Compositional Design of Dielectric, Ferroelectric and Piezoelectric Properties of (K, Na)NbO3 and (Ba, Na)(Ti, Nb)O3 Based Ceramics Prepared by Different Sintering Routes
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
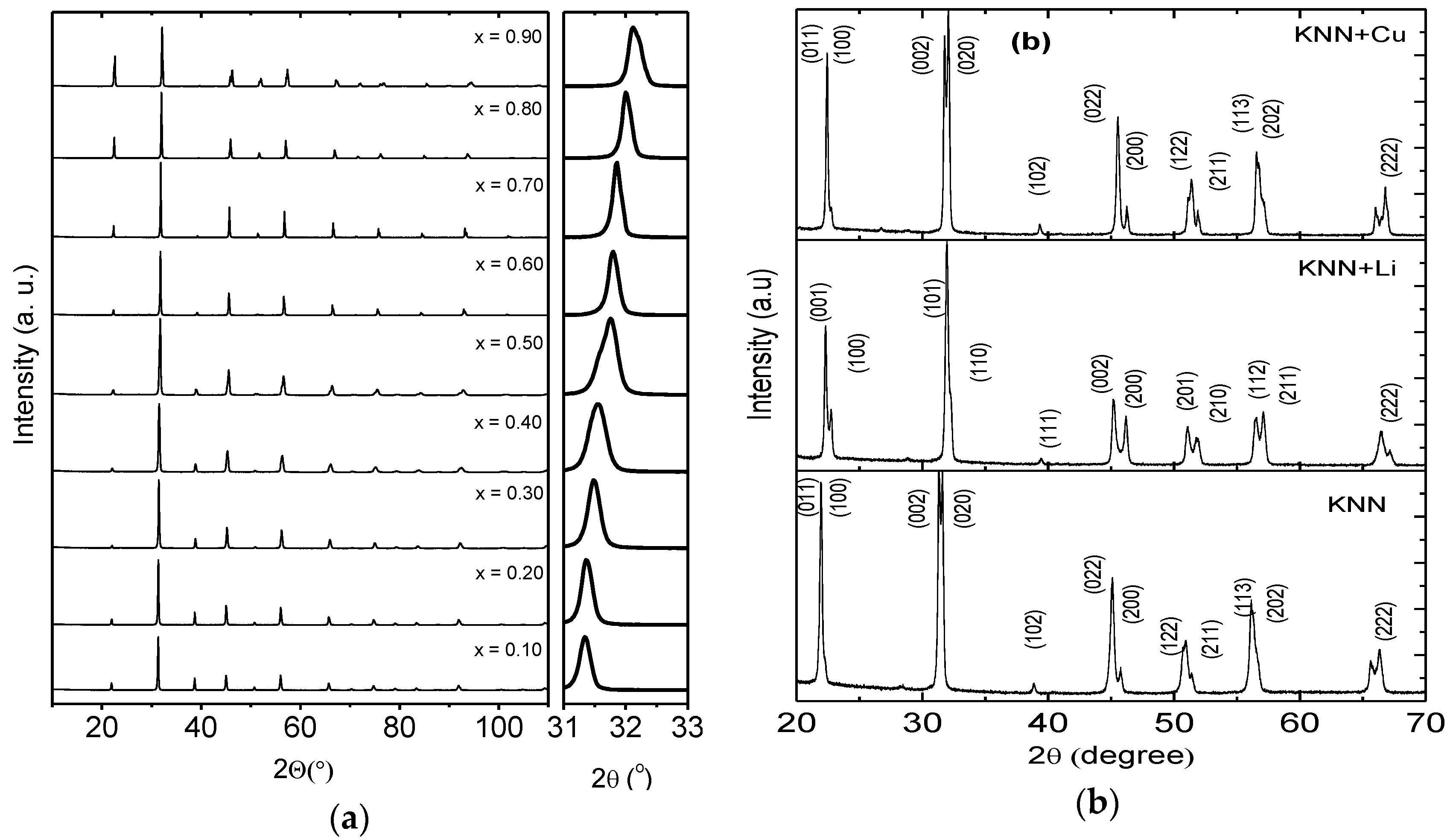
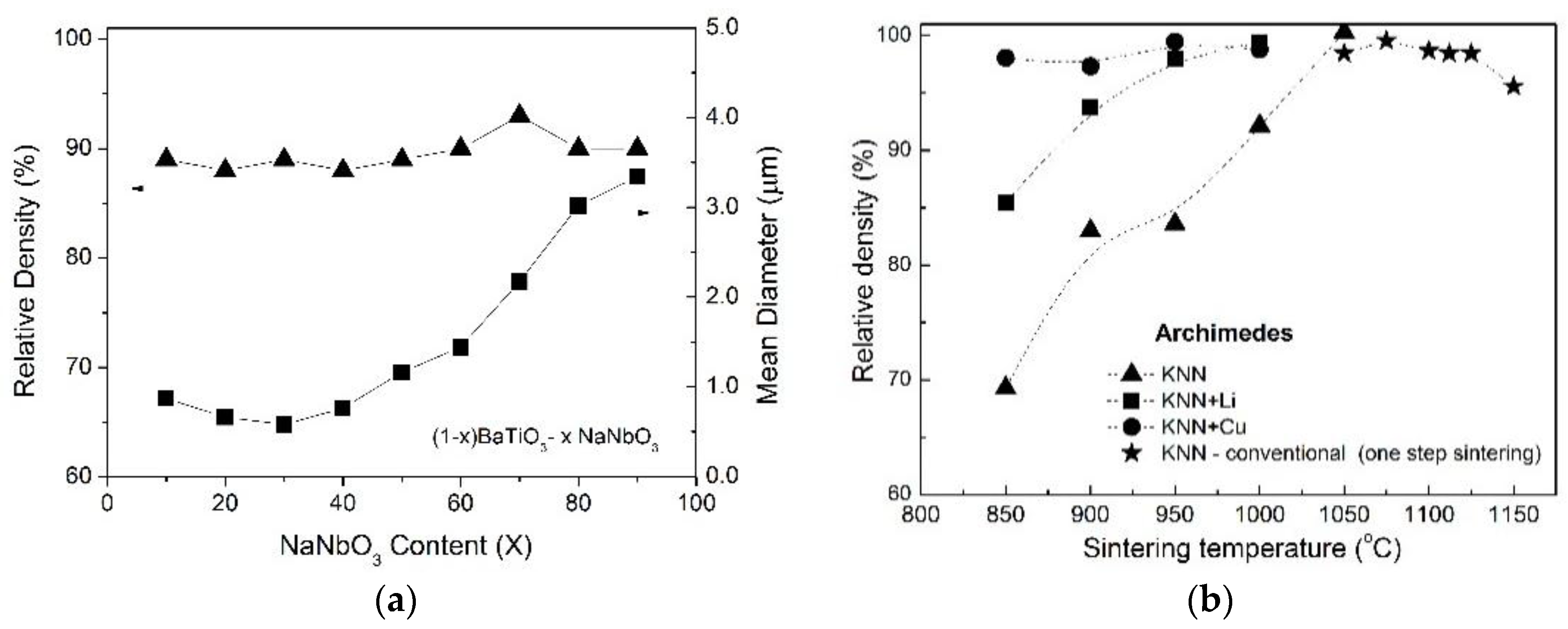
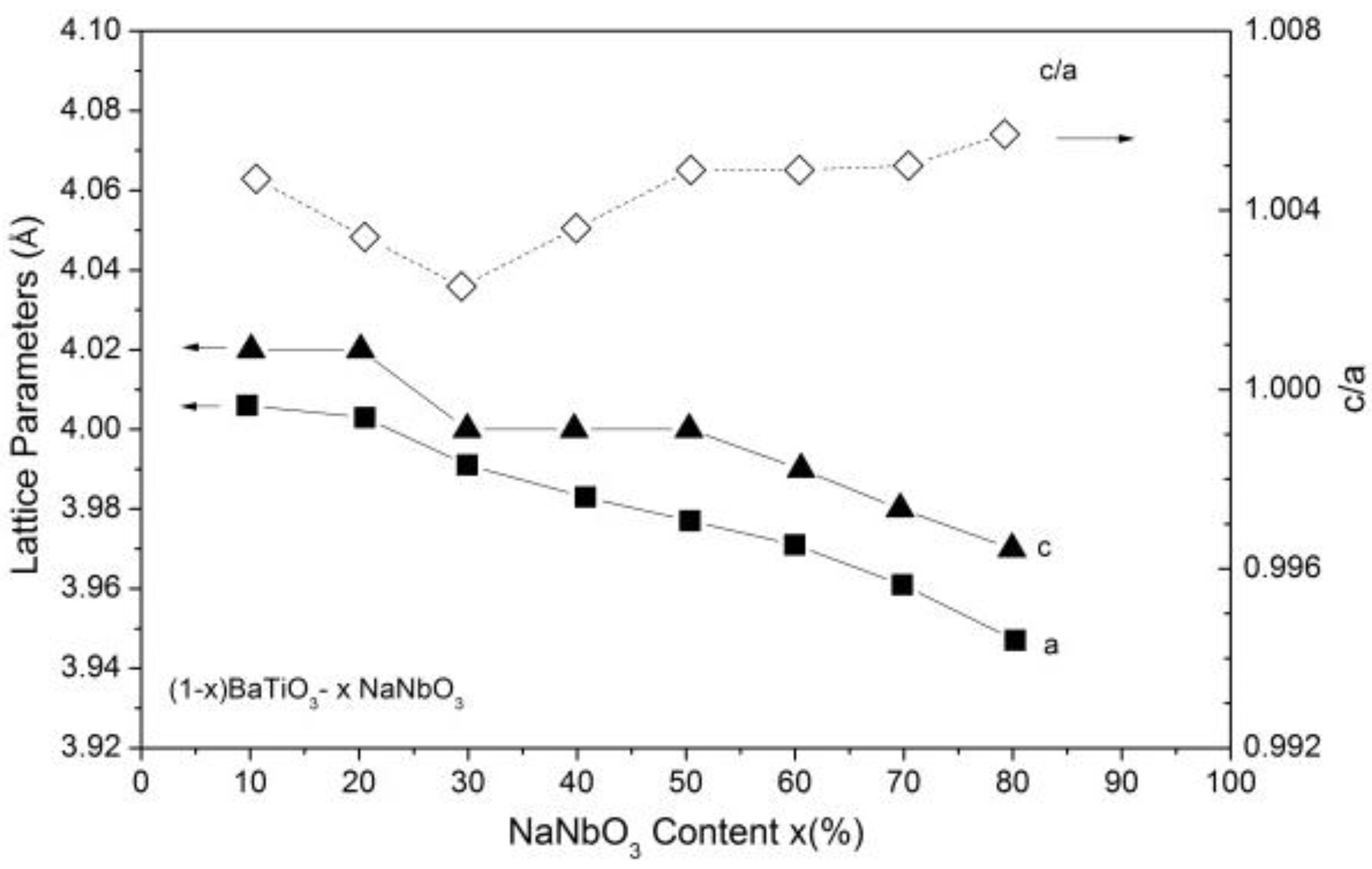
3.1. Structural and Microstructural Properties of BTNN and KNN Ceramics
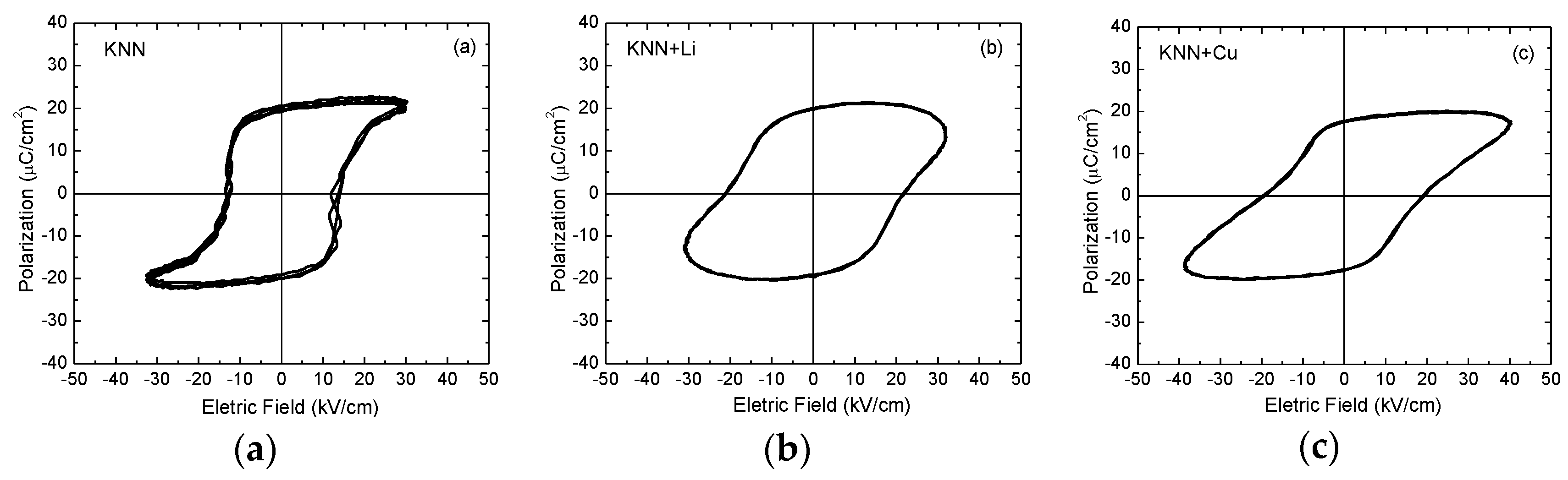
3.2. Dielectric, Piezoelectric and Ferroelectric Properties of BTNN
3.3. Dielectric, Piezoelectric and Ferroelectric Properties of KNN
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaffe, B.; Cook, W.R., Jr.; Jaffe, H. Piezoelectric Ceramics; Academic Press: London, UK; New York, NY, USA, 1971. [Google Scholar]
- Nuraje, N.; Su, K. Perovskite ferroelectric nanomaterials. Nanoscale 2013, 5, 8752–8780. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K. Ferroelectric Devices; Marcel Dekker, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Bowen, C.R.; Kim, H.A.; Weaver, P.M.; Dunn, S. Piezoelectric and ferroelectric materials and structures for energy harvesting applications. Energy Envorin. Sci. 2014, 7, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Takao, H.; Tani, T.; Nonoyama, T.; Takatori, K.; Homma, T.; Nagaya, T.; Nakamura, M. Lead-free piezoceramics. Nature 2004, 432, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.L.; Wang, C.L.; Li, J.C. Influence of compositional ratio K/Na on physical properties in (KxNa1−x)NbO3 ceramics. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Aksel, E.; Jones, J.L. Advances in lead-free piezoelectric materials for sensors and actuators. Sensors 2010, 10, 1935–1954. [Google Scholar] [CrossRef] [PubMed]
- Rodel, J.; Webber, K.; Dittmer, R.; Jo, W.; Kimura, M.; Damjanovic, D. Transferring lead-free piezoelectric ceramics into application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. [Google Scholar] [CrossRef]
- Pandaa, P.K.; Sahoo, B. PZT to lead free piezo ceramics: A Review. Ferroelectrics 2015, 474, 128–143. [Google Scholar] [CrossRef]
- Coondoo, I.; Panwar, N.; Kholkin, A. Lead-free piezoelectrics: Current status and perspectives. J. Adv. Dielect. 2013, 3, 1330002. [Google Scholar] [CrossRef]
- Egerton, L.; Dillon, D.M. Piezoelectric and dielectric properties of ceramics in the system potassium-sodium niobate. J. Am. Ceram. Soc. 1959, 42, 438–445. [Google Scholar] [CrossRef]
- Rodel, J.; Jo, W.; Seifert, K.T.P.; Anton, E.M.; Granzow, T.; Damjanovic, D. Perspective on the development of lead-free piezoceramics. J. Am. Ceram. Soc. 2009, 92, 1153–1160. [Google Scholar] [CrossRef]
- Bomlai, P.; Wichianrat, P.; Muensit, S.; Milne, S.J. Effect of calcination conditions and excess alkali carbonate on the phase formation and particle morphology of Na0.5K0.5NbO3 powders. J. Am. Ceram. Soc. 2007, 90, 1650–1655. [Google Scholar] [CrossRef]
- Malic, B.; Jenko, D.; Holc, J.; Hrovat, M.; Kosec, M. Synthesis of sodium potassium niobate: A diffusion couples study. J. Am. Ceram. Soc. 2008, 91, 1916–1920. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Ochoa, P.; Fernandez, J.F. Sintering and properties of lead-free piezoceramics. J. Eur. Ceram. Soc. 2007, 27, 4125–4129. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Del Campo, A.; López-Juárez, R.; Romero, J.J.; Fernández, J.F. High spatial resolution structure of (K, Na)NbO3 lead-free ferroelectric domains. J. Mater. Chem. 2012, 22, 9714–9720. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, D.; Zhu, J. Potassium-sodium niobate lead-free piezoelectric materials: Past, present, and future of phase boundaries. Chem. Rev. 2015, 115, 2559–2595. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.W.; Thomas, P.A.; Zhang, N.; Glazer, A.M. A comprehensive study of the phase diagram of KxNa1−xNbO3. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Hussain, A.; Ahn, C.W.; Ullah, A.; Lee, J.S.; Kim, I.W. Dielectric, ferroelectric and field-induced strain behavior of K0.5Na0.5NbO3–modified Bi–0.5(Na0.78K0.22)(0.5)TiO3 lead-free ceramics. Ceram. Int. 2012, 38, 4143–4150. [Google Scholar] [CrossRef]
- Shrout, T.R.; Zhang, S.J. Lead-free piezoelectric ceramics: Alternatives for PZT? J. Electroceram. 2007, 19, 111–114. [Google Scholar] [CrossRef]
- Malic, B.; Koruza, J.; Hrešcak, J.; Bernard, J.; Wang, K.; Fisher, J.G.; Bencan, A. Sintering of Lead-Free Piezoelectric Sodium Potassium Niobate Ceramics. Materials 2015, 8, 8117–8146. [Google Scholar] [CrossRef]
- Hagh, N.M.; Jadidian, B.; Safari, A. Property-processing relationship in lead-free (K, Na, Li) NbO3-solid solution system. J. Electroceram. 2007, 18, 339–346. [Google Scholar] [CrossRef]
- Acker, J.; Kung, H.M.; Hoffmann, J. Influence of alkaline and niobium excess on sintering and microstructure of sodium-potassium niobate (K0.5Na0.5)NbO3. J. Am. Ceram. Soc. 2010, 93, 1270–1281. [Google Scholar] [CrossRef]
- Koruza, J.; Malič, B. Initial stage sintering mechanism of NaNbO3 and implications regarding the densification of alkaline niobates. J. Eur. Ceram. Soc. 2014, 34, 1971–1979. [Google Scholar] [CrossRef]
- Jaeger, R.E.; Egerton, L. Hot pressing of potassium-sodium niobates. J. Am. Ceram. Soc. 1962, 45, 209–213. [Google Scholar] [CrossRef]
- Li, J.F.; Wang, K.; Zhang, B.P.; Zhang, L.M. Ferroelectric and piezoelectric properties of fine-grained Na0.5K0.5NbO3 lead-free piezoelectric ceramics prepared by spark plasma sintering. J. Am. Ceram. Soc. 2006, 89, 706–800. [Google Scholar] [CrossRef]
- Zhena, Y.; Li, J.-F.; Wang, K.; Yan, Y.; Yu, L. Spark plasma sintering of Li/Ta-modified (K, Na)NbO3 lead-free piezoelectric ceramics: Post-annealing temperature effect on phase structure, electrical properties and grain growth behavior. Mater. Sci. Eng. B 2011, 176, 1110–1114. [Google Scholar] [CrossRef]
- Wang, R.; Xie, R.; Sekiya, T.; Shimojo, Y. Fabrication and characterization of potassium–sodium niobate piezoelectric ceramics by spark-plasma-sintering method. Mater. Res. Bull. 2004, 39, 1709–1715. [Google Scholar] [CrossRef]
- Sen, C.; Alkan, B.; Akin, I.; Yucel, O.; Sahin, F.C.; Goller, G. Microstructure and ferroelectric properties of spark plasma sintered Li substituted K0.5Na0.5NbO3 ceramics. J. Ceram. Soc. Jpn. 2011, 119, 355–361. [Google Scholar] [CrossRef]
- Hrescak, J.; Bencan, A.; Rojac, T.; Malic, B. The influence of different niobium pentoxide precursors on the solid-state synthesis of potassium sodium niobate. J. Eur. Ceram. Soc. 2013, 33, 3065–3072. [Google Scholar] [CrossRef]
- Lente, M.H. Influence of niobium pentoxide phase and calcination route on the formation of potassium sodium niobate powders. Ferroelectrics 2015, 479, 15–21. [Google Scholar] [CrossRef]
- Hagha, N.M.; Kerman, K.; Jadidian, B.; Safari, A. Dielectric and piezoelectric properties of Cu2+-doped alkali Niobates. J. Eur. Ceram. Soc. 2009, 29, 2325–2332. [Google Scholar] [CrossRef]
- Rubio-Marcosa, F.; Reinosab, J.J.; Vendrellc, X.; Romerob, J.J.; Mestresc, L.; Leretb, P.; Fernándezb, J.F.; Marcheta, P. Structure, microstructure and electrical properties of Cu2+ doped (K, Na, Li)(Nb, Ta, Sb)O3 piezoelectric ceramics. Ceram. Int. 2013, 39, 4139–4149. [Google Scholar] [CrossRef]
- Abdelkefi, H.; Khemakhem, H.; Simon, A.; Darriet, J. X-ray diffraction study of Ba0.985Na0.015Ti0.985Nb0.015O3, Ba0.6Na0.4Ti0.6Nb0.4O3 and Ba0.3Na0.7Ti0.3Nb0.7O3 compositions. J. Alloys Comp. 2008, 463, 423–427. [Google Scholar] [CrossRef]
- Khemakhem, H.; Simon, A.; von der Mühll, R.; Ravez, J. Relaxor or classical ferroelectric behavior in ceramics with composition Ba1-xNaxTi1−xNbxO3. J. Phys. Condens. Matter 2000, 12, 5951–5959. [Google Scholar] [CrossRef]
- Bahri, F.; Khemakhem, H.; Gargouri, M.; Simon, A.; von der Mühll, R.; Ravez, J. Dielectric and Raman studies on the solid solution (1−x)BaTiO3–(x)NaNbO3 ceramics. Solid State Sci. 2003, 5, 1229–1234. [Google Scholar] [CrossRef]
- Khemakhem, S.; Yahyaoui, S.; Hassen, R.B.; Khemakhem, H.; Salah, A.B. Crystal structure and electrical behavior of the new ceramic Ba0.7Na0.3Ti0.7Nb0.3O3. Solid State Sci. 2003, 5, 367–371. [Google Scholar] [CrossRef]
- Zhang, S.T.; Lu, M.H.; Chen, Y.F.; Liu, Z.G.; Ming, N.B.; Wang, J.; Cheng, G.X. Composition-dependent structures and properties of (1−x)BaTiO3–(x)NaNbO3 thin films. Appl. Phys. Lett. 2006, 88. [Google Scholar] [CrossRef]
- Bąk, W.; Gabryś, M.; Kajtoch, C.; Tejchman, W.; Starzyk, F. The dielectric behavior of polycrystalline Ba0.96Na0.04Ti0.96Nb0.04O3 solid solution. Arch. Mater. Sci. Eng. 2009, 40, 13–16. [Google Scholar]
- Bąk, W.; Gabryś, M.; Kajtoch, C.; Stanuch, K.; Starzyk, F. Dielectric spectroscopy study of Ba0.98Na0.02Ti0.98Nb0.02O3 ceramic. Arch. Mater. Sci. Eng. 2009, 39, 107–110. [Google Scholar]
- Bak, W. Characterization of Ba1-xNaxTi1-xNbxO3 ceramic by dielectric spectroscopy. Arch. Mater. Sci. Eng. 2008, 34, 5–8. [Google Scholar]
- Alguero, M.; Moure, A.; Pardo, L.; Holc, J.; Kosec, M. Processing by mechanosynthesis and properties of piezoelectric Pb(Mg1/3Nb2/3)O3–PbTiO3 with different compositions. Acta Mater. 2006, 54, 501–511. [Google Scholar] [CrossRef]
- Stojanovic, B.D. Mechanochemical synthesis of ceramic powders with perovskite structure. J. Mater. Process. Technol. 2003, 143, 78–81. [Google Scholar] [CrossRef]
- Gotardo, R.A.; Santos, I.A.; Cotica, L.F.; Botero, E.R.; Garcia, D.; Eiras, J.A. Improved ferroelectric and magnetic properties of monoclinicstructured 0.8BiFeO3–0.2BaTiO3 magnetoelectric ceramics. Scr. Mater. 2009, 61, 508–511. [Google Scholar] [CrossRef]
- Azough, F.; Wegrzyn, M.; Freer, R.; Sharma, S.; Hall, D. Microstructure and piezoelectric properties of CuO added (K, Na, Li)NbO3 lead-free piezoelectric ceramics. J. Eur. Ceram. Soc. 2011, 31, 569–576. [Google Scholar] [CrossRef]
- Qian, S.; Zhu, K.; Pang, X.; Wang, J.; Liu, J.; Qiu, J. Influence of sintering temperature on electrical properties of (K0.4425Na0.52Li0.0375)(Nb0.8825Sb0.07Ta0.0475)O3 ceramics without phase transition induced by sintering temperature. J. Adv. Ceram. 2013, 2, 353–359. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Fernandez, J.F. Effect of the temperature on the synthesis of KNN-modified nanoparticles by a solid state reaction route. J. Nanopart. Res. 2010, 12, 2495–2502. [Google Scholar] [CrossRef]
- Souza, C.A.; Lente, M.H.; Eiras, J.A. Spark Plasma Sintering of doped (KxNa1−x)NbO3 piezoceramics. Ferroelectrics 2016, in press. [Google Scholar]
- Freitas, V.F.; Santos, I.A.; Botero, E.; Fraygola, B.M.; Garcia, D.; Eiras, J.A. Piezoelectric characterization of (0.6)BiFeO3–(0.4)PbTiO3 multiferroic ceramics. J. Am. Ceram. Soc. 2011, 94, 754–758. [Google Scholar] [CrossRef]
- Zampiere, R.B.; Dias, G.S.; Cotica, L.F.; Santos, I.A. Enhanced ferroism in mechanically processed and environmentally friendly Ba0.30Na0.70Ti0.30Nb0.70O3 ceramics. Scr. Mater. 2012, 66, 542–545. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, B.-P.; Li, J.-F. High piezoelectric d33 coefficient in Li-modified lead-free (Na, K)NbO3 ceramics sintered at optimal temperature. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Niu, X.K.; Zhang, J.L.; Wu, L.; Zheng, P.; Zhao, M.L.; Wang, C.L. Crystalline structural phase boundaries in (K,Na,Li)NbO3 ceramics. Solid State Commun. 2008, 146, 395–398. [Google Scholar] [CrossRef]
- Wang, K.; Li, J.-F. Analysis of crystallographic evolution in (Na, K)NbO3-based lead-free piezoceramics by X-ray diffraction. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Sun, X.; Deng, J.; Chen, J.; Sun, C.; Xing, X. Effects of Li substitution on the structure and ferroelectricity of (Na,K)NbO3. J. Am. Ceram. Soc. 2009, 92, 3033–3040. [Google Scholar] [CrossRef]
- Eriksson, M.; Yan, H.; Viola, G.; Ning, H.; Gruner, D.; Nygren, M.; Reece, M.J.; Shen, Z. Ferroelectric domain structures and electrical properties of fine-grained lead-free sodium potassium niobate ceramics. J. Am. Ceram. Soc. 2011, 94, 3391–3396. [Google Scholar] [CrossRef]
- Abdelkefi, H.; Khemakhem, H.; Vélu, G.; Carru, J.C.; von der Mühll, R. Dielectric proprieties of ferroelectric ceramics derived from the system BaTiO3-NaNbO3-based solid solutions. Solid State Sci. 2004, 6, 1347–1351. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Fang, J.; Zhang, Y.; Li, L. Piezoelectric properties of Li, Sb, and Ta co-doped (K, Na)NbO3 ceramics with fine grain size sintered by SPS method. J. Electroceram. 2013, 30, 217–220. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Li, J.F.; Wang, K.; Xu, S.; Jiang, W.; Deng, Q. Electrical and mechanical properties of fine-grained Li/Ta-modified (Na, K)NbO3-based piezoceramics prepared by spark plasma sintering. J. Am. Ceram. Soc. 2010, 93, 1378–1383. [Google Scholar] [CrossRef]
- Eichel, R.-A.; Erunal, E.; Jakes, P.; Korbel, S.; Elsasser, C.; Kungl, H.; Acker, J.; Hoffmann, M.J. Interactions of defect complexes and domain walls in CuO-doped ferroelectric (K, Na)NbO3. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Lente, M.H.; Eiras, J.A. Domain reorientation anisotropy in ferroelectric polycrystals. J. Appl. Phys. 2002, 92, 2112–2117. [Google Scholar] [CrossRef]
- Zhang, S.; Lim, J.B.; Lee, H.J.; Shrout, T.R. Characterization of hard piezoelectric lead-free ceramics. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Yoo, J.; Lee, K.; Kim, I.; Song, J.; Park, Y.-W. Dielectric and piezoelectric characteristics of the non-stoichiometric (Na, K)NbO3 ceramics doped with CuO. J. Alloy. Compd. 2010, 506, 872–876. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Xu, Y.; Li, J.F. Enhancement of Qm in CuO-doped compositionally optimized Li/Ta-modified (Na, K)NbO3 lead-free piezoceramics. Ceram. Int. 2012, 38, S331–S334. [Google Scholar] [CrossRef]
- Wook, J.; Ollagnier, J.B.; Park, J.-L.; Anton, E.M.; Kwon, O.-J.; Chan, P.; Seo, H.-H.; Lee, J.-S.; Erdem, E.; Eichel, R.-A.; et al. CuO as a sintering additive for (Bi1/2Na1/2)TiO3–BaTiO3–(K0.5Na0.5)NbO3 lead-free piezoceramics. J. Europ. Ceram. Soc. 2011, 31, 2107–2117. [Google Scholar]
- Rubio-Marcos, F.; Marchet, P.; Vendrell, X.; Romero, J.J.; Rémondiere, F.; Mestres, L.; Fernandez, J.F. Effect of MnO doping on the structure, microstructure and electrical properties of the (K, Na, Li)(Nb, Ta, Sb)O3 lead-free piezoceramics. J. Alloys Compd. 2011, 509, 8804–8811. [Google Scholar] [CrossRef]



| Parameters | x = 0.70 | x = 0.80 | x = 0.90 | PZT |
|---|---|---|---|---|
| Tm (K) | 298 | 352 | 612 | 598 |
| 2342 | 1240 | 1030 | 1300 | |
| k31 | 0.022 | 0.013 | 0.012 | 0.33 |
| k33 | 0.016 | 0.015 | 0.013 | 0.70 |
| k15 | 0.57 | 0.55 | 0.66 | 0.71 |
| kt | 0.02 | 0.014 | 0.013 | 0.51 |
| d31 (10−12 m/V) | 10.5 | 1.7 | 6.0 | 123 |
| d33 (10−12 m/V) | 5.0 | 3.1 | 3.3 | 289 |
| d15 (10−12 m/V) | 937 | 632 | 561 | 496 |
| g31 (10−3 Vm/N) | 0.49 | 0.15 | 0.66 | 11 |
| g33 (10−3 Vm/N) | 0.23 | 0.28 | 036 | 26 |
| g15 (10−3 Vm/N) | 47 | 65 | 64 | 39 |
| Qm | 119 | 182 | 217 | 500 |
| Parameters | KNN | KNN + Li | KNN + Cu |
|---|---|---|---|
| TC (K) | 406 | 454 | 420 |
| 1654 | 400 | 420 | |
| kp | 0.36 | 0.39 | 0.35 |
| kt | 0.39 | 0.30 | 0.28 |
| np (m/s) | 3439 | 3165 | 3510 |
| (m/s) | 2988 | 2686 | 2799 |
| Qpm | 166 | 114 | 112 |
| 15 | 15 | 14 | |
| Zp (MRayl) | 15.2 | 14.0 | 15.5 |
| Zt (MRayl) | 13.2 | 11.9 | 12.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiras, J.A.; Gerbasi, R.B.Z.; Rosso, J.M.; Silva, D.M.; Cótica, L.F.; Santos, I.A.; Souza, C.A.; Lente, M.H. Compositional Design of Dielectric, Ferroelectric and Piezoelectric Properties of (K, Na)NbO3 and (Ba, Na)(Ti, Nb)O3 Based Ceramics Prepared by Different Sintering Routes. Materials 2016, 9, 179. https://doi.org/10.3390/ma9030179
Eiras JA, Gerbasi RBZ, Rosso JM, Silva DM, Cótica LF, Santos IA, Souza CA, Lente MH. Compositional Design of Dielectric, Ferroelectric and Piezoelectric Properties of (K, Na)NbO3 and (Ba, Na)(Ti, Nb)O3 Based Ceramics Prepared by Different Sintering Routes. Materials. 2016; 9(3):179. https://doi.org/10.3390/ma9030179
Chicago/Turabian StyleEiras, José A., Rosimeire B. Z. Gerbasi, Jaciele M. Rosso, Daniel M. Silva, Luiz F. Cótica, Ivair A. Santos, Camila A. Souza, and Manuel H. Lente. 2016. "Compositional Design of Dielectric, Ferroelectric and Piezoelectric Properties of (K, Na)NbO3 and (Ba, Na)(Ti, Nb)O3 Based Ceramics Prepared by Different Sintering Routes" Materials 9, no. 3: 179. https://doi.org/10.3390/ma9030179






