Imaging Water Thin Films in Ambient Conditions Using Atomic Force Microscopy
Abstract
:1. Introduction
2. Electrostatic AFM
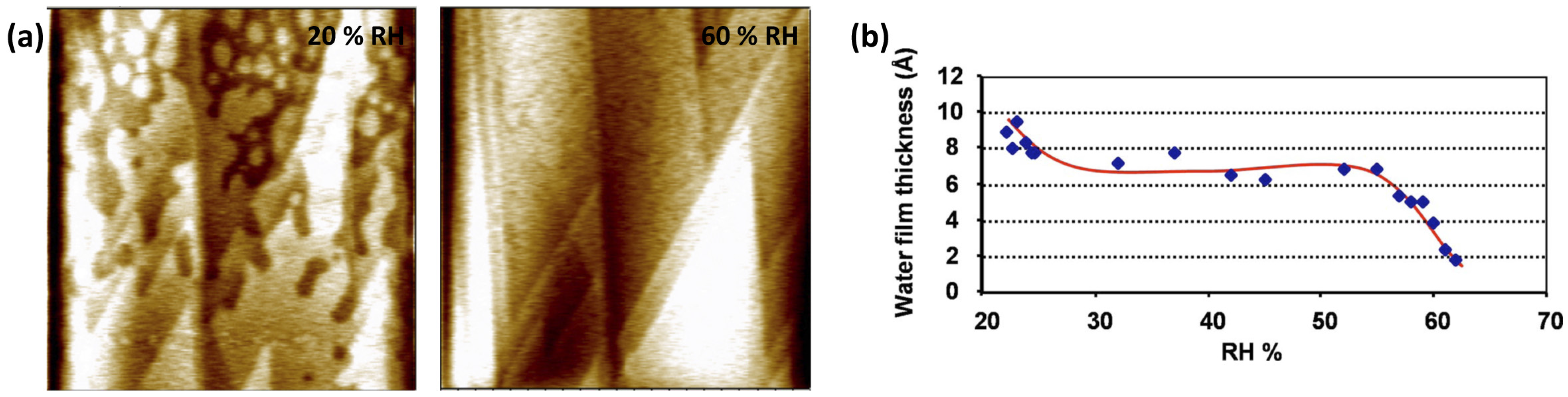
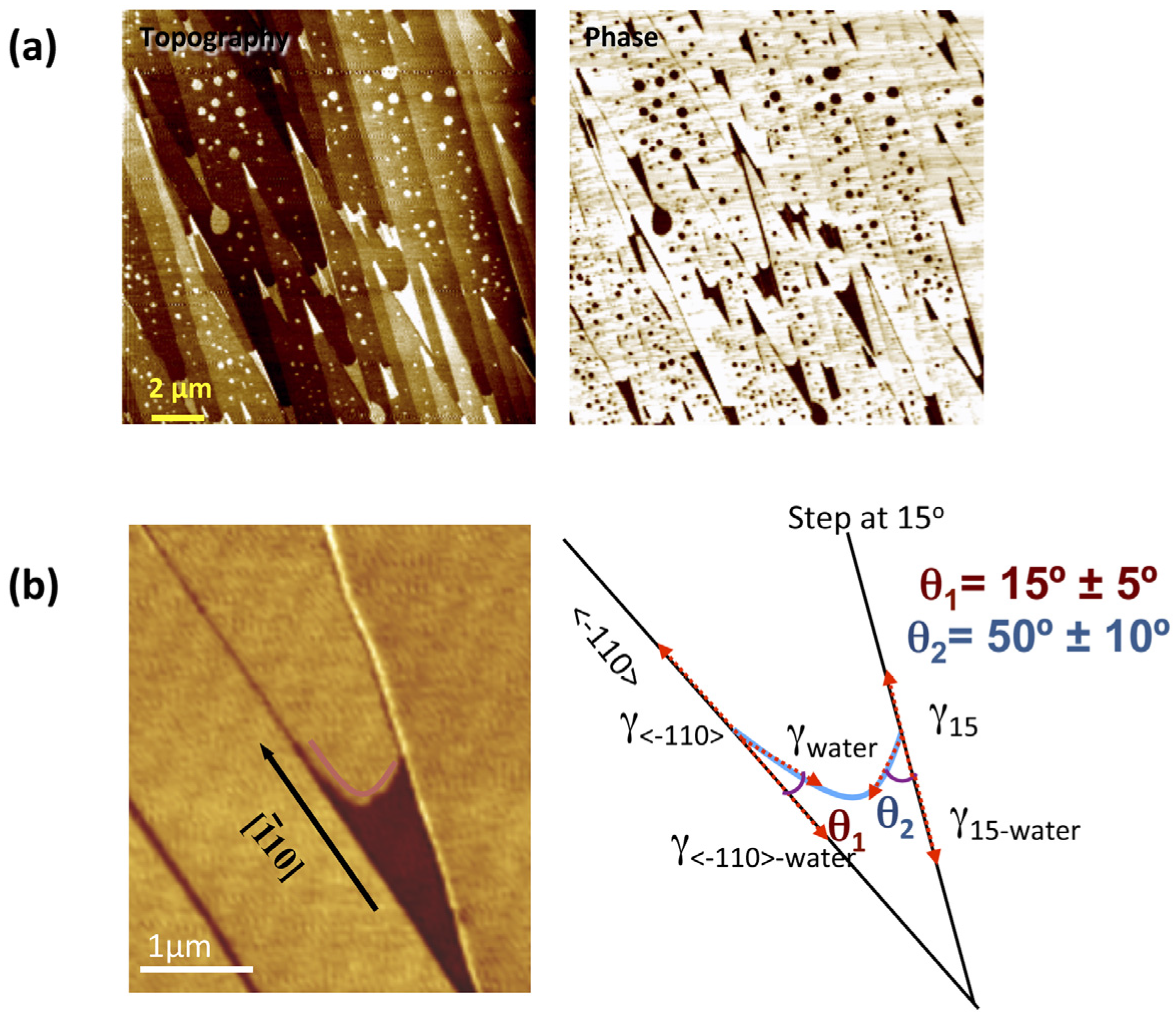
2.1. Imaging and Measuring Thickness
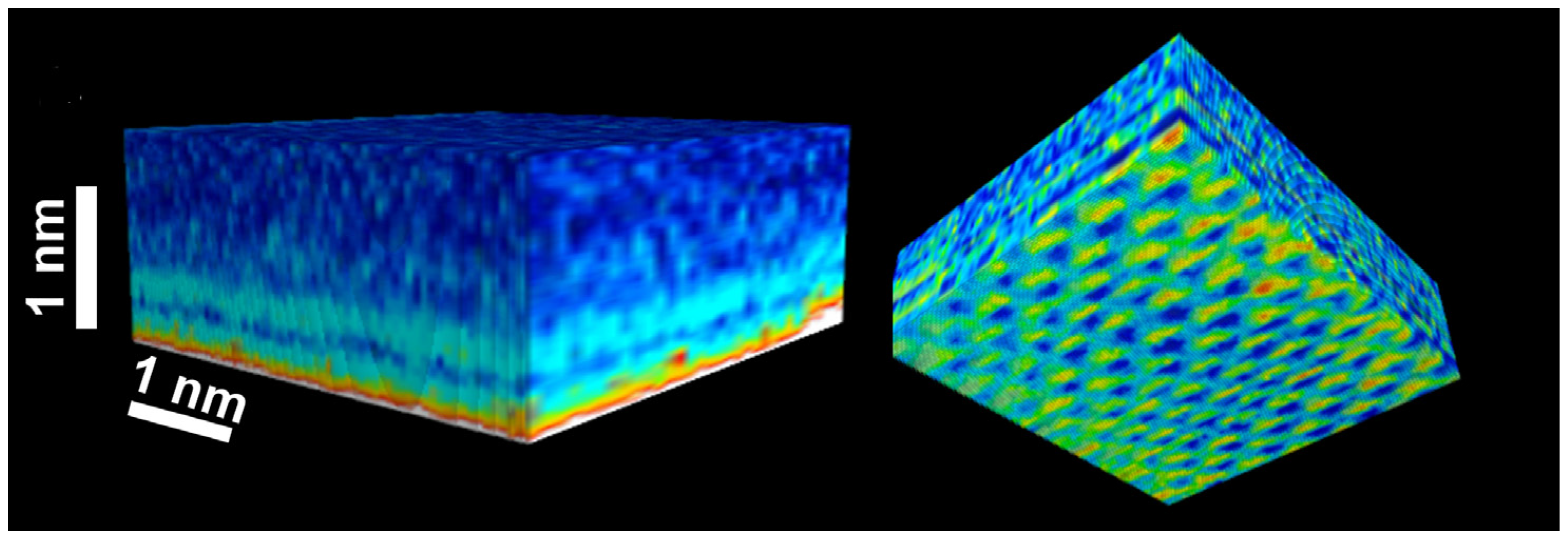
2.2. Ionic Mobility
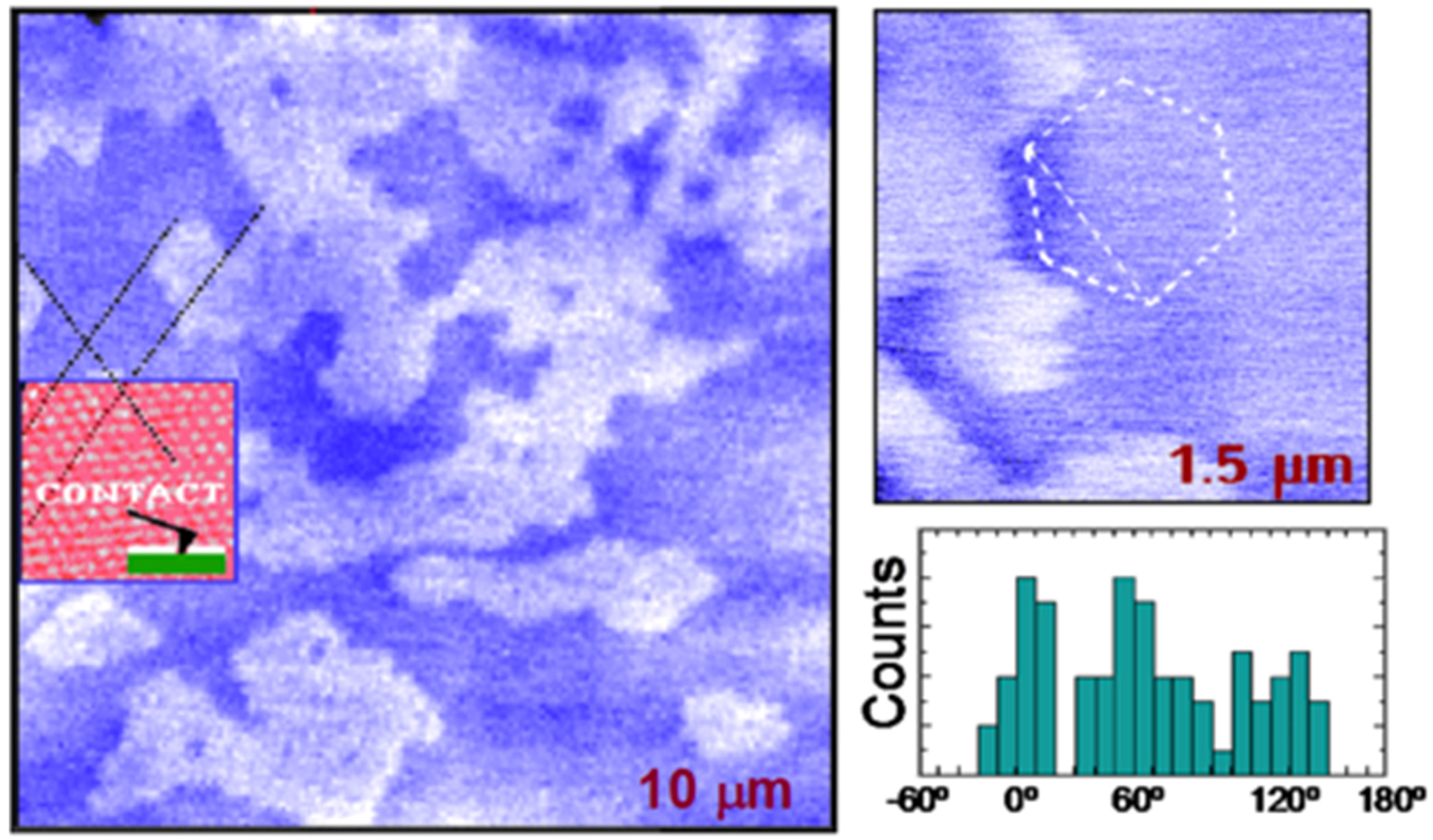
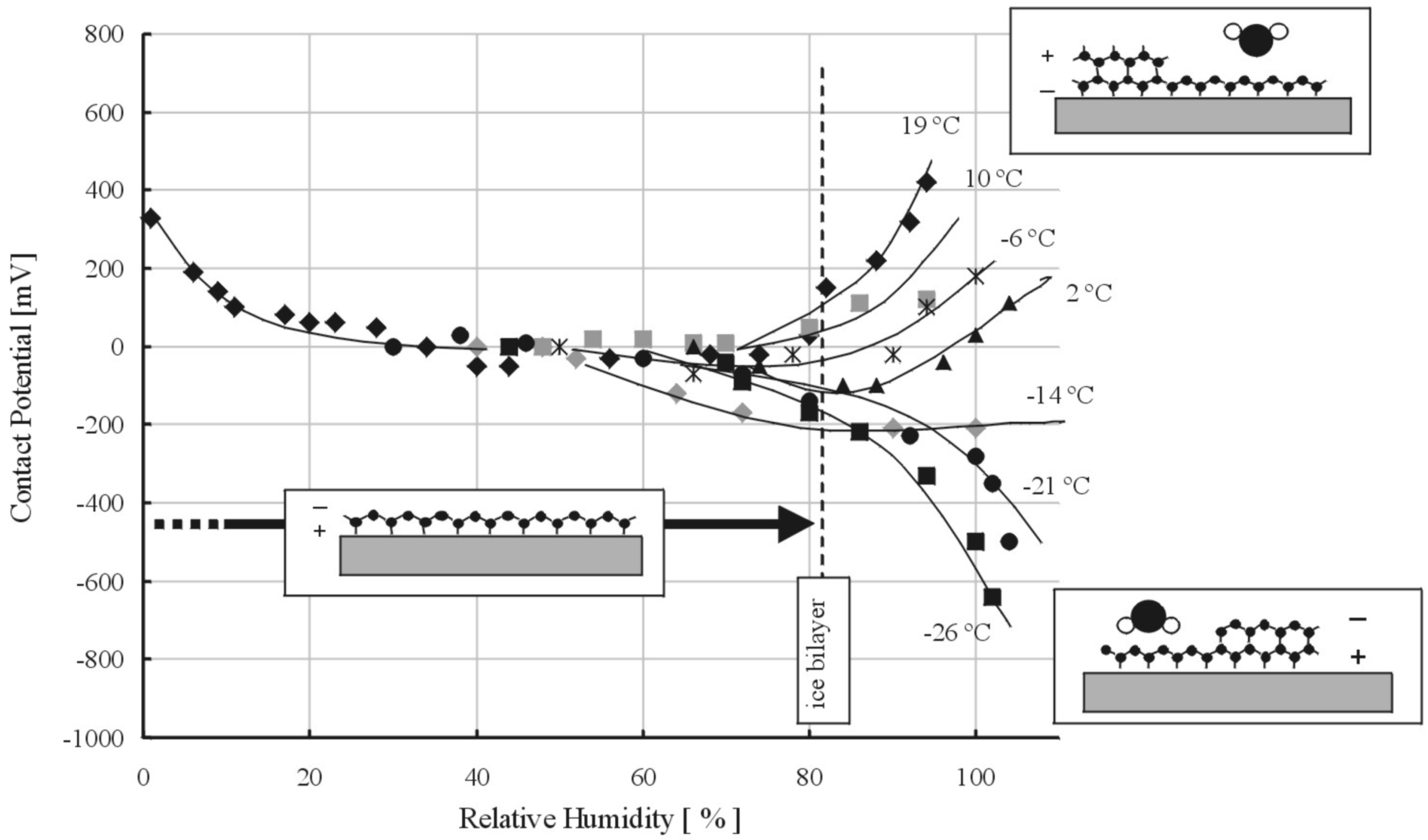
2.3. Molecular Orientation
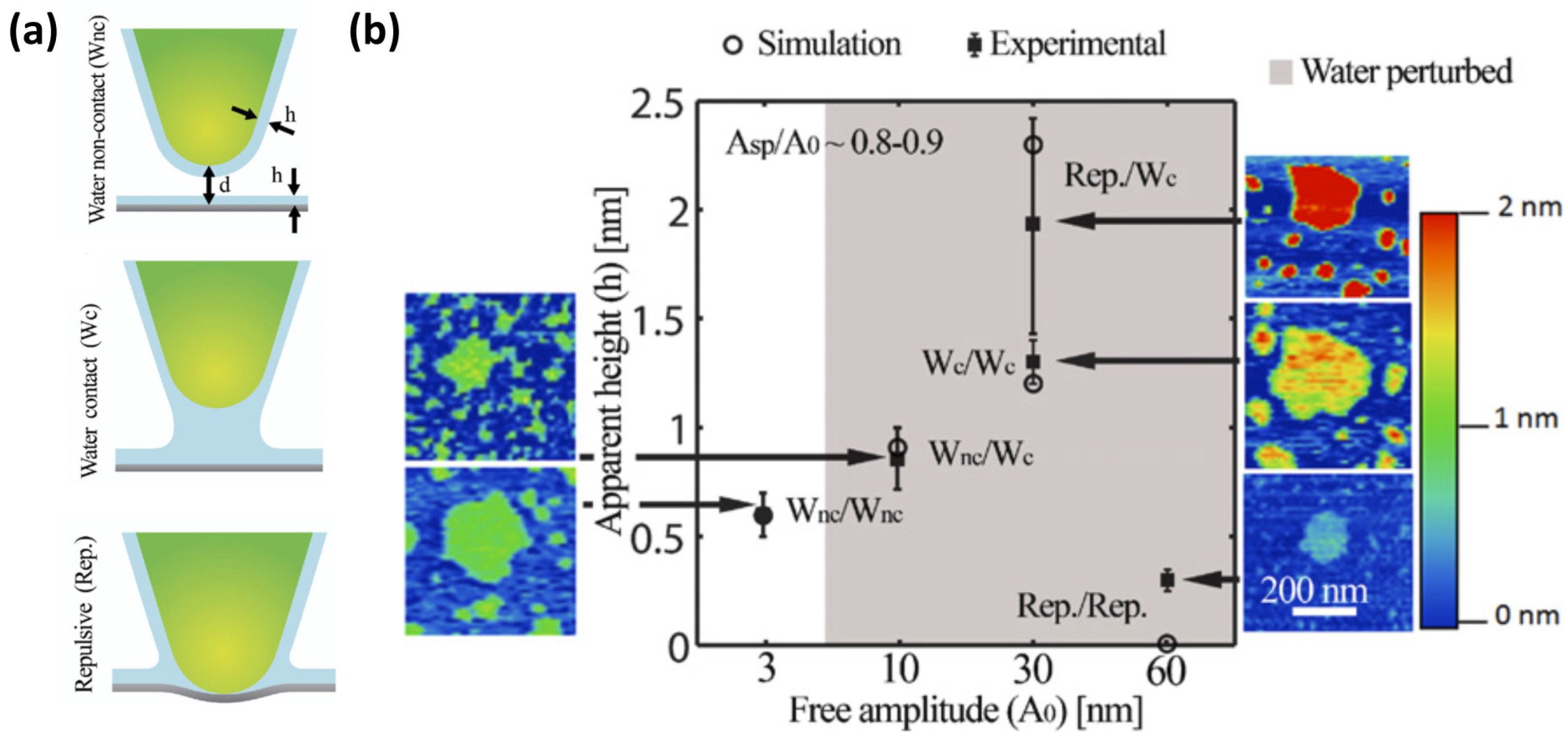
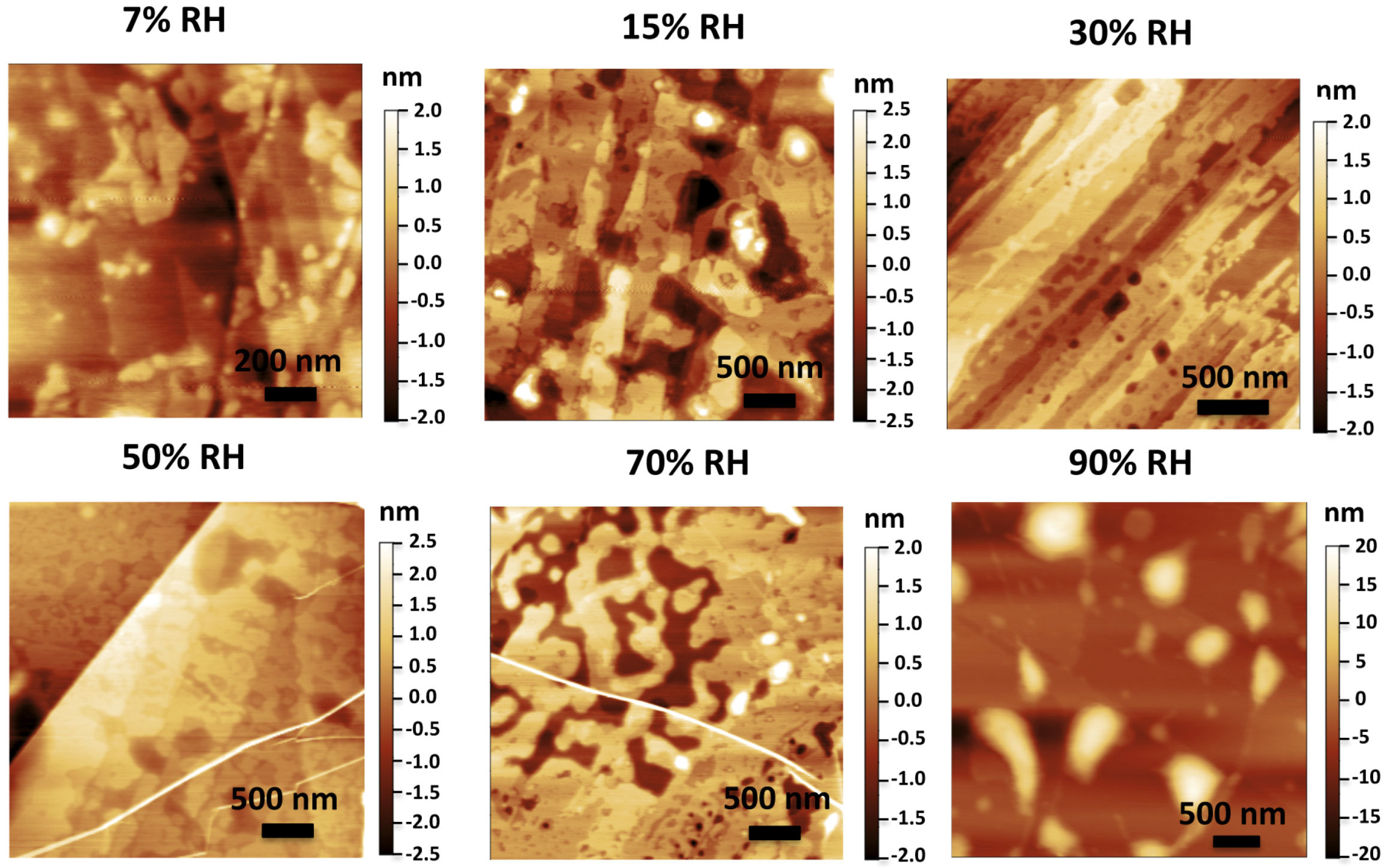
3. Dynamic AFM
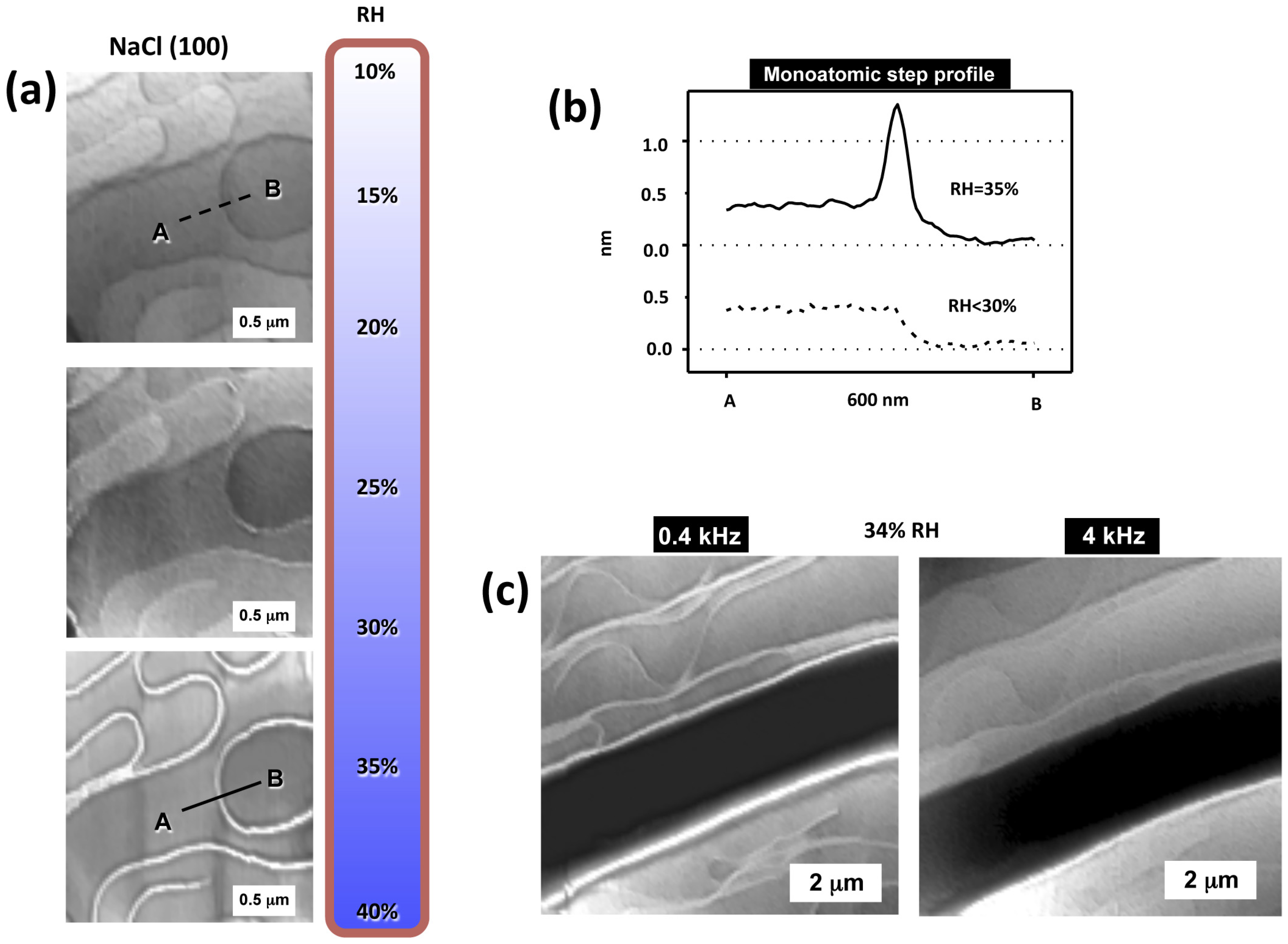
3.1. Imaging and Measuring Thickness
3.2. Spectroscopy
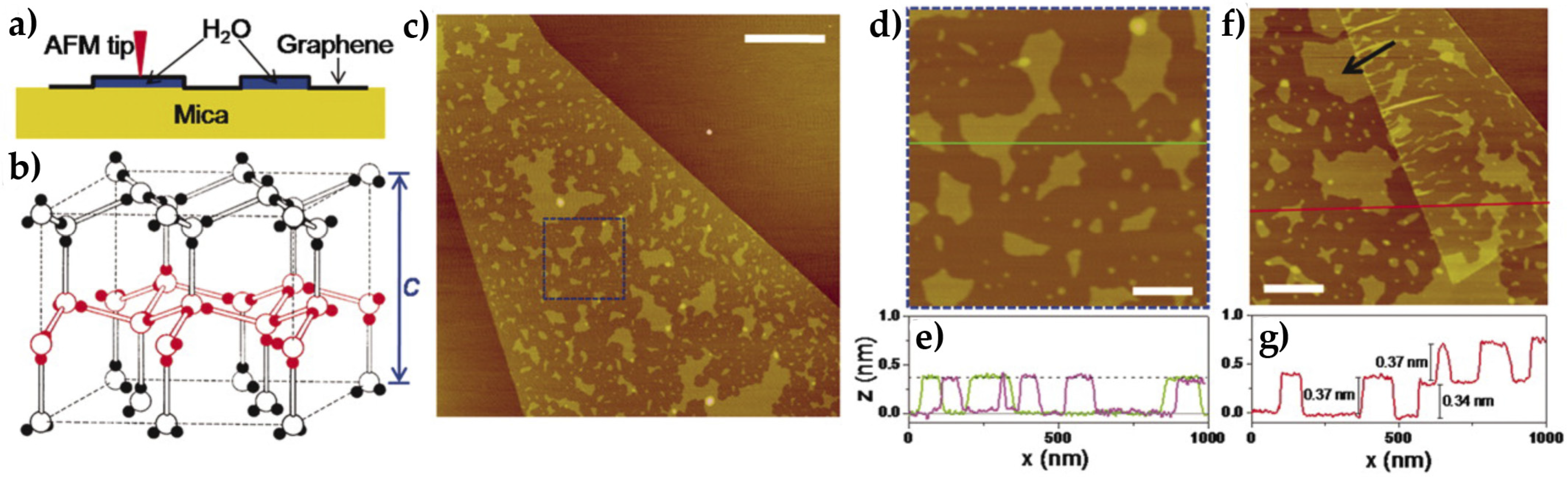
4. Graphene Template
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2D | Two-Dimensional |
| 3D-SPM | Three-Dimensional Scanning Probe Microscopy |
| AFM | Atomic Force Microscopy |
| AM-AFM | Amplitude Modulation Atomic Force Microscopy |
| dAFM | Dynamic Atomic Force Microscopy |
| FM-AFM | Frequency Modulation Atomic Force Microscopy |
| KPFM | Kelvin Probe Force Microscopy |
| RH | Relative Humidity |
| SPFM | Scaning Polarization Force Microscopy |
References
- Henderson, M.A. The interaction of water with solid surfaces: Fundamental aspects revisited. Surf. Sci. Rep. 2002, 46, 1–308. [Google Scholar] [CrossRef]
- Verdaguer, A.; Sacha, G.M.; Bluhm, H.; Salmeron, M. Molecular structure of water at interfaces: Wetting at the nanometer scale. Chem. Rev. 2006, 106, 1478–1510. [Google Scholar] [CrossRef] [PubMed]
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, USA, 2011. [Google Scholar]
- Ball, P. Water as an active constituent in cell biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Israelachvili, J.N.; Landman, U. Nanotribology—Friction, wear and lubrication at the atomic-scale. Nature 1995, 374, 607–616. [Google Scholar] [CrossRef]
- Ghosal, S.; Verdaguer, A.; Hemminger, J.C.; Salmeron, M. In Situ study of water induced segregation of bromide in bromide-doped sodium chloride by scanning polarization force microscopy. J. Phys. Chem. A 2005, 109, 4744–4749. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, A.; Weis, C.; Oncins, G.; Ketteler, G.; Bluhm, H.; Salmeron, M. Growth and structure of water on SiO2 films on Si investigated by kelvin probe microscopy and in situ X-ray spectroscopies. Langmuir 2007, 23, 9699–9703. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.L.; Vaughn, M.W.; DeYoreo, J.J. Direct imaging of meniscus formation in atomic force microscopy using environmental scanning electron microscopy. Langmuir 2005, 21, 8096–8098. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, X.D.; Salmeron, M. Scanning polarization force microscopy—A technique for imaging liquids and weakly adsorbed layers. Appl. Phys. Lett. 1995, 67, 476–478. [Google Scholar] [CrossRef]
- Santos, S.; Verdaguer, A.; Souier, T.; Thomson, N.H.; Chiesa, M. Measuring the true height of water films on surfaces. Nanotechnology 2011, 22. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Cao, P.G.; Heath, J.R. Graphene visualizes the first water adlayers on mica at ambient conditions. Science 2010, 329, 1188–1191. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, X.D.; Ogletree, D.F.; Salmeron, M. The structure of molecularly thin films of water on mica in humid environments. Surf. Sci. 1995, 344, 221–236. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, X.D.; Ogletree, D.F.; Salmeron, M. Imaging the condensation and evaporation of molecularly thin-films of water with nanometer resolution. Science 1995, 268, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, M.; Xu, L.; Hu, J.; Dai, Q. High-resolution imaging of liquid structures: Wetting and capillary phenomena at the nanometer scale. MRS Bull. 1997, 22, 36–41. [Google Scholar] [CrossRef]
- Gomez-Monivas, S.; Saenz, J.J.; Carminati, R.; Greffet, J.J. Theory of electrostatic probe microscopy: A simple perturbative approach. Appl. Phys. Lett. 2000, 76, 2955–2957. [Google Scholar] [CrossRef]
- Nonnenmacher, M.; Oboyle, M.P.; Wickramasinghe, H.K. Kelvin probe force microscopy. Appl. Phys. Lett. 1991, 58, 2921–2923. [Google Scholar] [CrossRef]
- Luna, M.; Rieutord, F.; Melman, N.A.; Dai, Q.; Salmeron, M. Adsorption of water on alkali halide surfaces studied by scanning polarization force microscopy. J. Phys. Chem. A 1998, 102, 6793–6800. [Google Scholar] [CrossRef]
- Verdaguer, A.; Sacha, G.M.; Luna, M.; Frank Ogletree, D.; Salmeron, M. Initial stages of water adsorption on Nacl (100) studied by scanning polarization force microscopy. J. Chem. Phys. 2005, 123. [Google Scholar] [CrossRef]
- Cardellach, M.; Verdaguer, A.; Santiso, J.; Fraxedas, J. Two-dimensional wetting: The role of atomic steps on the nucleation of thin water films on BaF2(111) at ambient conditions. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacha, G.M.; Cardellach, M.; Segura, J.J.; Moser, J.; Bachtold, A.; Fraxedas, J.; Verdaguer, A. Influence of the macroscopic shape of the tip on the contrast in scanning polarization force microscopy images. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Monivas, S.; Froufe, L.S.; Carminati, R.; Greffet, J.J.; Saenz, J.J. Tip-shape effects on electrostatic force microscopy resolution. Nanotechnology 2001, 12, 496–499. [Google Scholar] [CrossRef]
- Verdaguer, A.; Cardellach, M.; Fraxedas, J. Thin water films grown at ambient conditions on BaF2(111) studied by scanning polarization force microscopy. J. Chem. Phys. 2008, 129. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Salmeron, M. An XPS and scanning polarization force microscopy study of the exchange and mobility of surface ions on mica. Langmuir 1998, 14, 5841–5844. [Google Scholar] [CrossRef]
- Bluhm, H.; Inoue, T.; Salmeron, M. Formation of dipole-oriented water films on mica substrates at ambient conditions. Surf. Sci. 2000, 462, L599–L602. [Google Scholar] [CrossRef]
- Cardellach, M.; Verdaguer, A.; Fraxedas, J. Defect-induced wetting on Baf(2)(111) and Caf(2)(111) at ambient conditions. Surf. Sci. 2011, 605, 1929–1933. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Hayashi, K.; Saito, N.; Sugimura, H.; Takai, O. Imaging micropatterned organosilane self-assembled monolayers on silicon by means of scanning electron microscopy and kelvin probe force microscopy. Surf. Interface Anal. 2003, 35, 94–98. [Google Scholar] [CrossRef]
- Luna, M.; Colchero, J.; Gil, A.; Gomez-Herrero, J.; Baro, A.M. Application of non-contact scanning force microscopy to the study of water adsorption on graphite, gold and mica. Appl. Surf. Sci. 2000, 157, 393–397. [Google Scholar] [CrossRef]
- Miura, K.; Yamada, T.; Ishikawa, M.; Okita, S. Apparent contrast of molecularly thin films of water at ionic crystal surfaces. Appl. Surf. Sci. 1999, 140, 415–421. [Google Scholar] [CrossRef]
- Zitzler, L.; Herminghaus, S.; Mugele, F. Capillary forces in tapping mode atomic force microscopy. Phys. Rev. B 2002, 66, 155436–155438. [Google Scholar] [CrossRef]
- Santos, S.; Verdaguer, A.; Chiesa, M. The effects of adsorbed water layers on the apparent height of nanostructures in ambient amplitude modulation atomic force microscopy. J. Chem. Phys. 2012, 137. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, A.; Santos, S.; Sauthier, G.; Segura, J.J.; Chiesa, M.; Fraxedas, J. Water-mediated height artifacts in dynamic atomic force microscopy. Phys. Chem. Chem. Phys. 2012, 14, 16080–16087. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; San Paulo, A. Attractive and repulsive tip-sample interaction regimes in tapping mode atomic force microscopy. Phys. Rev. B 1999, 60, 4961–4967. [Google Scholar] [CrossRef]
- Barcons, V.; Verdaguer, A.; Font, J.; Chiesa, M.; Santos, S. Nanoscale capillary interactions in dynamic atomic force microscopy. J. Phys. Chem. C 2012, 116, 7757–7766. [Google Scholar] [CrossRef]
- Sahagun, E.; Garcıa-Mochales, P.; Sacha, G.M.; Saenz, J.J. Energy dissipation due to capillary interactions: Hydrophobicity maps in force microscopy. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef]
- Vitorino, M.V.; Carpentier, S.; Costa, L.; Rodrigues, M.S. Force feedback microscopy based on an optical beam deflection scheme. Appl. Phys. Lett. 2014, 105. [Google Scholar] [CrossRef]
- Carpentier, S.; Rodrigues, M.S.; Vitorino, M.V.; Costa, L.; Charlaix, E.; Chevrier, J. Out of equilibrium anomalous elastic response of a water nano-meniscus. Appl. Phys. Lett. 2015, 107. [Google Scholar] [CrossRef]
- Giessibl, F.J. Forces and frequency shifts in atomic-resolution dynamic-force microscopy. Phys. Rev. B 1997, 56, 16010–16015. [Google Scholar] [CrossRef]
- Sader, J.E.; Jarvis, S.P. Accurate formulas for interaction force and energy in frequency modulation force spectroscopy. Appl. Phys. Lett. 2004, 84, 1801–1803. [Google Scholar] [CrossRef]
- Dürig, U. Relations between interaction force and frequency shift in large-amplitude dynamic force microscopy. Appl. Phys. Lett. 1999, 75, 433–435. [Google Scholar] [CrossRef]
- Hölscher, H.; Schwarz, A.; Allers, W.; Schwarz, U.D.; Wiesendanger, R. Quantitative analysis of dynamic-force-spectroscopy data on graphite(0001) in the contact and noncontact regimes. Phys. Rev. B 2000, 61, 12678–12681. [Google Scholar] [CrossRef]
- Hu, S.; Raman, A. Inverting amplitude and phase to reconstruct tip-sample interaction forces in tapping mode atomic force microscopy. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jhe, W. General theory of amplitude-modulation atomic force microscopy. Phys. Rev. Lett. 2006, 97. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H. Quantitative measurement of tip-sample interactions in amplitude modulation atomic force microscopy. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Payam, A.F.; Martin-Jimenez, D.D.; Garcia, R. Force reconstruction from tapping mode force microscopy experiments. Nanotechnology 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Fukuma, T.; Ueda, Y.; Yoshioka, S.; Asakawa, H. Atomic-scale distribution of water molecules at the mica-water interface visualized by three-dimensional scanning force microscopy. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef] [PubMed]
- Herruzo, E.T.; Asakawa, H.; Fukuma, T.; Garcia, R. Three-dimensional quantitative force maps in liquid with 10 piconewton, angstrom and sub-minute resolutions. Nanoscale 2013, 5, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Katan, A.J.; van Es, M.H.; Oosterkamp, T.H. Quantitative force versus distance measurements in amplitude modulation Afm: A novel force inversion technique. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Amadei, C.A.; Verdaguer, A.; Chiesa, M. Size dependent transitions in nanoscale dissipation. J. Phys. Chem. C 2013, 117, 10615–10622. [Google Scholar] [CrossRef]
- Calo, A.; Domingo, N.; Santos, S.; Verdaguer, A. Revealing water films structure from force reconstruction in dynamic AFM. J. Phys. Chem. C 2015, 119, 8258–8265. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Komurasaki, H.; Tsukamoto, T.; Yamazaki, K.; Ogino, T. Layered structures of interfacial water and their effects on raman spectra in graphene-on-sapphire systems. J. Phys. Chem. C 2012, 116, 10084–10089. [Google Scholar] [CrossRef]
- Lee, M.J.; Choi, J.S.; Kim, J.S.; Byun, I.S.; Lee, D.H.; Ryu, S.; Lee, C.; Park, B.H. Characteristics and effects of diffused water between graphene and a SiO2 substrate. Nano Res. 2012, 5, 710–717. [Google Scholar] [CrossRef]
- Cao, P.G.; Xu, K.; Varghese, J.O.; Heath, J.R. The microscopic structure of adsorbed water on hydrophobic surfaces under ambient conditions. Nano Lett. 2011, 11, 5581–5586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.T.; Wood, J.D.; Doidge, G.P.; Pop, E.; Lyding, J.W. Scanning Tunneling microscopy study and nanomanipulation of graphene-coated water on mica. Nano Lett. 2012, 12, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Choi, J.S.; Lee, M.J.; Park, B.H.; Bukhvalov, D.; Son, Y.W.; Yoon, D.; Cheong, H.; Yun, J.N.; Jung, Y.; et al. Between scylla and charybdis: Hydrophobic graphene-guided water diffusion on hydrophilic substrates. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, A.; Segura, J.J.; Lopez-Mir, L.; Sauthier, G.; Fraxedas, J. Communication: Growing room temperature ice with graphene. J. Chem. Phys. 2013, 138. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.F.; Maier, S.; Salmeron, M. Water splits epitaxial graphene and intercalates. J. Am. Chem. Soc. 2012, 134, 5662–5668. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Mun, J.H.; Cho, B.J.; Kim, T.S. Penetration and lateral diffusion characteristics of polycrystalline graphene barriers. Nanoscale 2014, 6, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Ahn, G.; Ryu, S. Two-dimensional water diffusion at a graphene-silica interface. J. Am. Chem. Soc. 2014, 136, 6634–6642. [Google Scholar] [CrossRef] [PubMed]
- Severin, N.; Lange, P.; Sokolov, I.M.; Rabe, J.P. Reversible dewetting of a molecularly thin fluid water film in a soft graphene-mica slit pore. Nano Lett. 2012, 12, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Q.; Wang, X.F.; Li, J.Y.; Zhang, S.; Kjems, J.; Besenbacher, F.; Dong, M.D. Evidence of Stranski-Krastanov growth at the initial stage of atmospheric water condensation. Nat Commun 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, G.A.; Matthiesen, J.; Baer, M.; Mundy, C.J.; Petrik, N.G.; Smith, R.S.; Dohnalek, Z.; Kay, B.D. No confinement needed: Observation of a metastable hydrophobic wetting two-layer ice on graphene. J. Am. Chem. Soc. 2009, 131, 12838–12844. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, S.; Verdaguer, A. Imaging Water Thin Films in Ambient Conditions Using Atomic Force Microscopy. Materials 2016, 9, 182. https://doi.org/10.3390/ma9030182
Santos S, Verdaguer A. Imaging Water Thin Films in Ambient Conditions Using Atomic Force Microscopy. Materials. 2016; 9(3):182. https://doi.org/10.3390/ma9030182
Chicago/Turabian StyleSantos, Sergio, and Albert Verdaguer. 2016. "Imaging Water Thin Films in Ambient Conditions Using Atomic Force Microscopy" Materials 9, no. 3: 182. https://doi.org/10.3390/ma9030182




