Semisolid Microstructural Evolution during Partial Remelting of a Bulk Alloy Prepared by Cold Pressing of the Ti-Al-2024Al Powder Mixture
Abstract
:1. Introduction
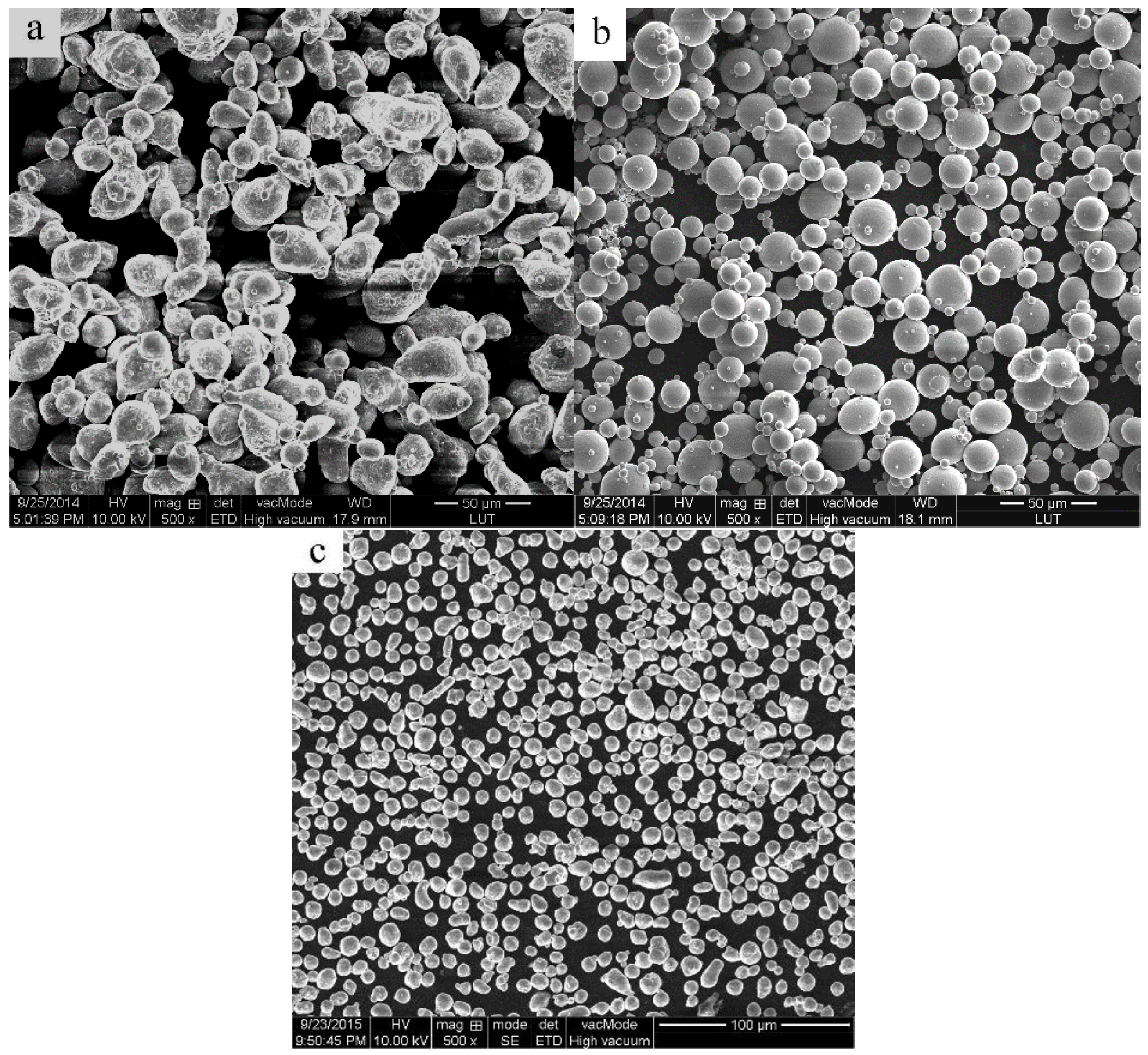
2. Experimental
3. Results and Discussion
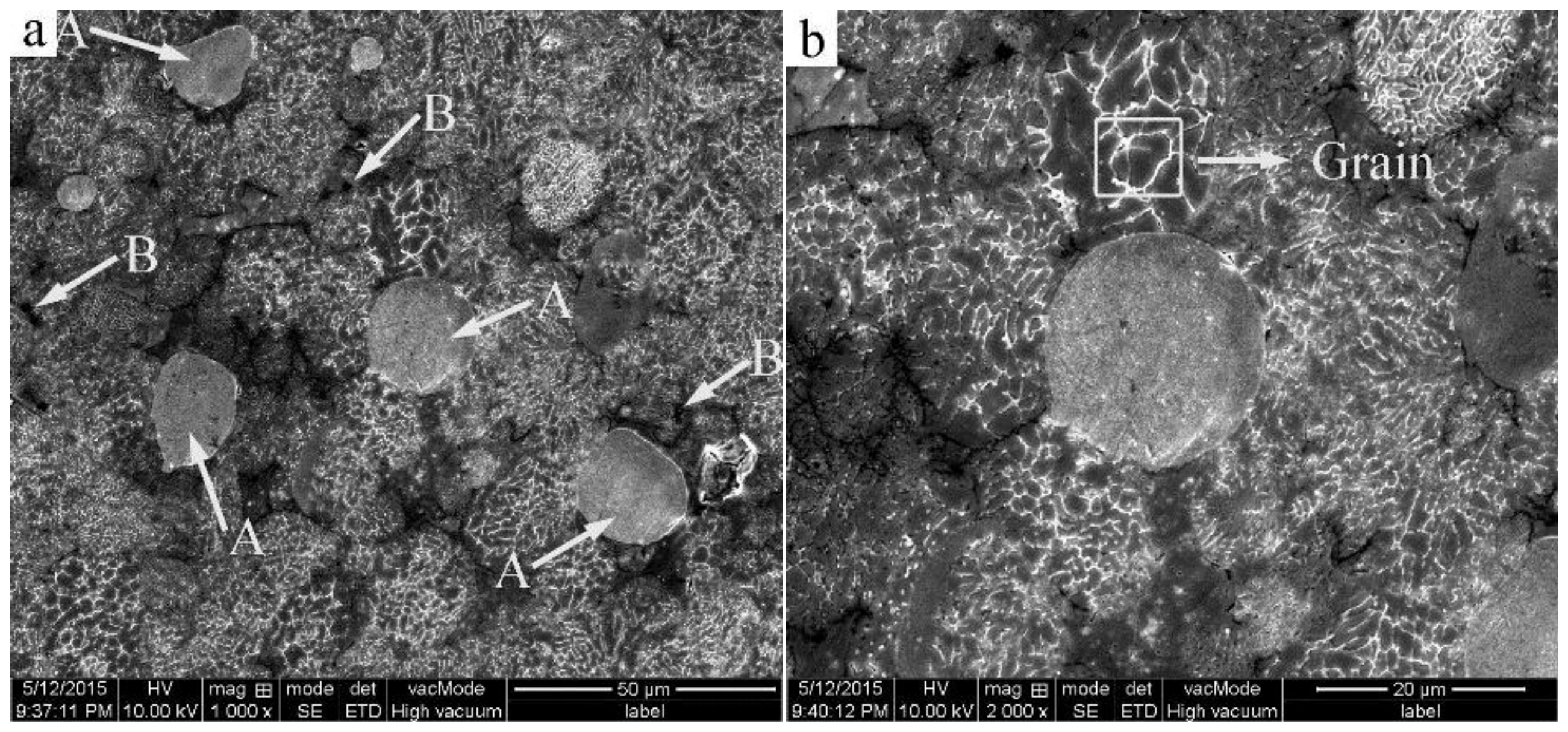
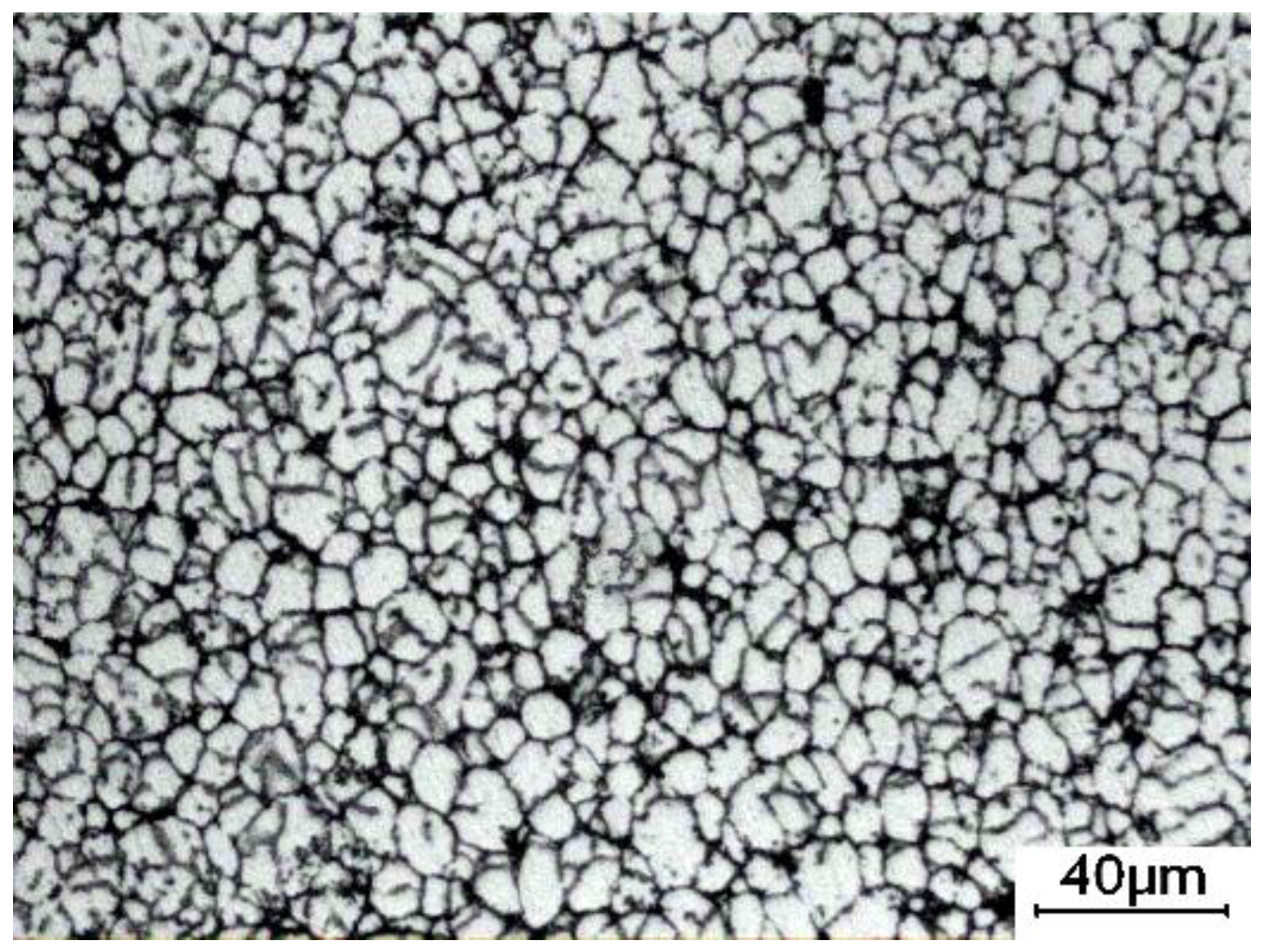
3.1. Microstructure of the Cold-Pressed Ingots
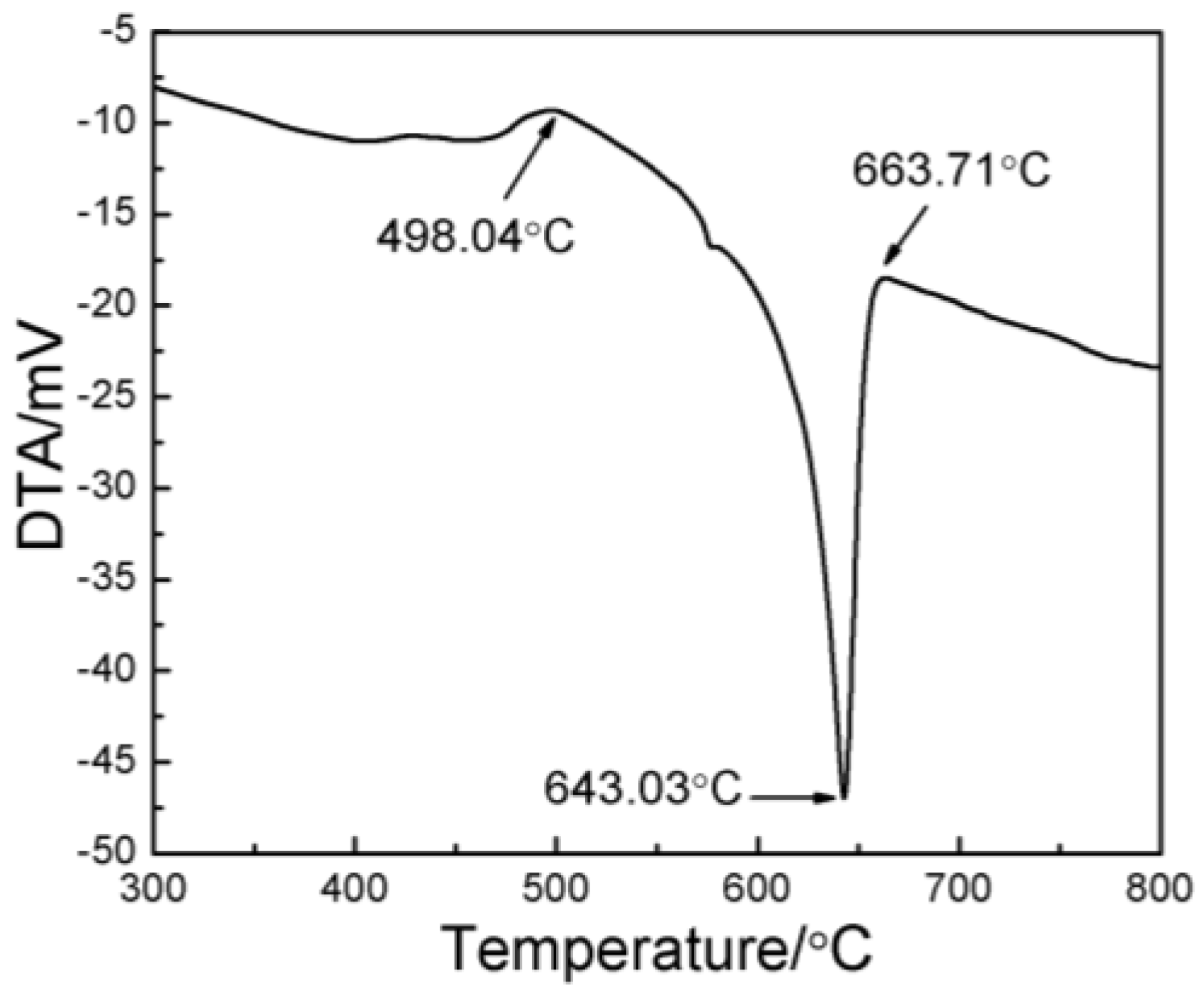
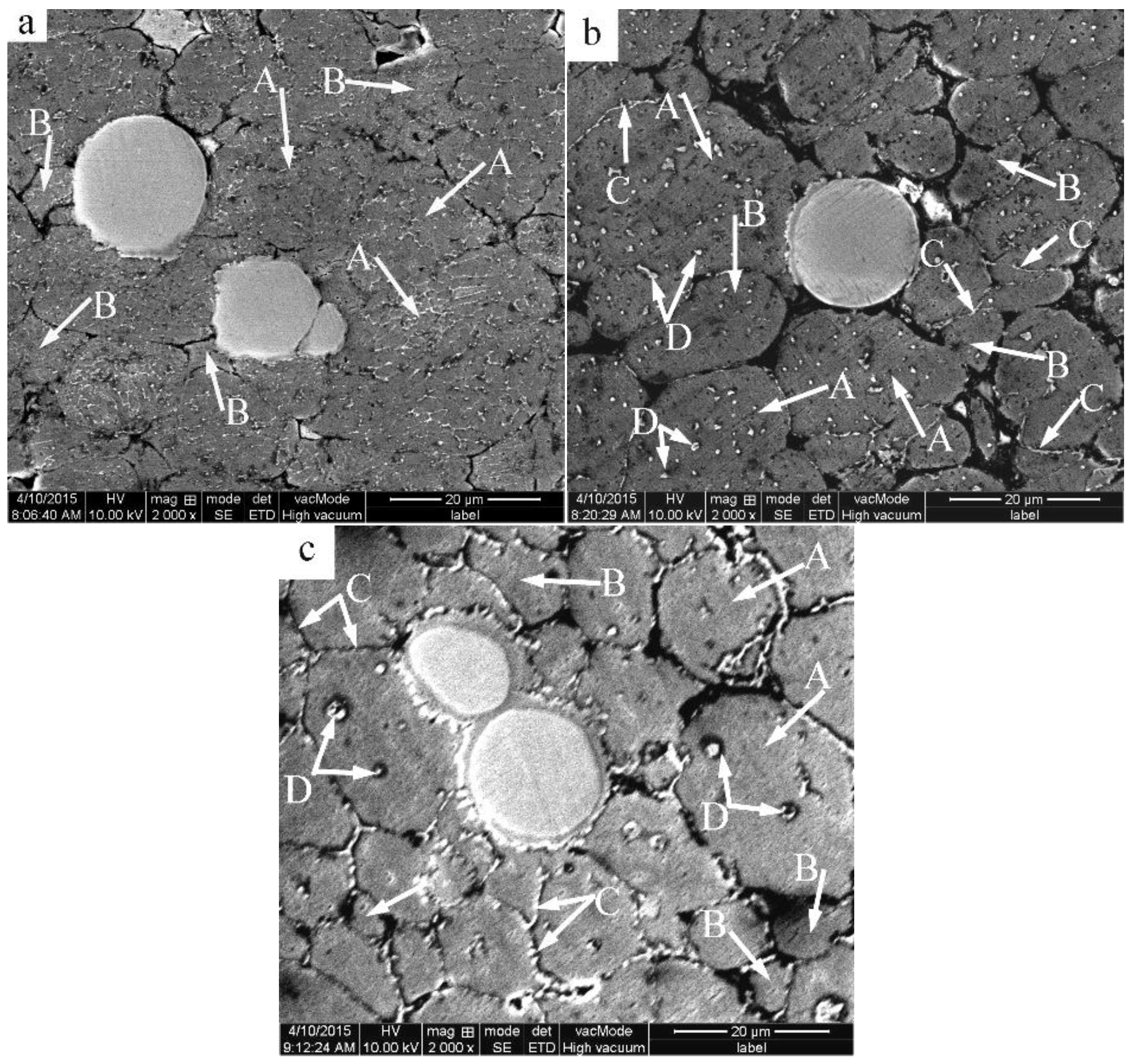
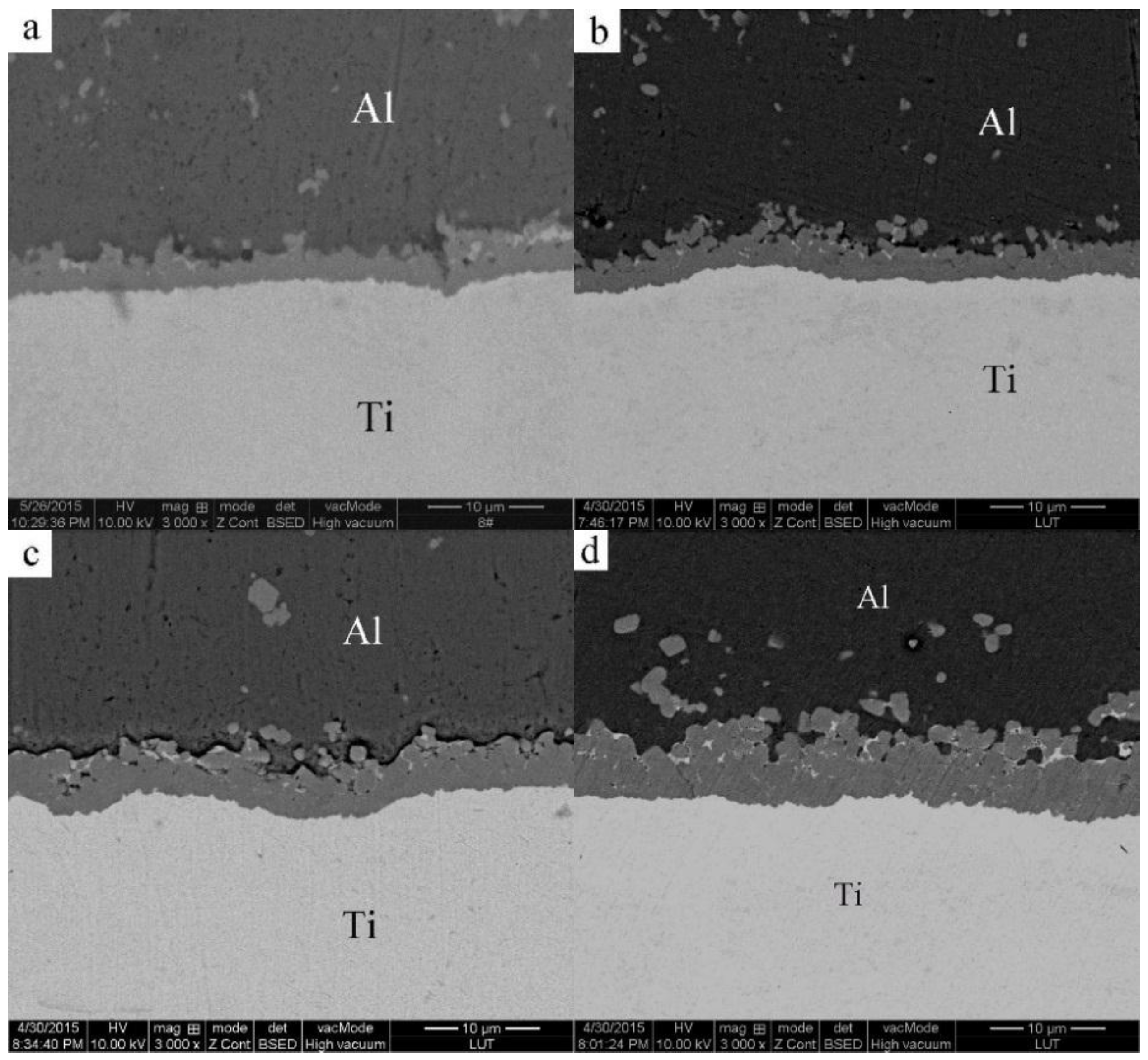
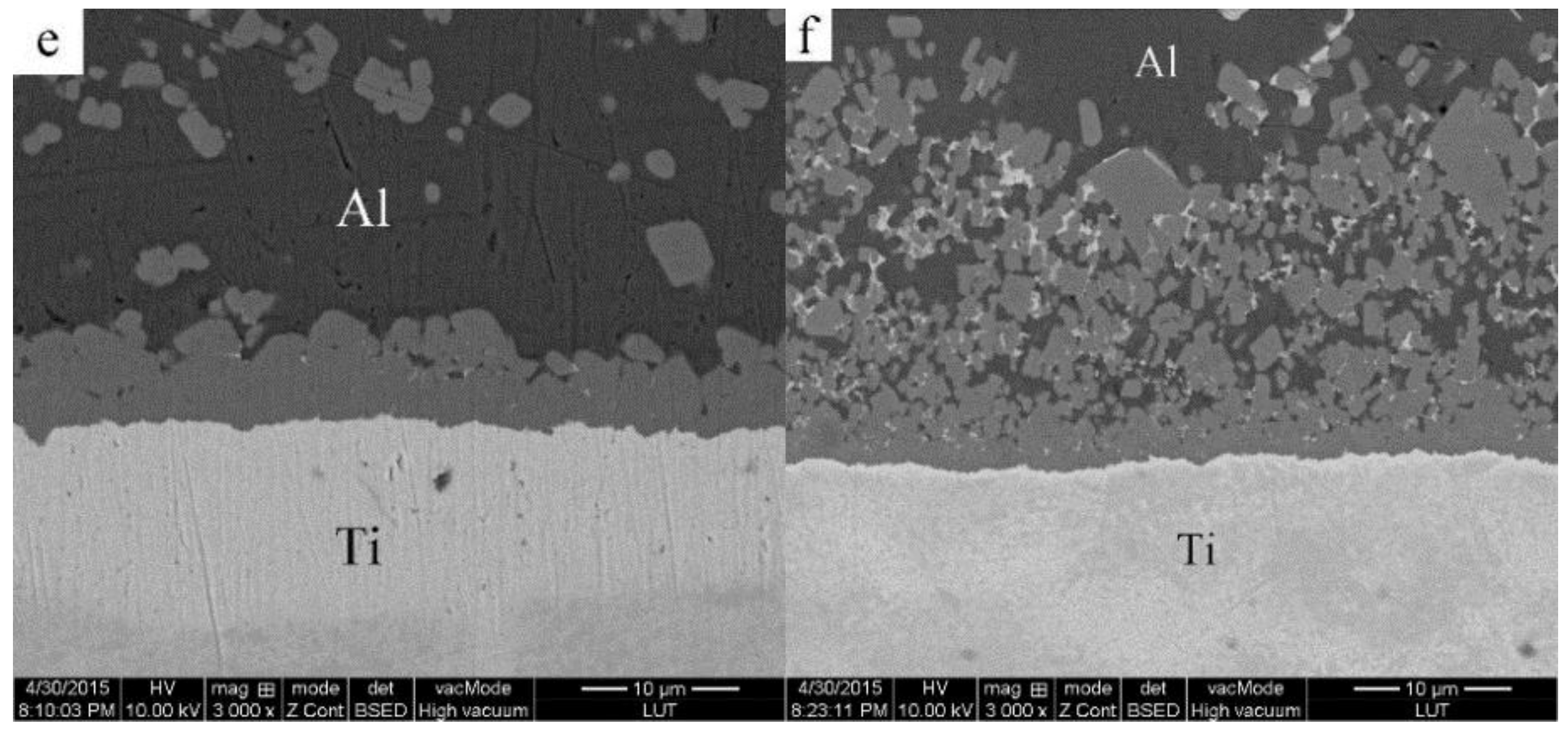
3.2. Microstructural Evolution of the 2024 Alloy Matrix during Partial Remelting
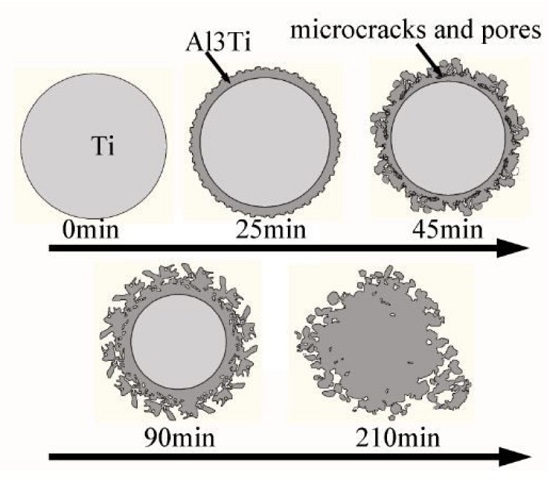
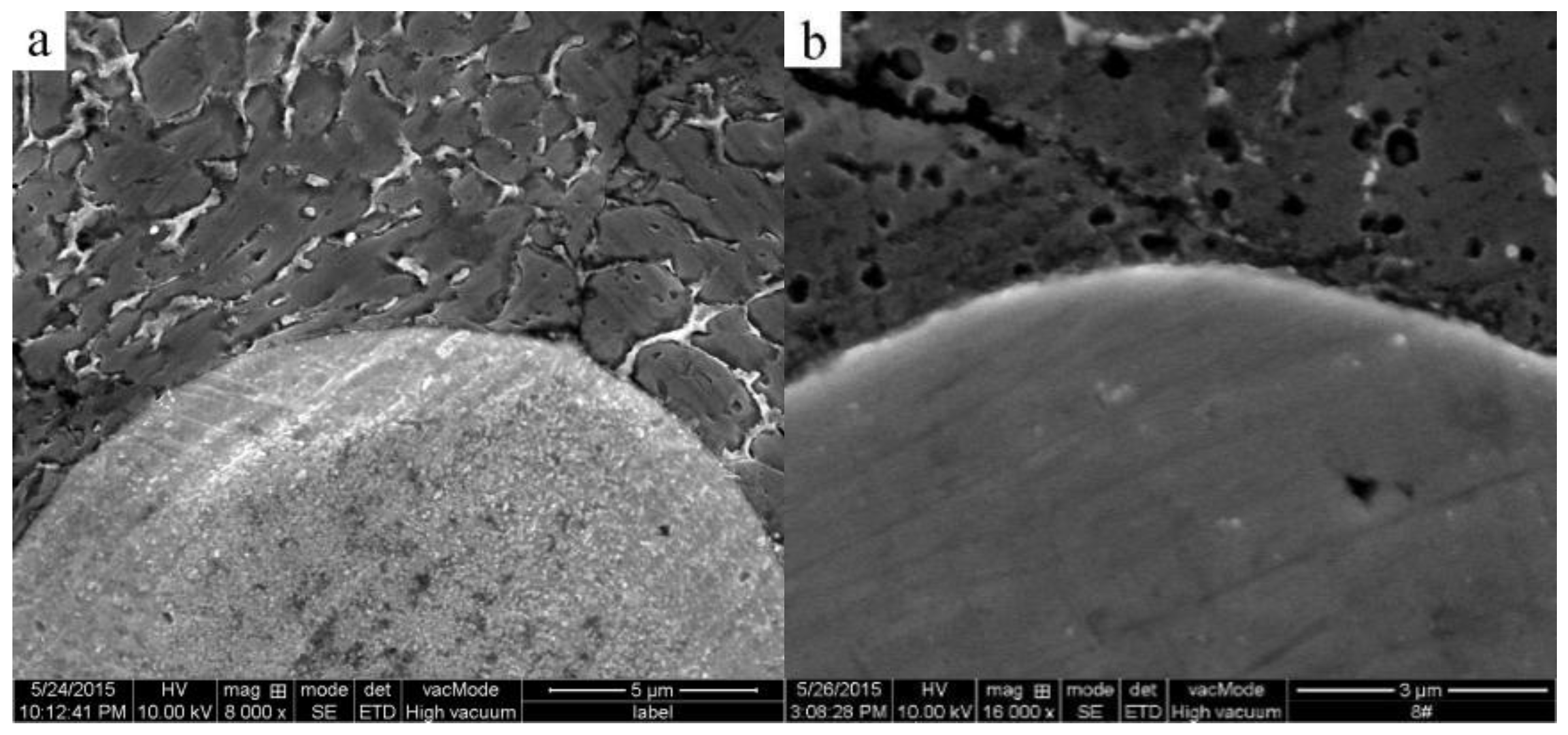
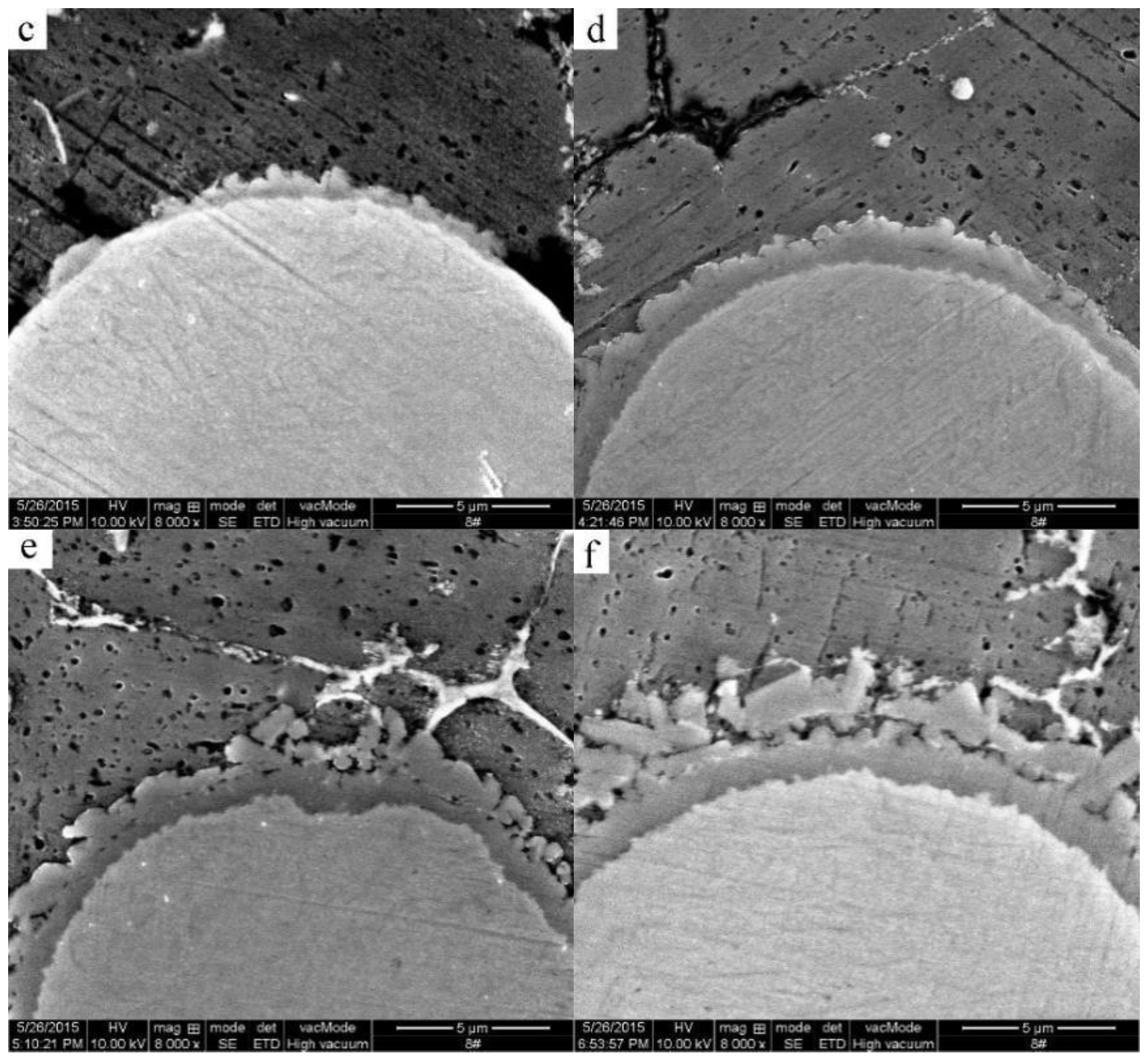
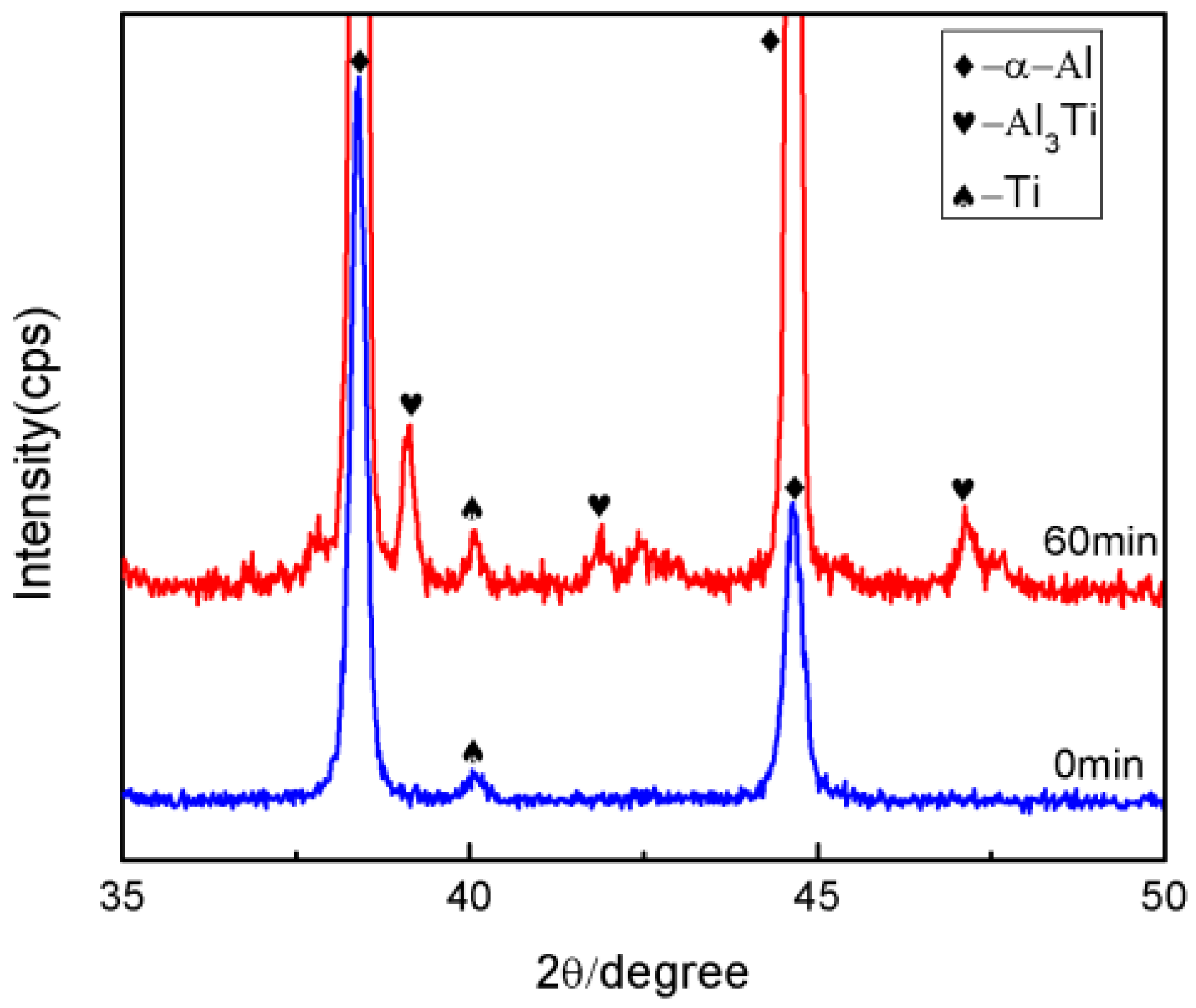
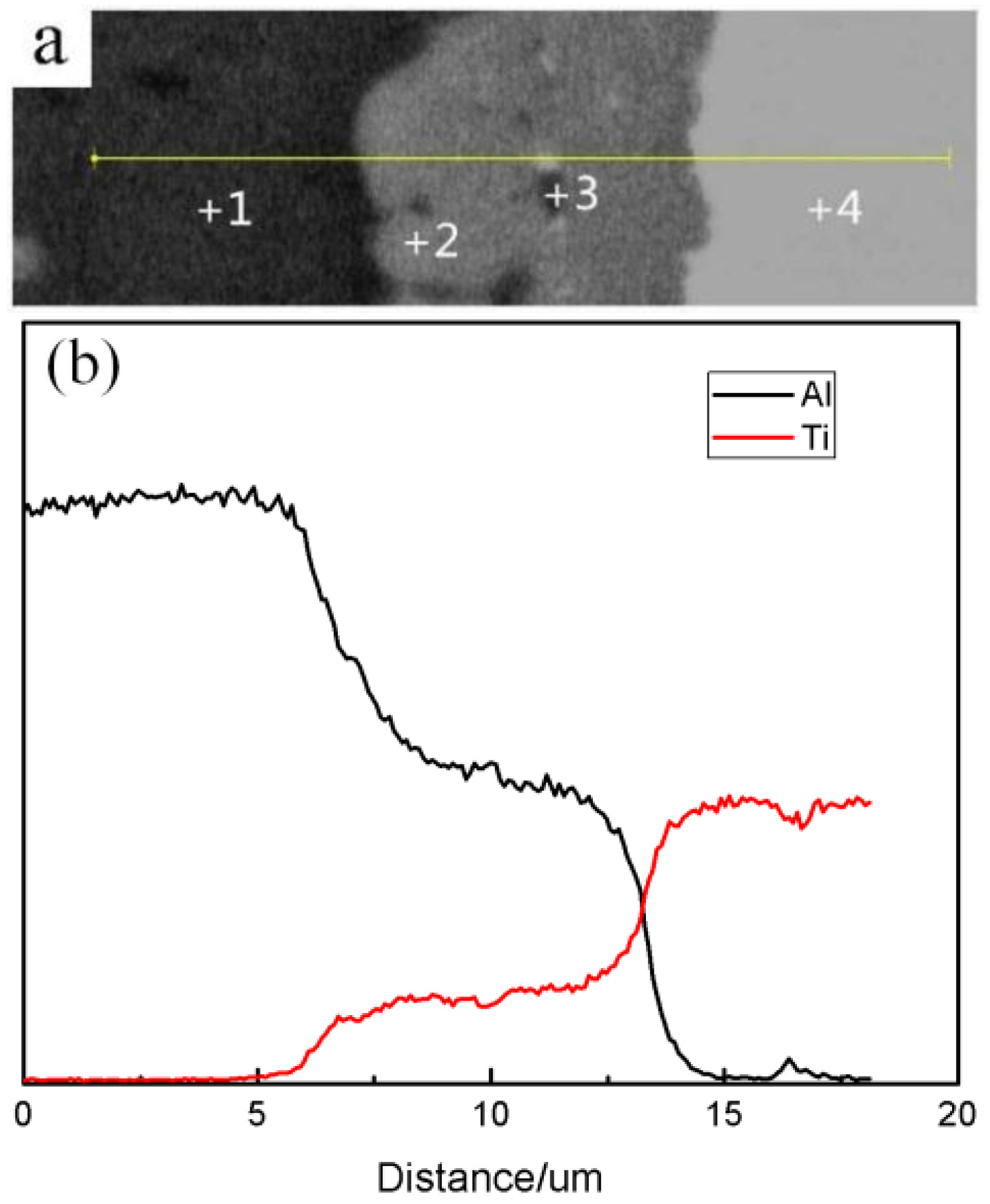
3.3. Formation of the Al3Ti Particles
3.4. Analysis of the Simulation Experiment Results for the Formation of the Al3Ti Phase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clyne, T.W.; Withers, P.J. An Introduction to Metal Matrix Composites; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Chawla, N.; Shen, Y.L. Mechanical behavior of particle reinforced metal matrix composites. Mater. Adv. Eng. 2001, 3, 357–370. [Google Scholar] [CrossRef]
- Torralba, J.M.; da Costa, C.E.; Velasco, F. P/M aluminum matrix composites: An overview. Mater. Process. Technol. 2003, 133, 203–206. [Google Scholar] [CrossRef]
- Wang, X.M.; Jha, A.; Brydson, R. In situ fabrication of Al3Ti particle reinforced aluminium alloy metal-matrix composites. Mater. Sci. Eng. A 2004, 364, 339–345. [Google Scholar] [CrossRef]
- Torralba, J.M.; Velasco, F.; Costa, C.E.; Vergara, I.; Cáceres, D. Mechanical behaviour of the interphase between matrix and reinforcement of Al 2014 matrix composites reinforced with (Ni3Al)p. Compos. A Appl. Sci. Manuf. 2002, 33, 427–434. [Google Scholar] [CrossRef]
- Lee, J.M.; Kang, S.B.; Sato, T.; Tezuka, H.; Kamio, A. Microstructures and mechanical properties of Al3Fe reinforced aluminum matrix composites fabricated by a plasma synthesis method. Mater. Trans. 2002, 43, 2487–2493. [Google Scholar] [CrossRef]
- Adamiak, M.; Fogagnolo, J.; Ruiz-Navas, E.; Dobrzañski, L.; Torralba, J. Mechanically milled AA6061/(Ti3Al) p MMC reinforced with intermetallics—The structure and properties. Mater. Process. Technol. 2004, 155, 2002–2006. [Google Scholar] [CrossRef]
- Reinhart, T.J.; Clements, L.L. Engineered Materials Handbook; ASM International: Detroit, MI, USA, 1993. [Google Scholar]
- Smith, C. ASM Handbook: Composites; ASM International: Detroit, MI, USA, 2001. [Google Scholar]
- Tjong, S.C.; Ma, Z. High temperature creep behavior of in-situ TiB2 particulate reinforced copper-based composite. Mater. Sci. Eng. 2000, 29, 49–113. [Google Scholar] [CrossRef]
- Abbasi Chianeh, V.; Madaah Hosseini, H.R.; Nofar, M. Microstructural features and mechanical properties of Al-Al3Ti composite fabricated by in-situ powder metallurgy route. J. Alloys Compd. 2009, 473, 127–132. [Google Scholar] [CrossRef]
- Nofar, M.; Madaah Hosseini, H.R.; Kolagar-Daroonkolaie, N. Fabrication of high wear resistant Al/Al3Ti metal matrix composite by in situ hot press method. Mater. Des. 2009, 30, 280–286. [Google Scholar] [CrossRef]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Ogel, B.; Gurbuz, R. Microstructural characterization and tensile properties of hot pressed Al-SiC composites prepared from pure Al and Cu powders. Mater. Sci. Eng. A 2001, 301, 213–220. [Google Scholar] [CrossRef]
- Zhao, N.Q.; Nash, P.; Yang, X.J. The effect of mechanical alloying on SiC distribution and the properties of 6061 Aluminum composite. J. Mater. Process. Technol. 2005, 170, 586–592. [Google Scholar] [CrossRef]
- Fan, Z. Semisolid metal processing. Int. Mater. Rev. 2002, 47, 49–85. [Google Scholar] [CrossRef]
- Chen, Y.S.; Chen, T.J.; Fu, W.; Li, P.B. Microstructural evolution during partial remelting of 6061 aluminum bulk alloy prepared by cold-pressing of alloy powder. Adv. Mater. Res. 2013, 820, 20–24. [Google Scholar] [CrossRef]
- Li, P.; Chen, T.; Zhang, S.; Guan, R. Research on semisolid microstructural evolution of 2024 Aluminum alloy prepared by powder thixoforming. Metals 2015, 5, 547–564. [Google Scholar] [CrossRef]
- Chen, T.J.; Jiang, X.D.; Ma, Y.; Li, Y.D.; Hao, Y. Microstructural evolution and phase transformations during partial remelting of AZ91D magnesium alloy refined by SiC. J. Alloys Compd. 2010, 497, 147–154. [Google Scholar] [CrossRef]
- Chen, T.J.; Jiang, X.D.; Ma, Y.; Li, Y.D.; Hao, Y. Effect of initial as-cast microstructure of AZ91D magnesium alloy on its semisolid microstructure. J. Alloys Compd. 2010, 505, 476–482. [Google Scholar] [CrossRef]
- Thuillard, M.; Tran, L.T.; Nicolet, M.A. Thermal reaction of Al/Ti bilayers with contaminated interface. Thin Solid Films 1989, 65, 2553–2556. [Google Scholar] [CrossRef]
- Bergner, D.; Hutte, N. Methods for determination of effective diffusion coefficients in ternary alloys (i). Direct measurement of ternary diffusion matrix. Crys. Res. Technol. 1985, 20, 1283–1300. [Google Scholar]
- Fu, E.K.Y.; Rawlings, R.D.; Mcshane, H.B. Reaction synthesis of titanium aluminides. J. Mater. Sci. 2001, 36, 5537–5542. [Google Scholar] [CrossRef]
- Peng, L.M.; Wang, J.H.; Li, H.; Zhao, J.H.; He, L.H. Synthesis and microstructural characterization of Ti-Al3Ti metal-intermetallic laminate (MIL) composites. Scr. Mater. 2005, 52, 243–248. [Google Scholar] [CrossRef]
- Gardner, D.S.; Flinn, P.A. Mechanical stress as a function of temperature for aluminum alloy films. J. Appl. Phys. 1990, 67, 1831–1844. [Google Scholar] [CrossRef]
- Qian, J.H.; Qi, X.Z. Application of TiAl (ɣ)-based titanium alloy. Rare Met. 2002, 26, 477–482. (In Chinese) [Google Scholar]
- Werst, R.C. Handbook of Chemistry and Physics; CRC: Boca Raton, FL, USA, 1984. [Google Scholar]
- Bolz, R.E.; Tuve, G.L. Handbook of Tables for Applied Engineering Science; CRC: Boca Raton, FL, USA, 1970. [Google Scholar]
- McClune, W.F. Inorganic Materials Powder Diffraction File (Joint Committee on Powder Diffraction Standards); ASTM: Philadelphia, PA, USA, 1979. [Google Scholar]
- Milmana, Y.V.; Miracleb, D.B.; Chugunovaa, S.I.; Voskoboinika, I.V.; Korzhovaa, N.P.; Legkayaa, T.N.; Podrezova, Y.N. Mechanical behaviour of Al3Ti intermetallic and L12 phases on its basis. Intermetallics 2001, 9, 839–845. [Google Scholar] [CrossRef]
- Okamoto, H. Al-Ti (aluminum-titanium). Phase Equil. 1993, 14, 120–121. [Google Scholar] [CrossRef]
- Shangguan, D. Lead-Free Solder Interconnect Reliability; ASM International: Detroit, MI, USA, 2005. [Google Scholar]
- Shen, J.; Zhao, M.; He, P.; Pu, Y. Growth behaviors of intermetallic compounds at Sn-3Ag-0.5Cu/Cu interface during isothermal and non-isothermal aging. J. Alloys Compd. 2013, 574, 451–458. [Google Scholar] [CrossRef]
- Laurila, T.; Vuorinen, V.; Kivilahti, J.K. Interfacial reactions between lead-free solders and common base materials. Mater. Sci. Eng. R Rep. 2005, 49, 1–60. [Google Scholar] [CrossRef]










| Heating Time/min | Structure | Composition (wt.%) | ||
|---|---|---|---|---|
| Al | Cu | Mg | ||
| 0 | Pure aluminum | 100 | 0 | 0 |
| 2024 aluminum | 90.2 | 8.3 | 1.5 | |
| 5 | Pure aluminum | 97.4 | 2.1 | 0.5 |
| 2024 aluminum | 94.1 | 4.8 | 1.1 | |
| 10 | Pure aluminum | 94.3 | 4.8 | 0.8 |
| 2024 aluminum | 94.2 | 5.0 | 0.8 | |
| Material | Molar Volume | Elastic Modulus |
|---|---|---|
| Al | 9.995 | 1.143 |
| Ti | 10.629 | 1.699 |
| TiAl3 | 38.408 | 3.086 |
| Point | Composition (wt.%) | |||
|---|---|---|---|---|
| Al | Ti | Mg | Cu | |
| 1 | 63.0 | 35.6 | 0.5 | 0 |
| 2 | 75.2 | 24.6 | 0.2 | 0 |
| 3 | 88.3 | 11.3 | 0.4 | 0 |
| 4 | 92.1 | 7.8 | 0.1 | 0 |
| Point | Composition (wt.%) | Phase | |
|---|---|---|---|
| Al | Ti | ||
| 1 | 98.6 | 0 | Al |
| 2 | 75.2 | 24.6 | Al3Ti |
| 3 | 92.8 | 5.8 | (Al) |
| 4 | 0 | 99.4 | Ti |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Chen, T.; Wang, Y.; Zhang, X.; Li, P. Semisolid Microstructural Evolution during Partial Remelting of a Bulk Alloy Prepared by Cold Pressing of the Ti-Al-2024Al Powder Mixture. Materials 2016, 9, 199. https://doi.org/10.3390/ma9030199
Qin Y, Chen T, Wang Y, Zhang X, Li P. Semisolid Microstructural Evolution during Partial Remelting of a Bulk Alloy Prepared by Cold Pressing of the Ti-Al-2024Al Powder Mixture. Materials. 2016; 9(3):199. https://doi.org/10.3390/ma9030199
Chicago/Turabian StyleQin, Yahong, Tijun Chen, Yingjun Wang, Xuezheng Zhang, and Pubo Li. 2016. "Semisolid Microstructural Evolution during Partial Remelting of a Bulk Alloy Prepared by Cold Pressing of the Ti-Al-2024Al Powder Mixture" Materials 9, no. 3: 199. https://doi.org/10.3390/ma9030199