Preparation and Characterization of Ni Spines Grown on the Surface of Cubic Boron Nitride Grains by Electroplating Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
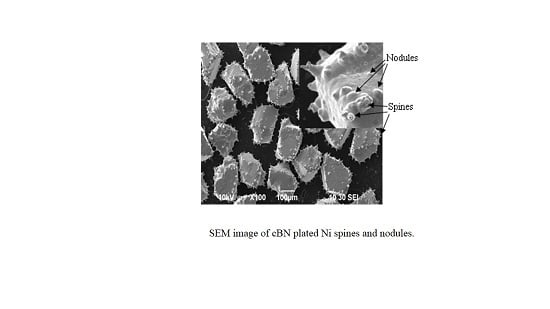
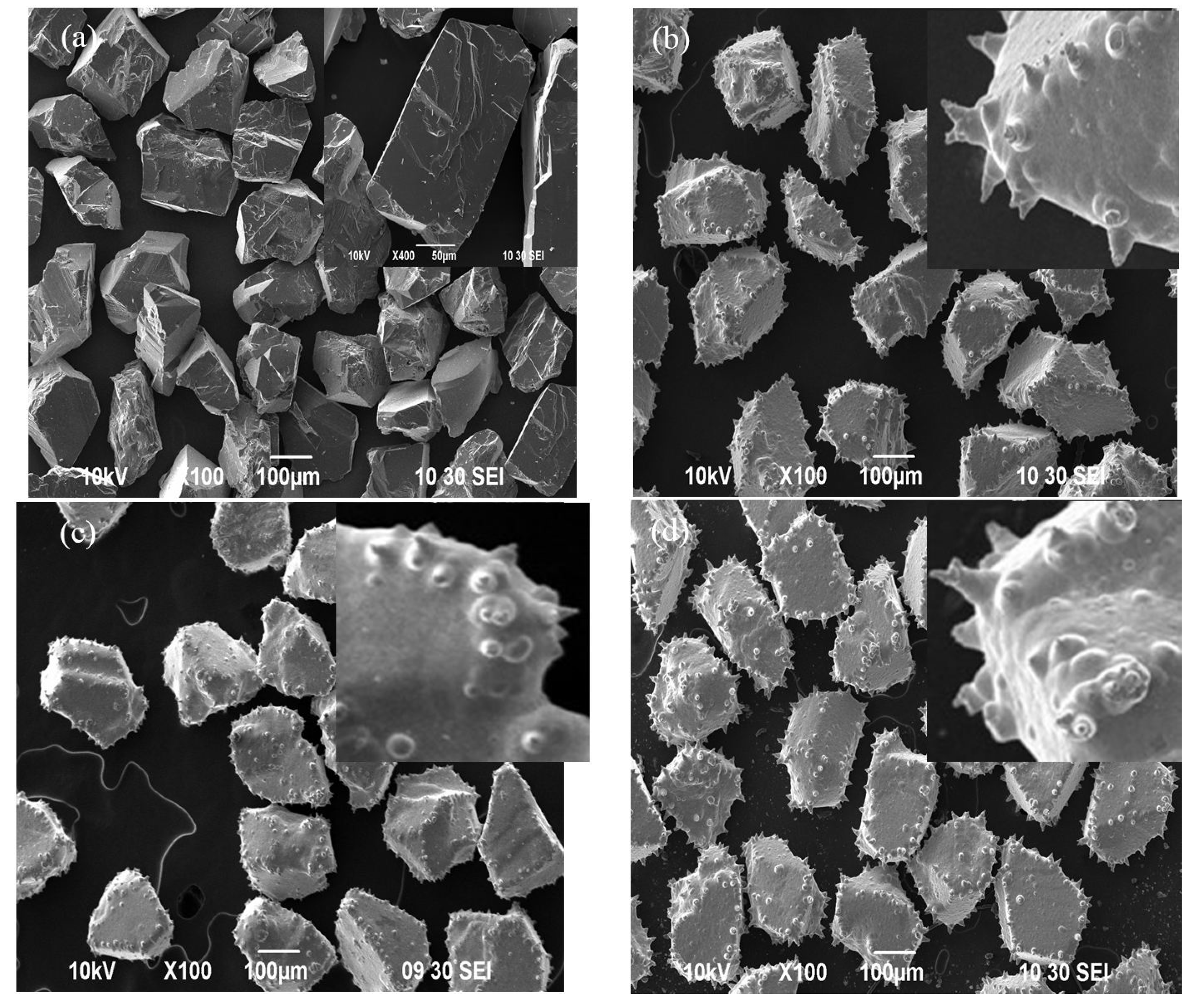
3.1. The Morphologies of Plated cBN Grains
3.2. The Weight Increment of Plated cBN Grains
3.3. The Phase Composition of Plated cBN Grains
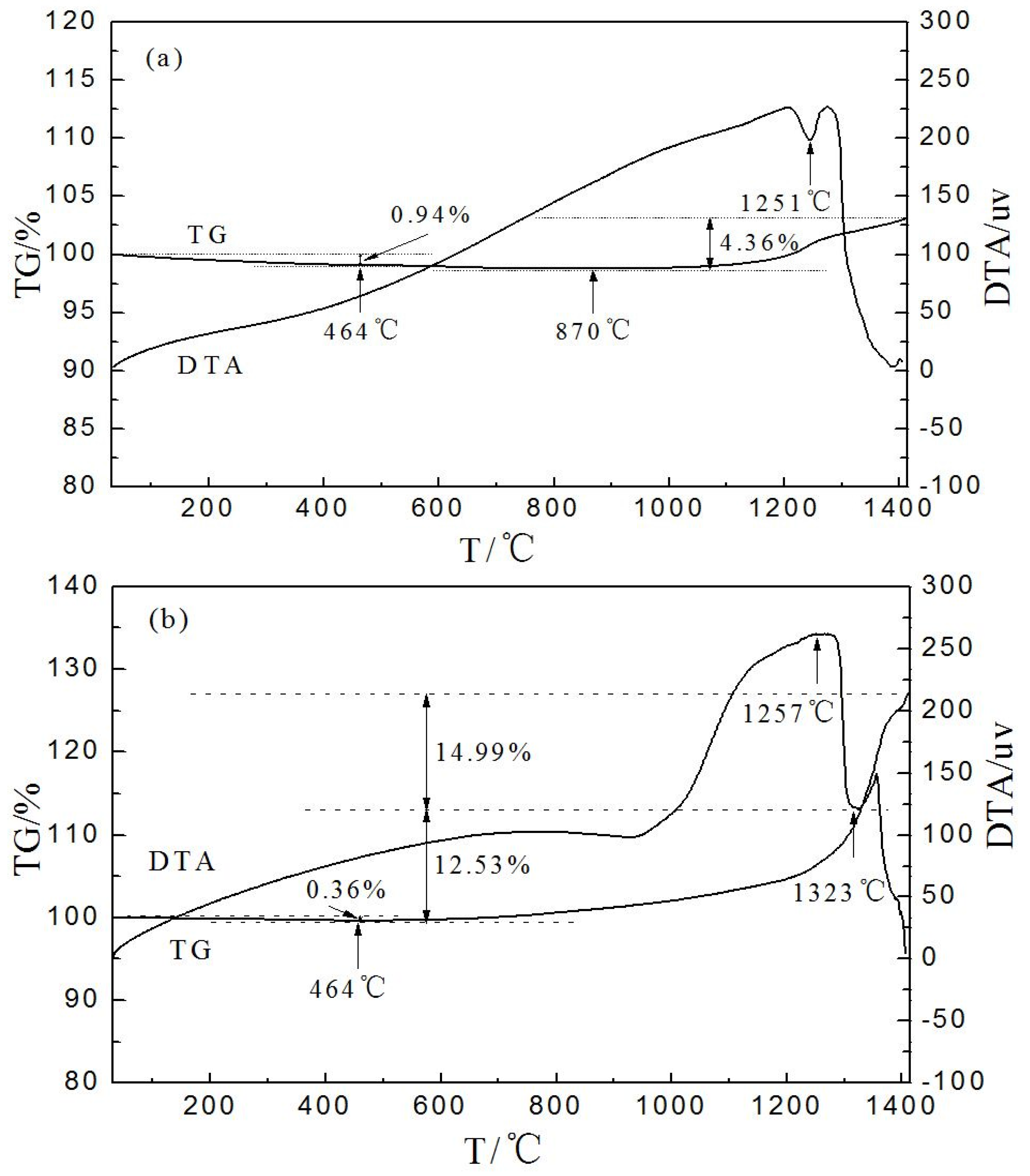
3.4. Thermal Stability of Plated cBN Grains
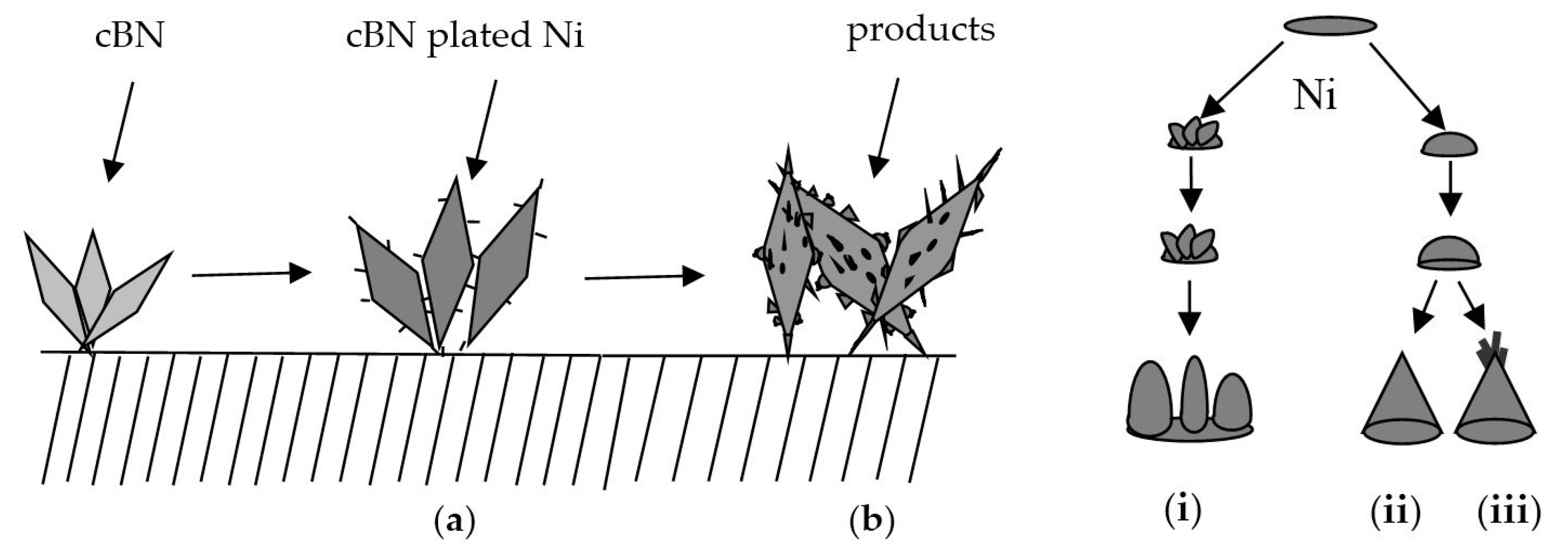
3.5. The Forming Process of Ni Spines Plated cBN Grains
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luo, X.T.; Li, C.J. Large sized cubic BN reinforced nanocomposite with improved abrasive wear resistance deposited by cold spray. Mater. Des. 2015, 83, 249–256. [Google Scholar] [CrossRef]
- Alessandra, C.; Roberto, T. CBN grinding performance improvement in aircraft engine components manufacture. Procedia CIRP 2013, 9, 109–114. [Google Scholar]
- Zhang, J.; Tu, R.; Goto, T. Densification of SiO2-cBN composites by using Ni nanoparticle and SiO2 nanolayer coated cBN powder. Ceram. Int. 2012, 38, 4961–4966. [Google Scholar] [CrossRef]
- Brookes, K. Making hardmetal even harder with dispersed cBN. Met. Powder Rep. 2007, 62, 14–17. [Google Scholar] [CrossRef]
- Hamdi, A.; Mohamed, A.Y.; Kamel, C.; Tarek, M.; Jean-François, R. Analysis of surface roughness and cutting force components in hard turning with CBN tool: Prediction model and cutting conditions optimization. Measurement 2012, 45, 344–353. [Google Scholar]
- Amanda, M.; Jami, W.; Iakovos, S.; Mathias, H.; Ludwig, W.; Jürgen, R.; Nedret, C. Mechanical properties of cBN-Al composite materials. Ceram. Int. 2011, 7, 1–8. [Google Scholar]
- Ding, W.; Xu, J.; Chen, Z.; Miao, Q.; Yang, C. Interface characteristics and fracture behavior of brazed polycrystalline CBN grains using Cu-Sn-Ti alloy. Mat. Sci. Eng. A 2013, 55, 629–634. [Google Scholar] [CrossRef]
- Shan, D.; Li, Z.; Zhu, Y.; Ye, H.; Gao, K.; Yu, Y. Influence of TiO2 on the physical properties of low-temperature ceramic vitrified bond and mechanical properties of CBN composites. Ceram. Int. 2012, 38, 4573–4578. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic bath. J. Electrochm. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Zimmerman, A.F.; Palumbo, G.; Aust, K.T.; Erb, U. Mechanical properties of nickel silicon carbide nanocomposites. Mat. Sci. Eng. A 2002, 328, 137–146. [Google Scholar] [CrossRef]
- Ghaziof, S.; Kilmartin, P.A.; Gao, W. Electrochemical studies of sol-enhanced Zn-Ni-Al2O3 composite and Zn-Ni alloy coatings. J. Electroanal. Chem. 2015, 755, 63–70. [Google Scholar] [CrossRef]
- Ghaziof, S.; Gao, W. Zn-Ni-Al2O3 nano-composite coatings prepared by sol-enhanced electroplating. Appl. Surf. Sci. 2015, 351, 869–879. [Google Scholar] [CrossRef]
- Zhang, L.S.; Gui, Y.H.; Yan, L.; Wang, W. Study of Ni-P alloy electroless plating on boron nitride particles. Plat. Finish. 2009, 31, 38–40. [Google Scholar]
- Chen, J.; Wan, L. Manufacture and grinding performance of a polyimide resin-bonded cBN wheel for precision grinding of ferrous materials. Adv. Mater. Res. 2012, 496, 443–448. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, C.; Li, B.; Wang, S.; Xie, Z.; Song, Y. Ablation behavior of boron nitride based ceramic composites reinforced by continuous silicon oxynitride fiber. Ceram. Int. 2015, 41, 4768–4774. [Google Scholar] [CrossRef]


| Item | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| ρ(NiSO4·6H2O)/(g·L−1) | 275 | 275 | 275 | 275 |
| ρ(NiCl2·6H2O)/(g·L−1) | 45 | 45 | 45 | 45 |
| ρ(H3BO3)/(g·L−1) | 37.5 | 37.5 | 37.5 | 37.5 |
| ρ(SiC)/(g·L−1) | 0 | 5 | 10 | 15 |
| pH | 4.7~5.5 | 4.7~5.5 | 4.7~5.5 | 4.7~5.5 |
| Temperature/(°C) | 25~35 | 25~35 | 25~35 | 25~35 |
| Velocity/(rpm) | 3 | 3 | 3 | 3 |
| Current/(A) | 1.0~1.5 | 1.0~1.5 | 1.0~1.5 | 1.0~1.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, Y.; Zhao, J.; Chen, J.; Jiang, Y. Preparation and Characterization of Ni Spines Grown on the Surface of Cubic Boron Nitride Grains by Electroplating Method. Materials 2016, 9, 153. https://doi.org/10.3390/ma9030153
Gui Y, Zhao J, Chen J, Jiang Y. Preparation and Characterization of Ni Spines Grown on the Surface of Cubic Boron Nitride Grains by Electroplating Method. Materials. 2016; 9(3):153. https://doi.org/10.3390/ma9030153
Chicago/Turabian StyleGui, Yanghai, Jianbo Zhao, Jingbo Chen, and Yuanli Jiang. 2016. "Preparation and Characterization of Ni Spines Grown on the Surface of Cubic Boron Nitride Grains by Electroplating Method" Materials 9, no. 3: 153. https://doi.org/10.3390/ma9030153