; Gao, C.L.; Chang, W.; Lu, M.X. Corrosion behavior of novel 3% Cr pipeline steel in CO2 Lop-of-Line Corrosion environment. Mater. Des. 2012, 36, 54–57. [Google Scholar] [CrossRef]
- Chen, T.H.; Xu, L.N.; Lu, M.X.; Chang, W.; Zhang, L. Study on factors affecting low Cr alloy steels in a CO2 corrosion. In CORROSION 2011; NACE International: Houston, TA, USA, 2011. [Google Scholar]
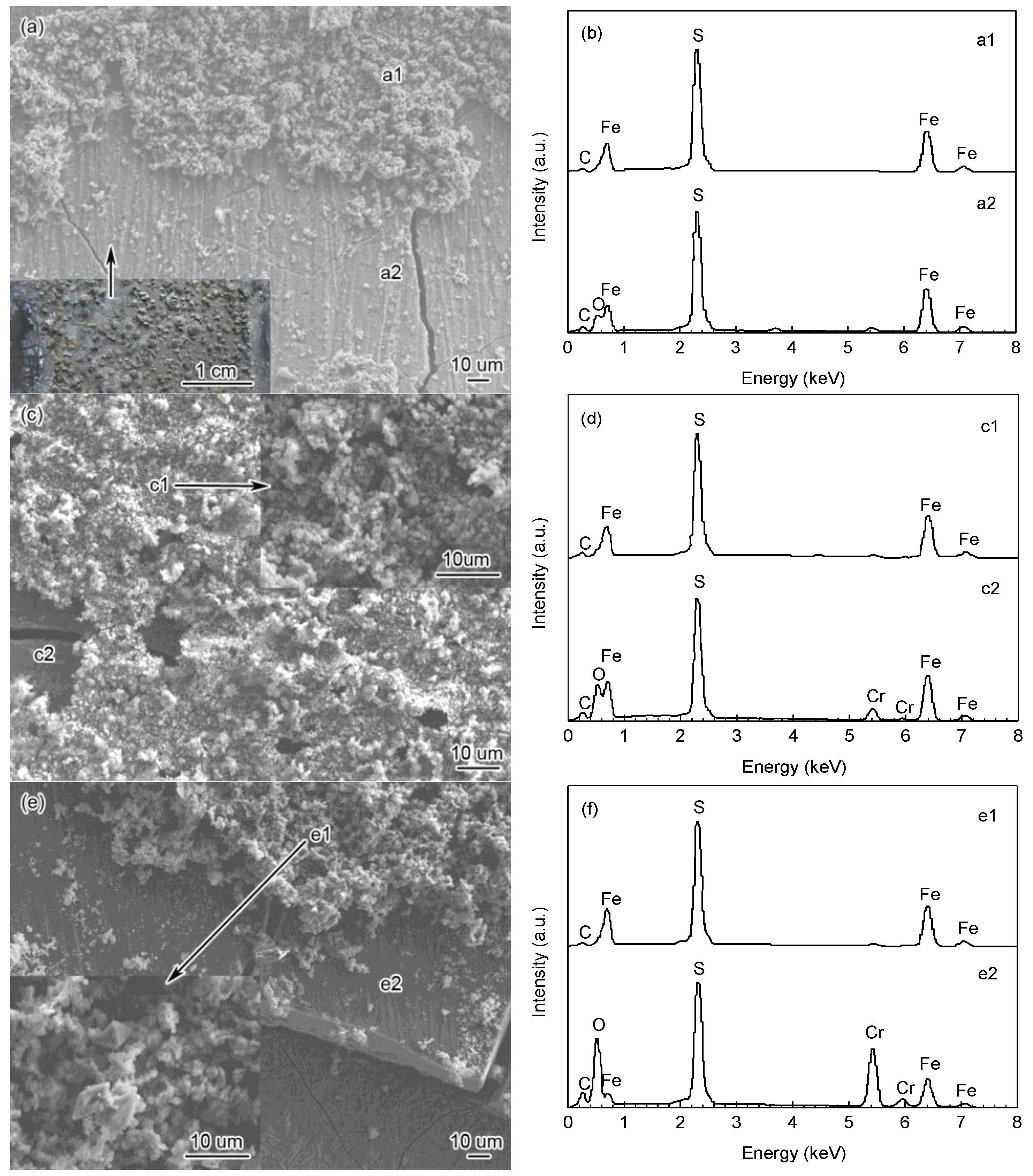


| Steel | C | Si | Cr | Mn | Mo | Ni | P | S | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| P110 | 0.25 | 0.29 | 0.15 | 0.76 | 0.27 | 0.032 | 0.009 | 0.004 | 0.004 | Bal. |
| 3Cr-P110 | 0.26 | 0.27 | 2.99 | 0.58 | 0.19 | 0.043 | 0.011 | 0.004 | 0.008 | Bal. |
| 5Cr-P110 | 0.25 | 0.23 | 5.11 | 0.54 | 0.21 | 0.041 | 0.008 | 0.007 | 0.009 | Bal. |
| Element | P110 | 3Cr-P110 | 5Cr-P110 |
|---|---|---|---|
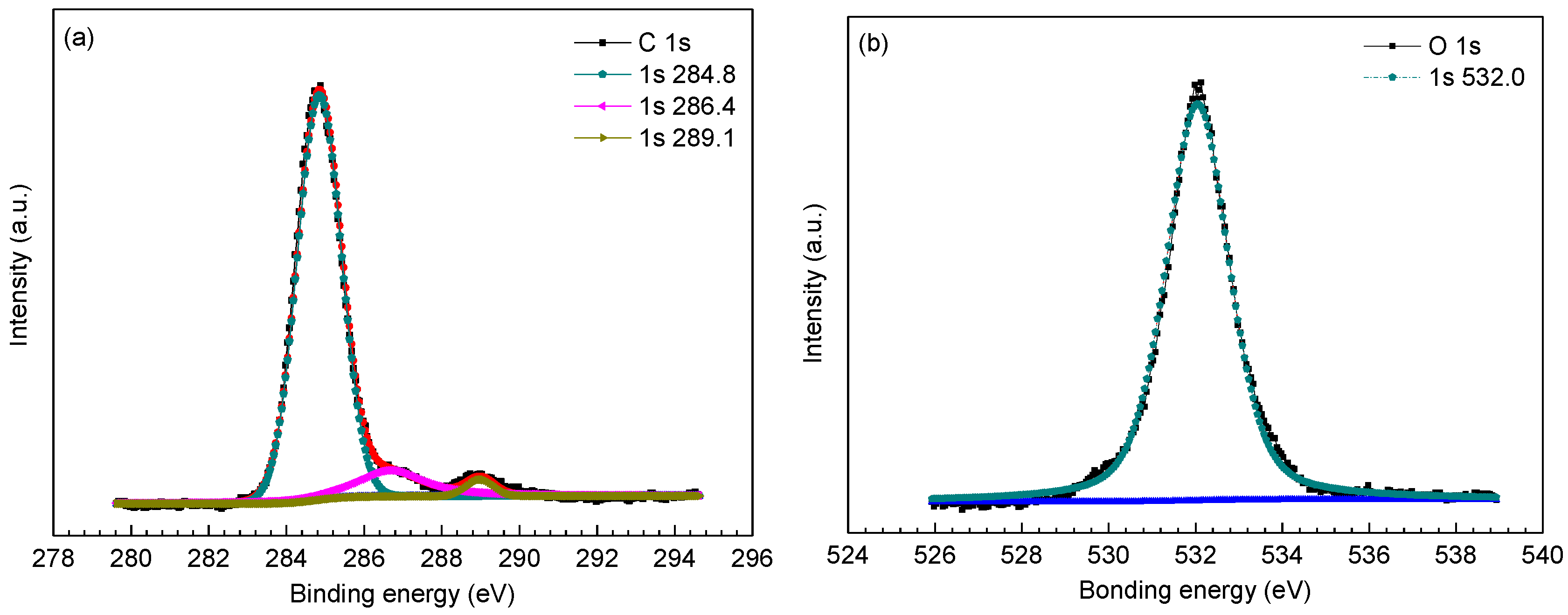
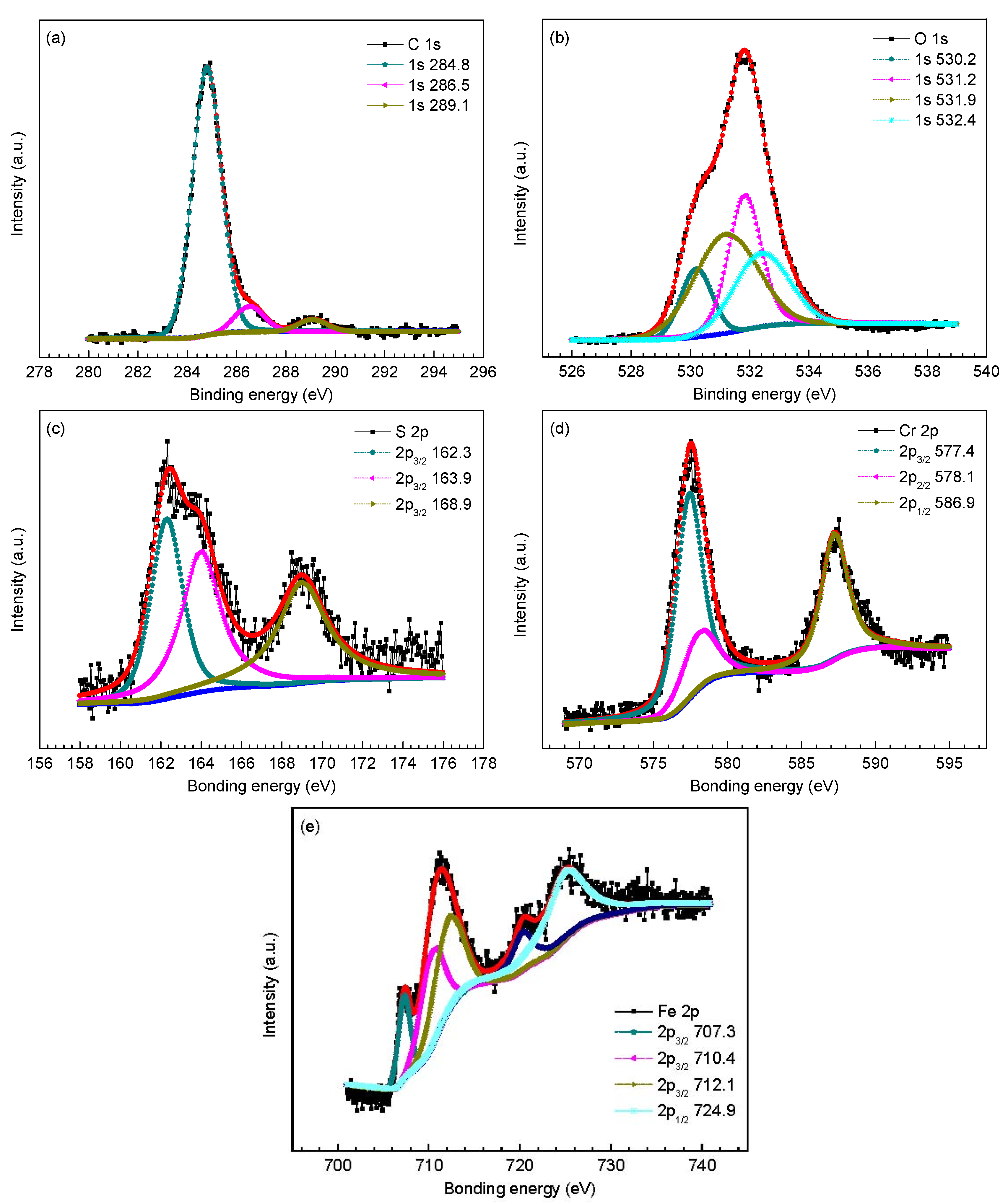
| C 1s | 284.8 (adventitious) [28] | 284.8 (adventitious) [28] | 284.8 (adventitious) [28] |
| 286.4 (adventitious) [28] | 286.5 (adventitious) [28] | 286.5 (adventitious) [28] | |
| 289.1 (FeCO3) [2,27] | 289.0 (FeCO3) [2,27] | 289.1 (FeCO3) [2,27] | |
| O 1s | 532.0 FeCO3 [2,27,28] | 530.2 (Cr2O3 ) [32] | 530.2 (Cr2O3 ) [32] |
| - | 531.9 (FeCO3) [27] | 531.2 Cr(OH)3 [9] | |
| - | 532.4 (Cr(OH)3) [32] | 531.9 (FeCO3) [27] | |
| - | - | 532.4 (Cr(OH)3) [32] | |
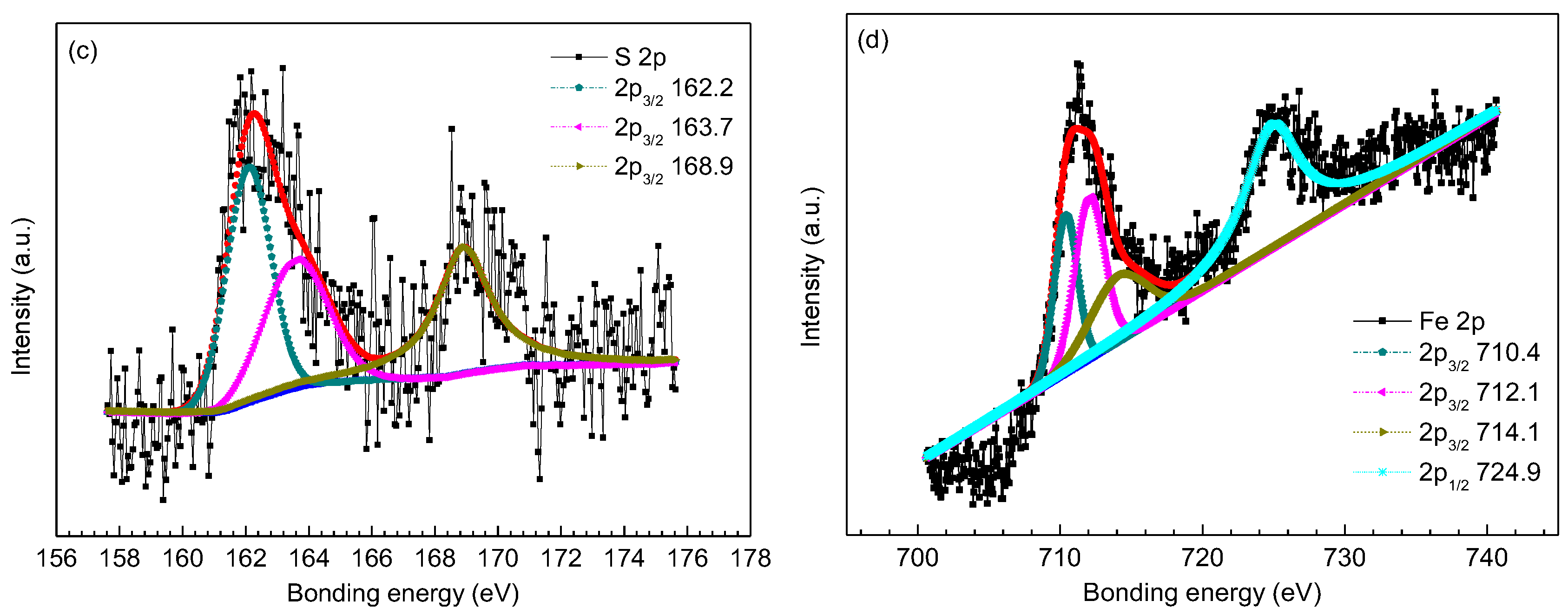
| S 2p | 162.2 (FeS1−x) [29] | 162.3 (FeS1−x) [29] | 162.3 (FeS1−x) [29] |
| 163.7 (elemental sulfur) [3] | 163.9 (elemental sulfur) [3] | 163.9 (elemental sulfur) [3] | |
| 168.9 (adventitious) [30] | 169.2 (adventitious) [30] | 168.9 (adventitious) [30] | |
| Fe 2p | 710.4 (FeCO3) [9,27] | 710.4 (FeCO3) [9,27] | 707.3 (iron sulfide) [31] |
| 712.1 (FeS1−x) [29] | 712.1 (FeS1−x) [29] | 710.4 (FeCO3) [9,27] | |
| 714.1 (FeCO3) [9,27] | 714.9 (FeCO3) [9,27] | 712.1 (FeS1−x) [29] | |
| 724.9(FeCO3) [9,27] | 724.6(FeCO3) [9,27] | 724.9(FeCO3) [9,27] | |
| Cr 2p | - | 577.4 (Cr(OH)3) [9,32,33] | 577.4 (Cr(OH)3) [9,32,33] |
| - | 586.9 (Cr(OH)3) [9,32,33] | 578.1(Cr2O3) [32] | |
| - | - | 586.9 (Cr(OH)3) [9,32,33] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Sun, C.; Lin, X.; Cheng, X.; Liu, H. Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature. Materials 2016, 9, 200. https://doi.org/10.3390/ma9030200
Sun J, Sun C, Lin X, Cheng X, Liu H. Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature. Materials. 2016; 9(3):200. https://doi.org/10.3390/ma9030200
Chicago/Turabian StyleSun, Jianbo, Chong Sun, Xueqiang Lin, Xiangkun Cheng, and Huifeng Liu. 2016. "Effect of Chromium on Corrosion Behavior of P110 Steels in CO2-H2S Environment with High Pressure and High Temperature" Materials 9, no. 3: 200. https://doi.org/10.3390/ma9030200






