Spatial Frequency Responses of Anisotropic Refractive Index Gratings Formed in Holographic Polymer Dispersed Liquid Crystals
Abstract
:1. Introduction
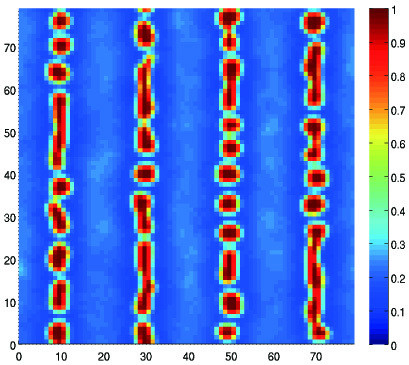
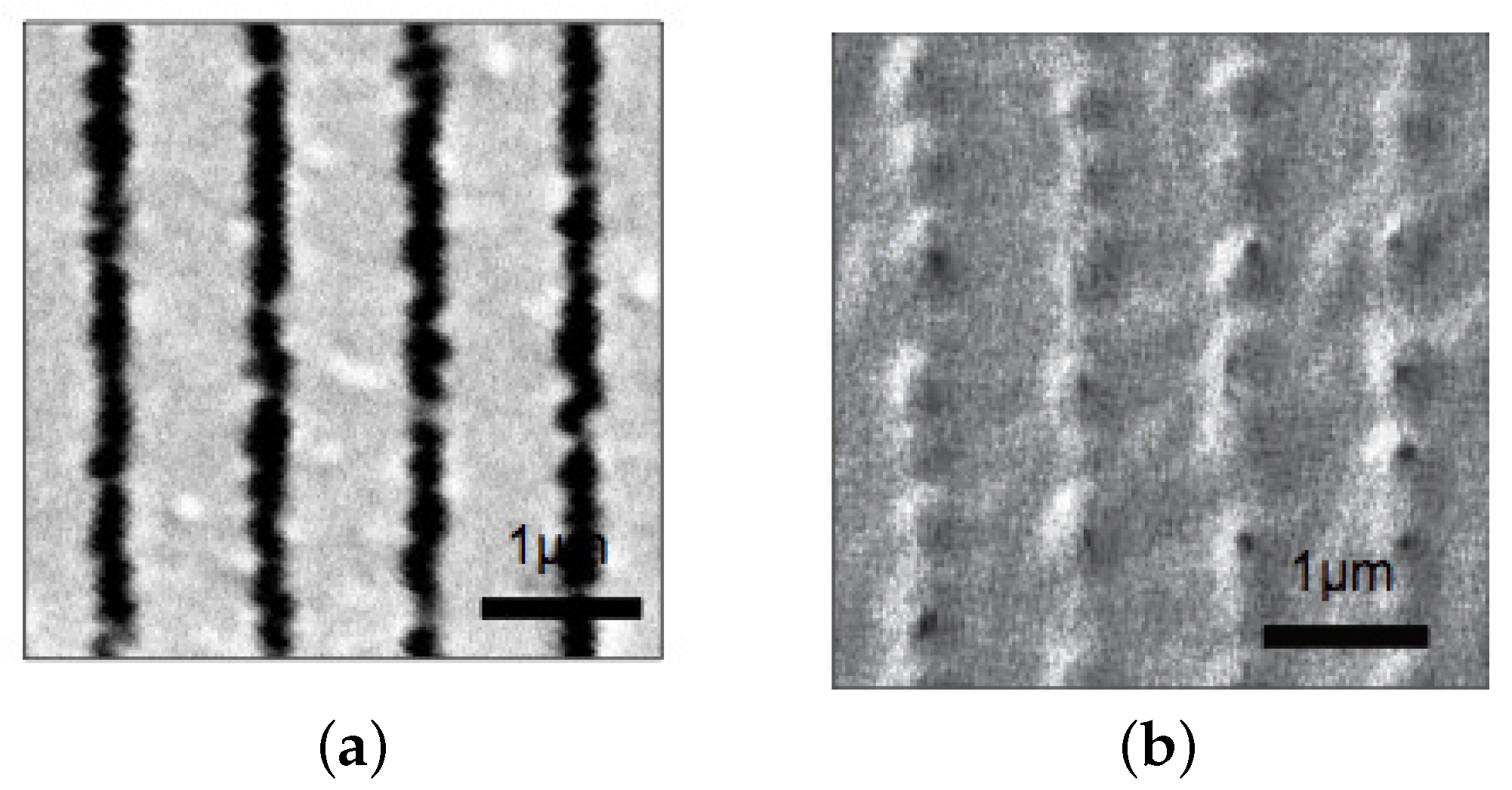
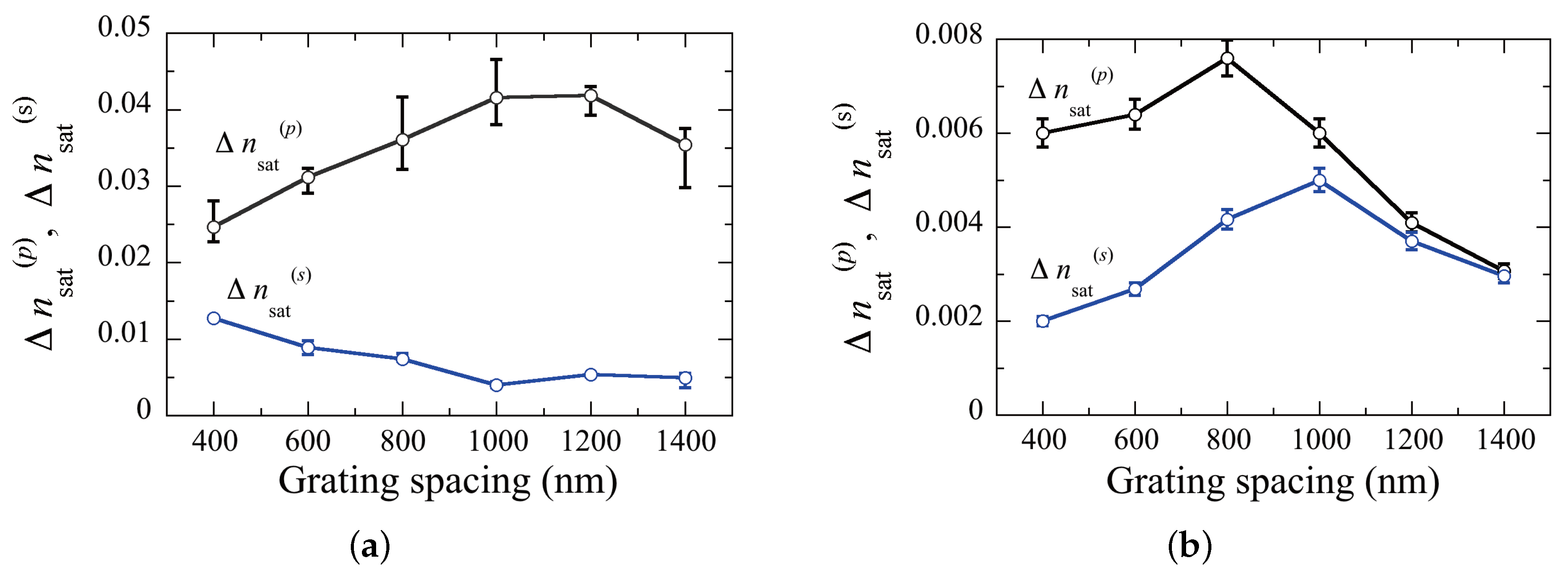
2. Experimental Results and Discussion
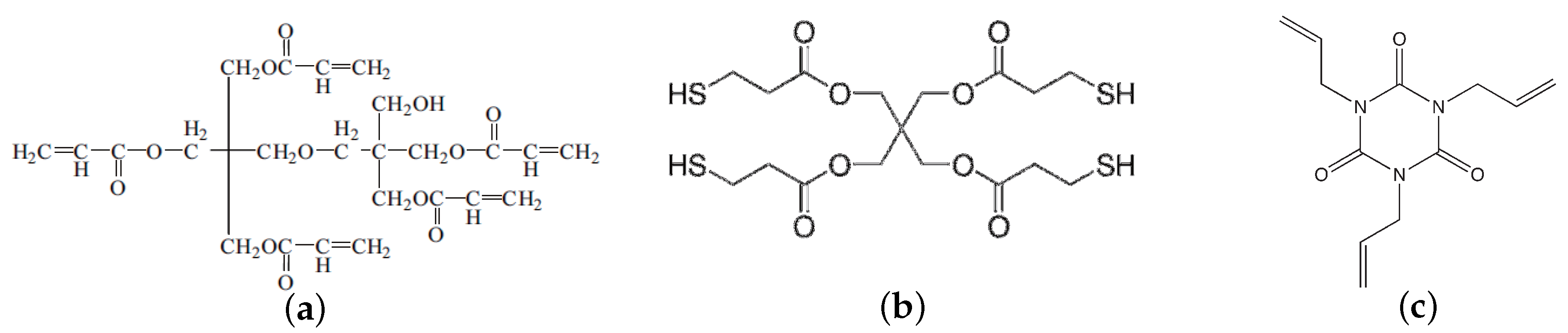
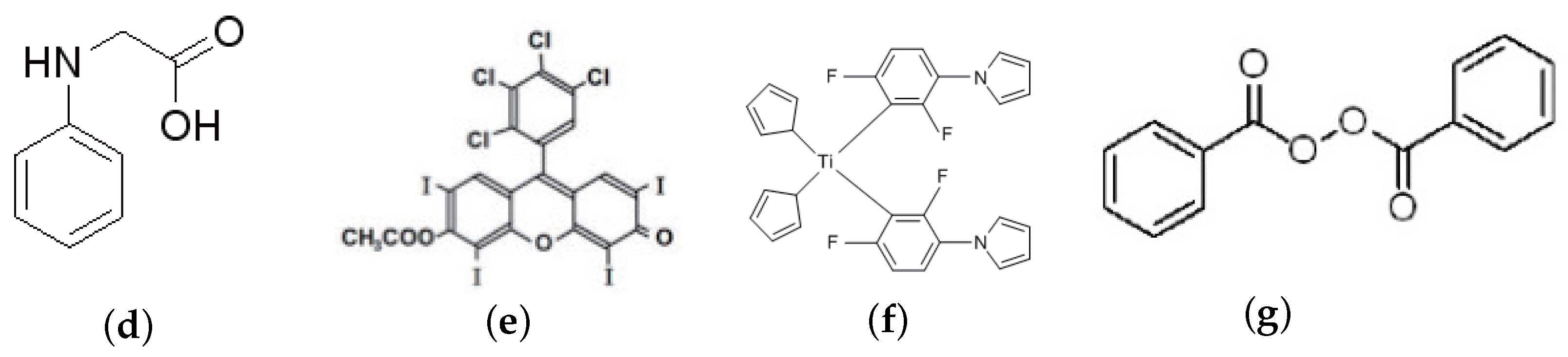
2.1. Materials
2.2. Photopolymerization Kinetics
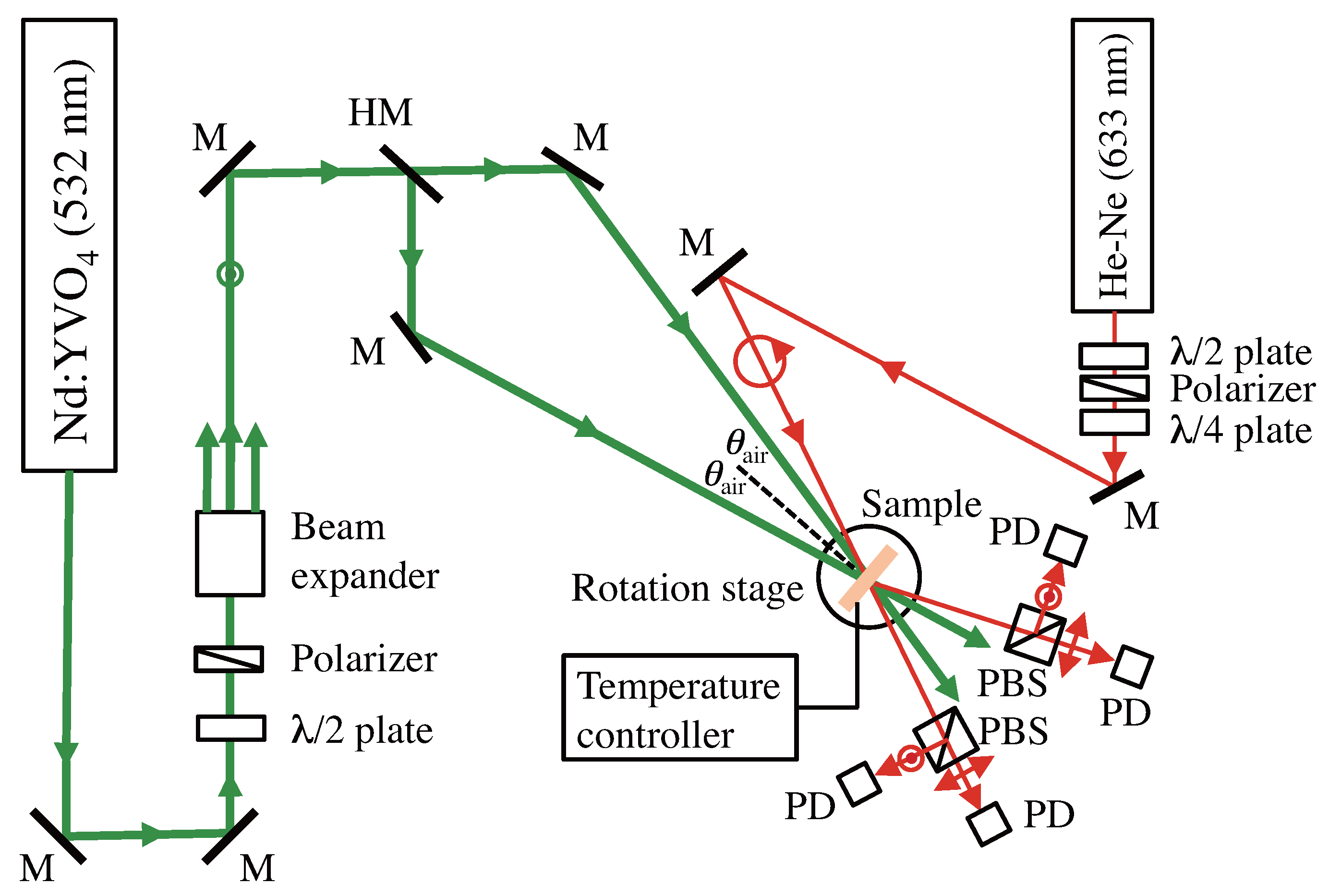
2.3. Holographic Recording
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
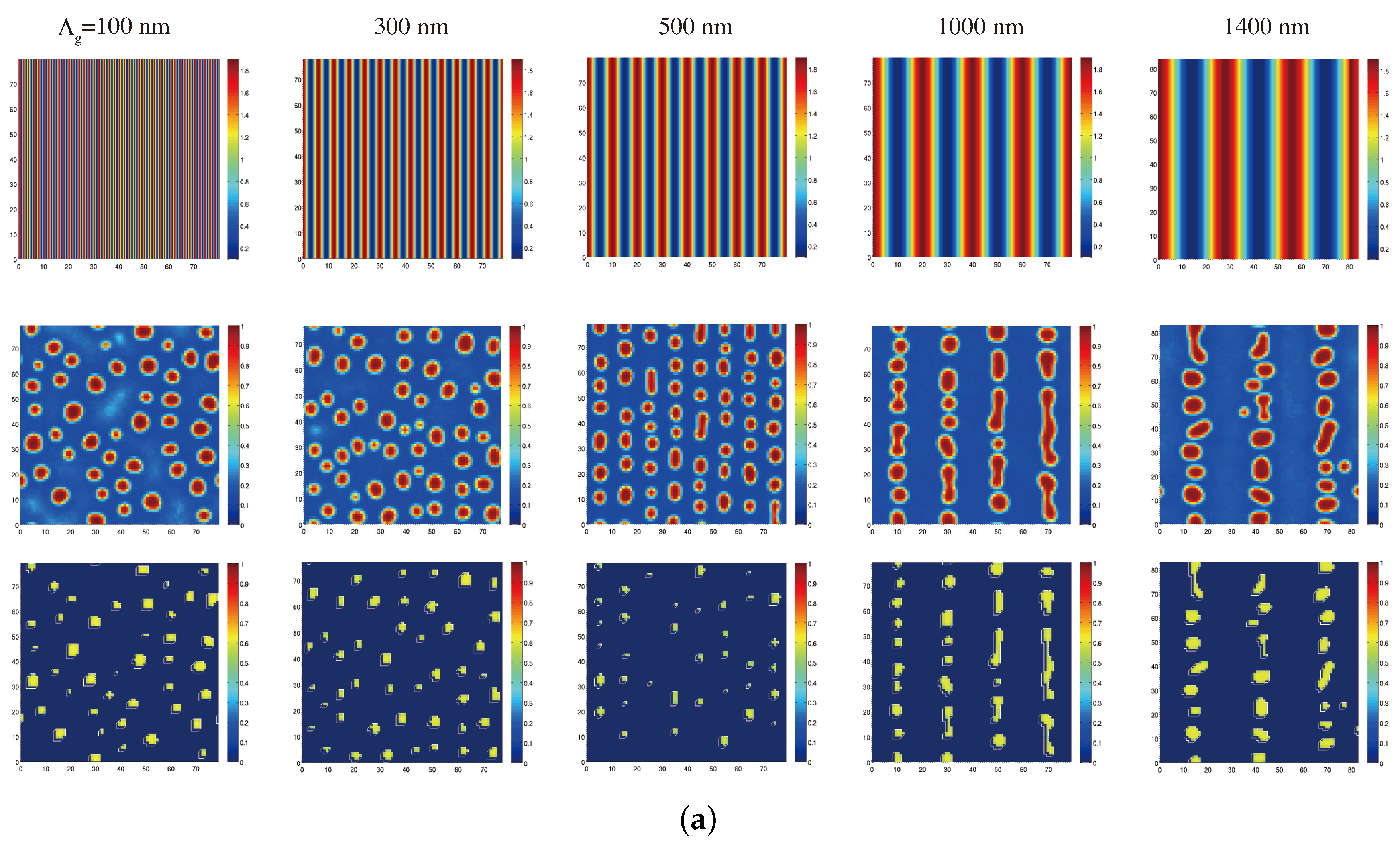
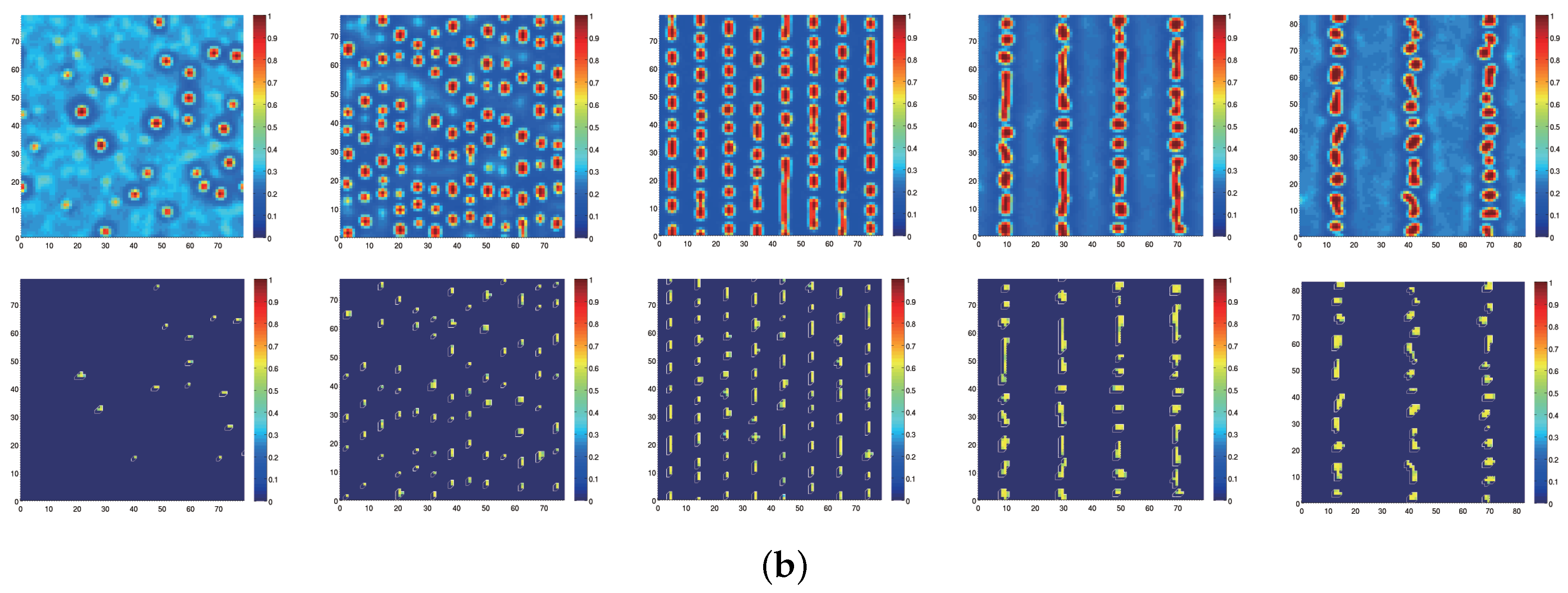
Appendix A : Theoretical Formulation for Statistical Thermodynamic Simulations
References
- Chen, R.H. Liquid Crystal Displays: Fundamental Physics and Technology; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Yang, D.K.; Wu, S.T. Fundamentals of Liquid Crystal Devices; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Coles, H.; Morris, S. Liquid-crystal lasers. Nat. Photonics 2010, 4, 676–685. [Google Scholar] [CrossRef]
- Sutherland, R.L.; Natarajan, L.V.; Tondiglia, V.P. Bragg gratings in an acrylate polymer consisting of periodic polymer-dispersed liquid-crystal planes. Chem. Mater. 1993, 5, 1553–1538. [Google Scholar] [CrossRef]
- Bunning, T.J.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L. Holographic polymer-dispersed liquid crystals (H-PDLCs). Annu. Rev. Mater. Sci. 2000, 30, 83–115. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, X.W. Holographic polymer-dispersed liquid crystals: Materials, formation, and applications. Adv. Optoelectron. 2008, 2008. [Google Scholar] [CrossRef]
- Crawford, G.P. Electrically switchable Bragg gratings. Opt. Photonics News 2003, 14, 54–59. [Google Scholar] [CrossRef]
- Sutherland, R.L.; Tondiglia, V.P.; Natarajan, L.V.; Bunning, T.J. Evolution of anisotropic reflection gratings formed in holographic polymer dispersed liquid crystals. Appl. Phys. Lett. 2001, 79, 1420–1422. [Google Scholar] [CrossRef]
- Want, K.; Zheng, J.; Gao, H.; Lu, F.; Sun, L.; Yin, S.; Zhuang, S. Tri-color composite volume H-PDLC grating and its application to 3D color autostereoscopic display. Opt. Express 2015, 23, 31436–31445. [Google Scholar]
- Shriyan, S.K.; Schundler, E.; Schwarze, C.; Fontecchio, A.K. Electro-optic polymer liquid crystal thin films for hyperspectral imaging. J. Appl. Remote Sens. 2012, 6. [Google Scholar] [CrossRef]
- Riquelme, M.; Ortuño, M.; Márquez, A.; Gallego, S.; Pascual, I.; Beléndez, A. A dynamic beam splitter using polymer dispersed liquid crystals materials. In Proceedings of the SPIE 8498: Optics and Photonics for Information Processing VI, San Diego, CA, USA, 15–16 August 2012; Volume 8498.
- Rai, K.; Fontecchio, A.K. Optimization of pressure response in HPDLC gratings based on polymer composition. Mol. Cryst. Liquid Cryst. 2006, 450, 183–190. [Google Scholar] [CrossRef]
- Lucchetta, D.E.; Vita, F.; Simoni, F. All-optical switching of diffraction gratings infiltrated with dye-doped liquid crystals. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- Criante, L.; Lucchetta, D.E.; Vita, F.; Castagna, R.; Simoni, F. Distributed feedback all-organic microlaser based on holographic polymer dispersed liquid crystals. Appl. Phys. Lett. 2009, 94. [Google Scholar] [CrossRef]
- Ogiwara, A.; Watanabe, M.; Moriwaki, R. Formation of temperature dependable holographic memory using holographic polymer-dispersed liquid crystal. Opt. Lett. 2013, 38, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Tondiglia, V.P.; Natarajan, L.V.; Sutherland, R.L.; Tomlin, D.; Bunning, T.J. Holographic formation of electro-optical polymer-liquid crystal photonic crystals. Adv. Mater. 2002, 14, 187–191. [Google Scholar] [CrossRef]
- Jakubiak, R.; Bunning, T.J.; Vaia, R.A.; Natarajan, L.V.; Tondiglia, V.P. Electrically switchable, one-dimensional polymeric resonators from holographic photopolymerization: A new approach for active photonic bandgap materials. Adv. Mater. 2003, 15, 241–244. [Google Scholar] [CrossRef]
- Escuti, M.J.; Qi, J.; Crawford, G.P. Tunable face-centered-cubic photonic crystal formed in holographic polymer dispersed liquid crystals. Opt. Lett. 2003, 28, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Escuti, M.J.; Crawford, G.P. Mesoscale three dimensional lattices formed in polymer dispersed liquid crystals: A diamond-like face centered cubic. Mol. Cryst. Liquid Cryst. 2004, 421, 23–36. [Google Scholar] [CrossRef]
- Gorkhali, S.P.; Qi, J.; Crawford, G.P. Switchable quasi-crystal structures with five-, seven-, and ninefold symmetries. J. Opt. Soc. Am. B 2006, 23, 149–158. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, X.W. Electrically tunable two-dimensional holographic photonic crystal fabricated by a single diffractive element. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Liu, Y.J.; Sun, X.W. Electrically tunable three-dimensional holographic photonic crystal made of polymer-dispersed liquid crystals using a single prism. Jpn. J. Appl. Phys. 2007, 46, 6634–6638. [Google Scholar] [CrossRef]
- Sun, X.H.; Tao, X.M.; Ye, T.J.; Xue, P.; Szeto, Y.S. Optics design and fabrication of 3D electrically switchable hexagonal photonic crystal. Appl. Phys. B 2007, 87, 65–69. [Google Scholar] [CrossRef]
- Miki, M.; Ohira, R.; Tomita, Y. Optical properties of electrically tunable two-dimensional photonic lattice structures formed in a holographic polymer-dispersed liquid crystal film: Analysis and experiment. Materials 2014, 7, 3677–3698. [Google Scholar] [CrossRef]
- Zhao, G.; Mouroulis, P. Diffusion model of hologram formation in dry photopolymer materials. J. Modern Opt. 1994, 41, 1929–1939. [Google Scholar] [CrossRef]
- Sheridan, J.T.; Lawrence, J.R. Nonlocal response diffusion model of holographic recording in photopolymer. J. Opt. Soc. Am. A 2000, 17, 1108–1114. [Google Scholar] [CrossRef]
- Guo, J.; Gleeson, M.R.; Liu, S.; Sheridan, J.T. Non-local spatial frequency response of photopolymer materials containing chain transfer agents: II. Experimental results. J. Opt. 2011, 13. [Google Scholar] [CrossRef]
- Caputo, R.; Sukhov, A.V.; Tabiryan, N.V.; Umeton, C.; Ushakov, R.F. Mass transfer processes induced by inhomogeneous photo-polymerisation in a multicomponent medium. Chem. Phys. 2001, 271, 323–335. [Google Scholar] [CrossRef]
- Caputo, R.; De Sio, L.; Veltri, A.; Umeton, C.P. POLICRYPS switchable holographic grating: A promising grating electro-optical pixel for high resolution display application. IEEE J. Disp. Technol. 2006, 2, 38–51. [Google Scholar] [CrossRef]
- Suzuki, N.; Tomita, Y.; Kojima, Y. Holographic recording in TiO2 nanoparticle-dispersed methacrylate photopolymer films. Appl. Phys. Lett. 2002, 81, 4121–4123. [Google Scholar] [CrossRef]
- Suzuki, N.; Tomita, Y. Silica-nanoparticle-dispersed methacrylate photopolymers with net diffraction efficiency near 100%. Appl. Opt. 2004, 43, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Caputo, R.; Umeton, C.; Sukhov, A.V. Model for the photoinduced formation of diffraction gratings in liquid-crystalline composite materials. Appl. Phys. Lett. 2004, 84, 3492–3494. [Google Scholar] [CrossRef]
- Smith, D.M.; Li, C.Y.; Bunning, T.J. Light-directed mesoscale phase separation via holographic polymerization. J. Polym. Sci. B Polym. Phys. 2014, 52, 232–250. [Google Scholar] [CrossRef]
- Van Nostrum, C.F.; Nolte, R.J.; Broer, D.J.; Fuhrman, T.; Wendorff, J.H. Photoinduced opposite diffusion of nematic and isotropic monomers during patterned photopolymerization. Chem. Mater. 1998, 10, 135–145. [Google Scholar] [CrossRef]
- Caputo, R.; Sukhov, A.V.; Tabiryan, N.V.; Umeton, C. Efficiency dynamics of diffraction gratings recorded in liquid crystalline composite materials by a UV interference pattern. Chem. Phys. 1999, 245, 463–471. [Google Scholar] [CrossRef]
- Qi, J.; Li, L.; Sarkar, M.D.; Crawford, G.P. Nonlocal photopolymerization effect in the formation of reflective holographic polymer-dispersed liquid crystals. J. App. Phys. 2004, 96, 2443–2450. [Google Scholar] [CrossRef]
- Leewis, C.M.; de Jong, A.M.; van IJzendoorn, L.J.; Broer, D.J. Reaction-diffusion model for the preparation of polymer gratings by patterned ultraviolet illumination. J. Appl. Phys. 2004, 95, 4125–4139. [Google Scholar] [CrossRef]
- Sutherland, R.L.; Tondiglia, V.P.; Natarajan, L.V.; Bunning, T.J. Phenomenological model of anisotropic volume hologram formation in liquid-crystal-photopolymer mixtures. J. Appl. Phys. 2004, 96, 951–965. [Google Scholar] [CrossRef]
- Kyu, T.; Nwabunma, D.; Chiu, H.W. Theoretical simulation of holographic polymer-dispersed liquid-crystal films via pattern photopolymerization-induced phase separation. Phys. Rev. E 2001, 63. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L.; Bunning, T.J. Holographic photopolymerization-induced phase separation in reference to the phase diagram of a mixture of photocurable monomer and nematic liquid crystal. Macromolecules 2005, 38, 4844–4854. [Google Scholar] [CrossRef]
- Yandek, G.R.; Meng, S.; Sigalov, G.M.; Kyu, T. Three-dimensional switchable polymer photonic crystals via various optical wave interference techniques. Liquid Cryst. 2006, 33, 775–788. [Google Scholar] [CrossRef]
- Kyu, T.; Meng, S.; Duran, H.; Nanjundiah, K.; Yandek, G.R. Holographic polymer-dispersed liquid crystals and polymeric photonic crystals formed by holographic photolithography. Macromol. Res. 2006, 14, 155–165. [Google Scholar] [CrossRef]
- Meng, S.; Duran, H.; Hu, J.; Kyu, T.; Natarajan, L.V.; Tondiglia, V.P.; Sutherland, R.L.; Bunning, T.J. Influence of photopolymerization reaction kinetics on diffraction efficiency of H-PDLC undergoing photopatterning reaction in mixtures of acrylic monomer/nematic liquid crystals. Macromolecules 2007, 40, 3190–3197. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Fukuka, Y.; Tomita, Y. Computer simulation of Bragg grating formation in holographic polymer-dispersed liquid crystals based on the density functional theory. In Proceedings of the SPIE Photonics Europe 2012, Optical Modelling and Design II, Brussels, Belgium, 19 April 2012.
- Odian, G. Principles of Polymerization, 4th ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Lu, H.; Carioscia, J.A.; Stansbury, J.W.; Bowman, C.N. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent. Mater. 2005, 21, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Natarajan, L.V.; Tondiglia, V.P.; Lloyd, P.F.; Bunning, T.J.; Guymon, C.A. Holographic polymer dispersed liquid crystals (HPDLCs) containing triallyl isocyanurate monomer. Polymer 2007, 48, 5979–5987. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Hoyle, C.E. The effect of thiol and ene structures on thiol-ene networks: Photopolymerization, physical, mechanical and optical properties. Polymer 2009, 50, 2237–2245. [Google Scholar] [CrossRef]
- Ellabban, M.A.; Fally, M.; Urši̇č, H.; Drevenšek-Olenik, I. Holographic scattering in photopolymer-dispersed liquid crystals. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef]
- Omura, K.; Tomita, Y. Photopolymerization kinetics and volume holographic recording in ZrO2 nanoparticle-polymer composites at 404 nm. J. Appl. Phys. 2010, 107. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Kamal, M.R. Thermoset characterization for moldability analysis. Polym. Eng. Sci. 1974, 14, 231–239. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Nwabunma, D.; Kim, K.J.; Lin, Y.; Chien, L.C.; Kyu, T. Phase diagram and photopolymerization behavior of mixtures of UV-curable multifunctional monomer and low molar mass nematic liquid crystal. Macromolecules 1998, 31, 6806–6812. [Google Scholar] [CrossRef]
- Nwabunma, D.; Chiu, H.W.; Kyu, T. Theoretical investigation on dynamics of photopolymerization-induced phase separation and morphology development in nematic liquid crystal/polymer mixtures. J. Chem. Phys. 2000, 113, 6429–6436. [Google Scholar] [CrossRef]
- Kwon, J.H.; Hwang, H.C.; Woo, K.C. Analysis of temporal behavior of beams diffracted by volume gratings formed in photopolymers. J. Opt. Soc. Am. B 1999, 16, 1651–1657. [Google Scholar] [CrossRef]
- Kelly, J.V.; O’Neill, F.T.; Sheridan, J.T. Holographic photopolymer materials: Nonlocal polymerization-driven diffusion under nonideal kinetic conditions. J. Opt. Soc. Am. B 2005, 22, 407–416. [Google Scholar] [CrossRef]
- Kyu, T.; Chiu, H.W. Morphology development during polymerization-induced phase separation in a polymer dispersed liquid crystal. Polymer 2001, 42, 9173–9185. [Google Scholar] [CrossRef]
- Hata, E.; Tomita, Y. Order-of-magnitude polymerization-shrinkage suppression of volume gratings recorded in nano particle-polymer composites. Opt. Lett. 2010, 35, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Hata, E.; Mitsube, K.; Momose, K.; Tomita, Y. Holographic nanoparticle-polymer composites based on step-growth thiol-ene photopolymerization. Opt. Mater. Express 2011, 1, 207–222. [Google Scholar] [CrossRef]
- Hata, E.; Tomita, Y. Stoichiometric thiol-to-ene ratio dependences of refractive index modulation and shrinkage of volume gratings recorded in photopolymerizable nano particle-polymer composites based on step-growth polymerization. Opt. Mater. Express 2011, 1, 1113–1120. [Google Scholar] [CrossRef]
- Fukuda, Y. Statistical Thermodynamic Analysis of Photopolymerization and Holographic Grating Formation Processes in Holographic Polymer Dispersed Liquid Crystals. Master's Thesis, University of Electro-Communications, Tokyo, Japan, 28 February 2013. [Google Scholar]
- De Sio, L.; Caputo, R.; De Luca, A.; Veltri, A.; Umeton, C.P.; Sukhov, A.V. In situ optical control and stabilization of the curing process of holographic gratings with a nematic film-polymer-slice sequence structure. Appl. Opt. 2006, 45, 3721–3727. [Google Scholar] [CrossRef] [PubMed]
- Caputo, R.; De Sio, L.; Veltri, A.; Umeton, C.P.; Sukhov, A.V. Observation of two-wave coupling during the formation of POLICRYPS diffraction gratings. Opt. Lett. 2005, 30, 1840–1842. [Google Scholar] [CrossRef] [PubMed]
- Kogelnik, H. Coupled wave theory for thick hologram gratings. Bell Syst. Tech. J. 1969, 48, 2909–2947. [Google Scholar] [CrossRef]
- Jazbinšek, M.; Drevenšek-Olenik, I.; Zgonik, M.; Fontecchio, A.K.; Crawford, G.P. Characterization of holographic polymer dispersed liquid crystal transmission gratings. J. Appl. Phys. 2001, 90, 3831–3837. [Google Scholar] [CrossRef]
- Drzaic, P.S. Liquid Crystal Dispersions; World Scientific: Singapore, Singapore, 1995. [Google Scholar]
- Natarajan, L.V.; Shepherd, C.K.; Brandelik, D.M.; Sutherland, R.L.; Chandra, S.; Tondiglia, V.P.; Tomlin, D.; Bunning, T.J. Switchable holographic polymer-dispersed liquid crystal reflection gratings based on thiol-ene photopolymerization. Chem. Mater. 2003, 15, 2477–2484. [Google Scholar] [CrossRef]
- Natarajan, L.V.; Brown, D.P.; Wofford, J.M.; Tondiglia, V.P.; Sutherland, R.L.; Lloyd, P.F.; Bunning, T.J. Holographic polymer dispersed liquid crystal reflection gratings formed by visible light initiated thiol-ene photopolymerization. Polymer 2006, 47, 4411–4420. [Google Scholar] [CrossRef]
- Tomita, Y.; Suzuki, N.; Chikama, K. Holographic manipulation of nanoparticle distribution morphology in nanoparticle-dispersed photopolymers. Opt. Lett. 2005, 30, 839–841. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Chikama, K.; Nohara, Y.; Suzuki, N.; Furushima, K.; Endoh, Y. Two-dimensional imaging of atomic distribution morphology created by holographically induced mass transfer of monomer molecules and nanoparticles in a silica-nanoparticle-dispersed photopolymer film. Opt. Lett. 2006, 31, 1402–1404. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Rey, A.D. Texture formation under phase ordering and phase separation in polymer-liquid crystal mixtures. J. Chem. Phys. 2004, 121, 9733–9743. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, T. Density functional simulation of spontaneous formation of vesicle in block copolymer solutions. J. Chem. Phys. 2007, 126, 114902–114917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, M.; Hashimoto, T. Computer-simulation of the spinodal decomposition for a polydisperse polymer mixture. Phys. Rev. E 1993, 48, R647–R650. [Google Scholar] [CrossRef]
- Doi, M.; Edwards, S.F. Theory of Polymer Dynamics; Oxford University Press: Oxford, UK, 1988. [Google Scholar]
- Flory, P.J. Principles of Polymer Chemistry; Cornel University Press: Ithaca, NY, USA, 1953. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuda, Y.; Tomita, Y. Spatial Frequency Responses of Anisotropic Refractive Index Gratings Formed in Holographic Polymer Dispersed Liquid Crystals. Materials 2016, 9, 188. https://doi.org/10.3390/ma9030188
Fukuda Y, Tomita Y. Spatial Frequency Responses of Anisotropic Refractive Index Gratings Formed in Holographic Polymer Dispersed Liquid Crystals. Materials. 2016; 9(3):188. https://doi.org/10.3390/ma9030188
Chicago/Turabian StyleFukuda, Yoshiaki, and Yasuo Tomita. 2016. "Spatial Frequency Responses of Anisotropic Refractive Index Gratings Formed in Holographic Polymer Dispersed Liquid Crystals" Materials 9, no. 3: 188. https://doi.org/10.3390/ma9030188