Cu-Doped ZnO Thin Films Deposited by a Sol-Gel Process Using Two Copper Precursors: Gas-Sensing Performance in a Propane Atmosphere
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Deposition Conditions
2.2. Characterization Conditions
3. Results and Discussion
3.1. Crystal Structure of the Films
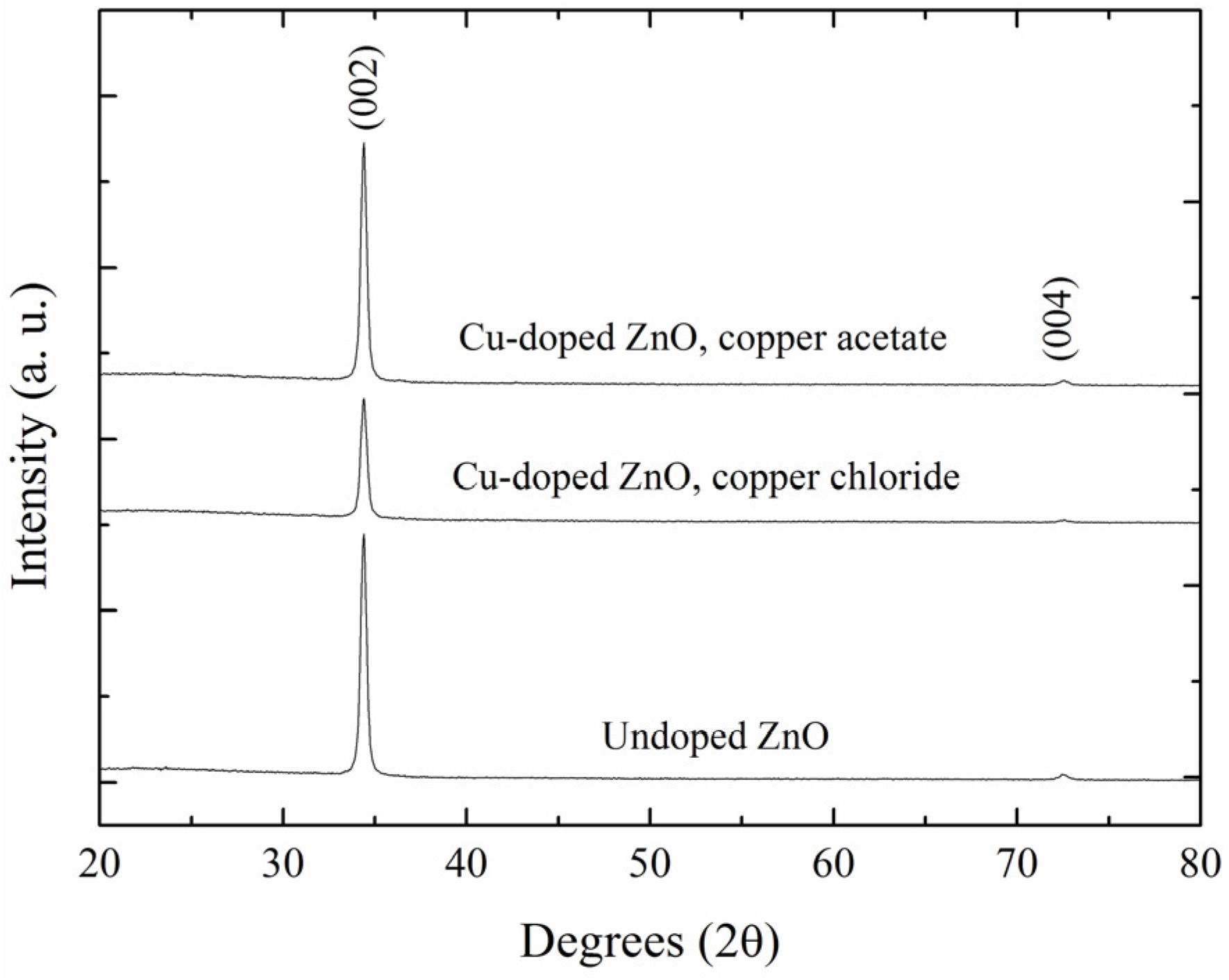
| ID Sample | FWHM (°) | 2θ (°) | c (Å) | D (Å) |
|---|---|---|---|---|
| Cu-doped ZnO, copper chloride | 0.2048 | 34.37 | 5.212 | 401.4 |
| Cu-doped ZnO, copper acetate | 0.2036 | 34.38 | 5.210 | 403.8 |
| Undoped ZnO | 0.2032 | 34.39 | 5.207 | 405.5 |
3.2. Morphological Properties
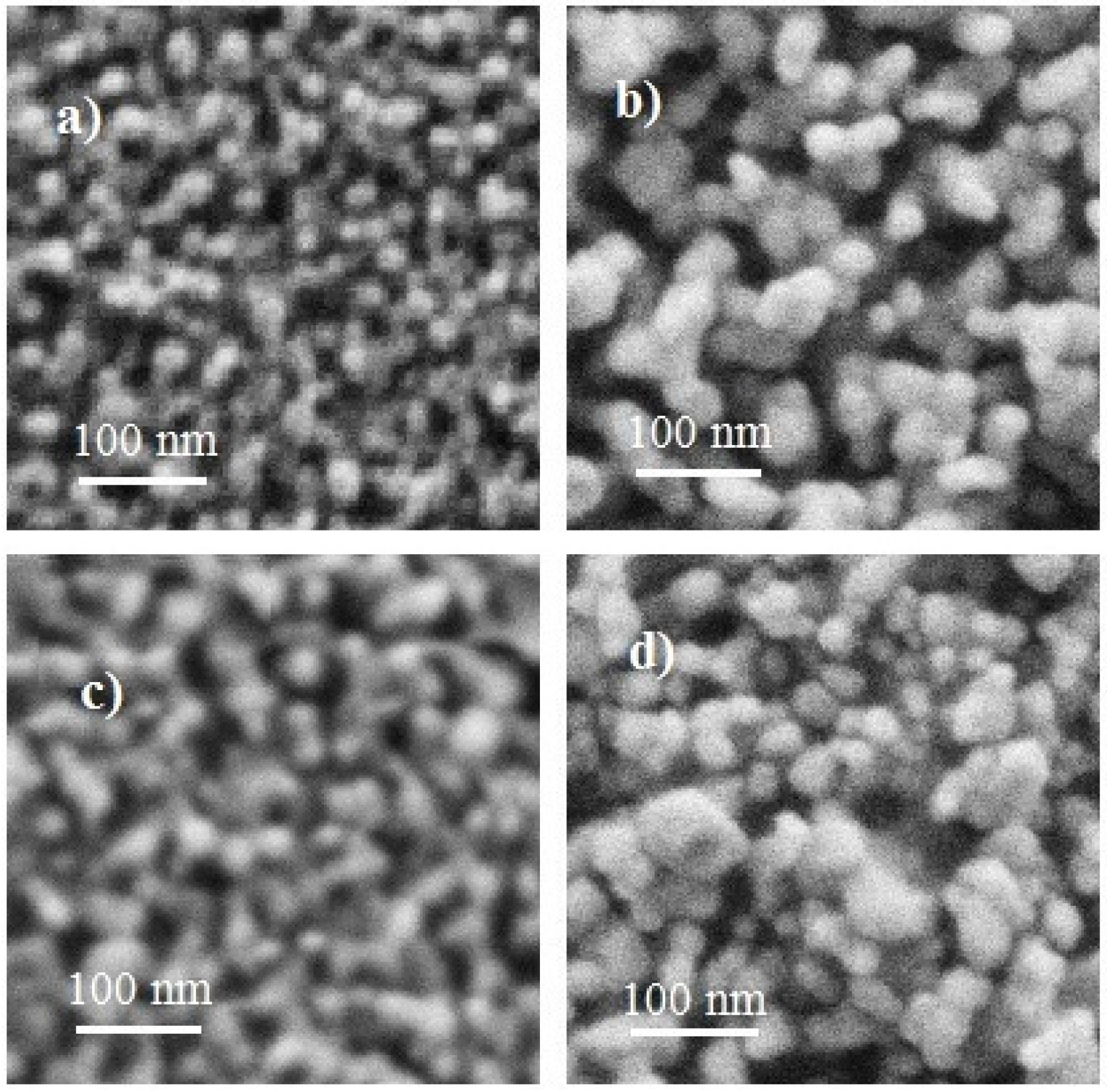
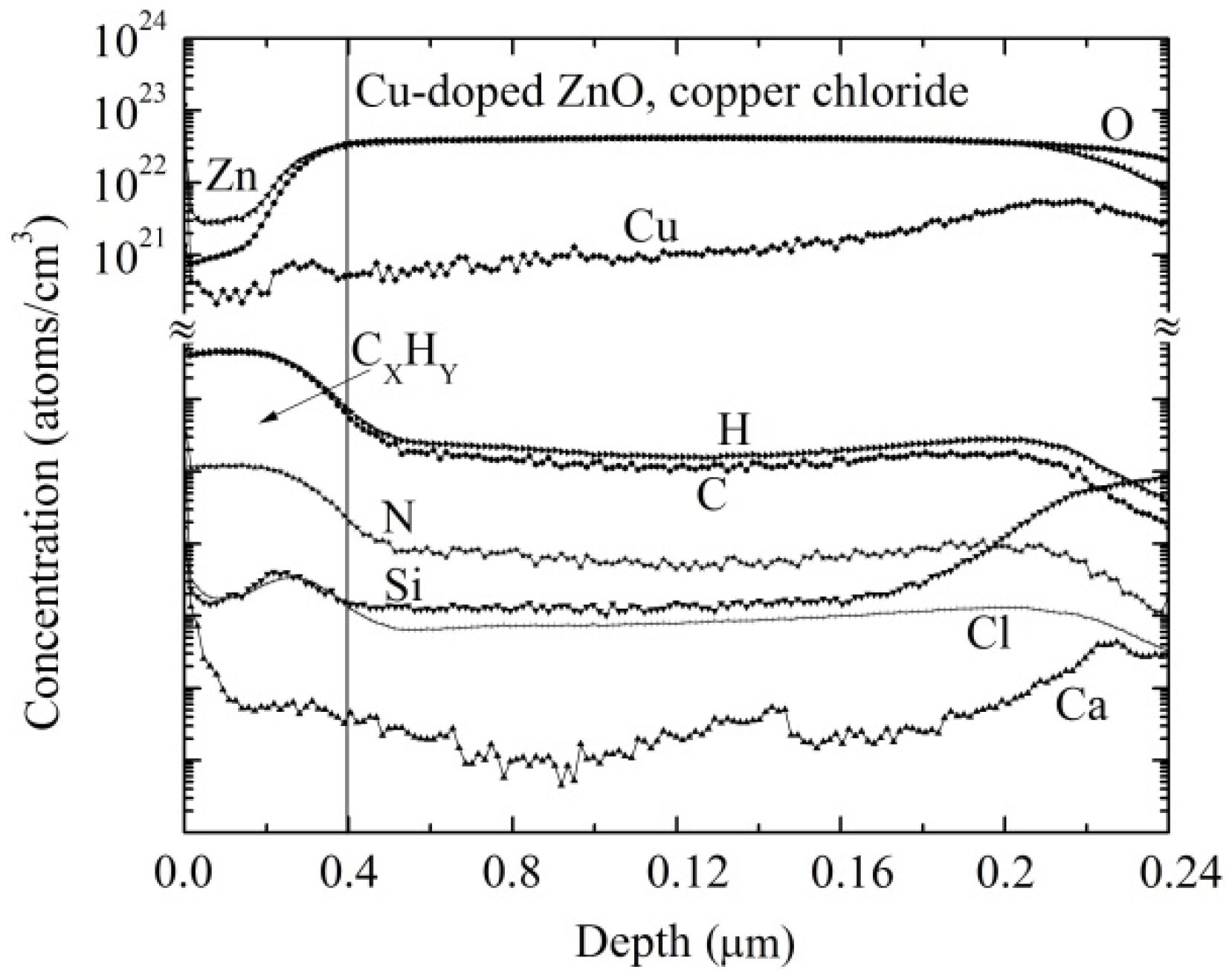
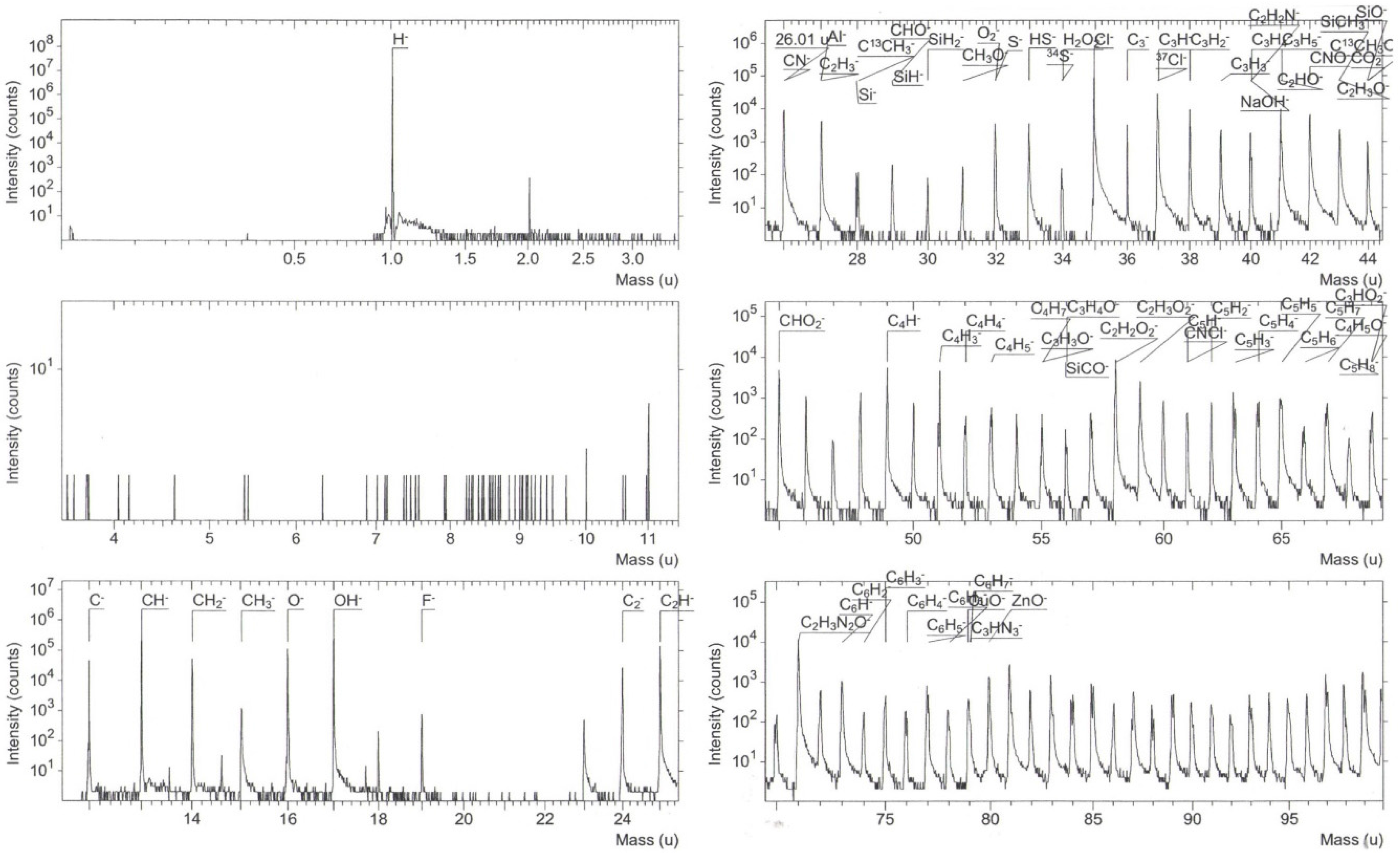
3.3. Composition Analysis
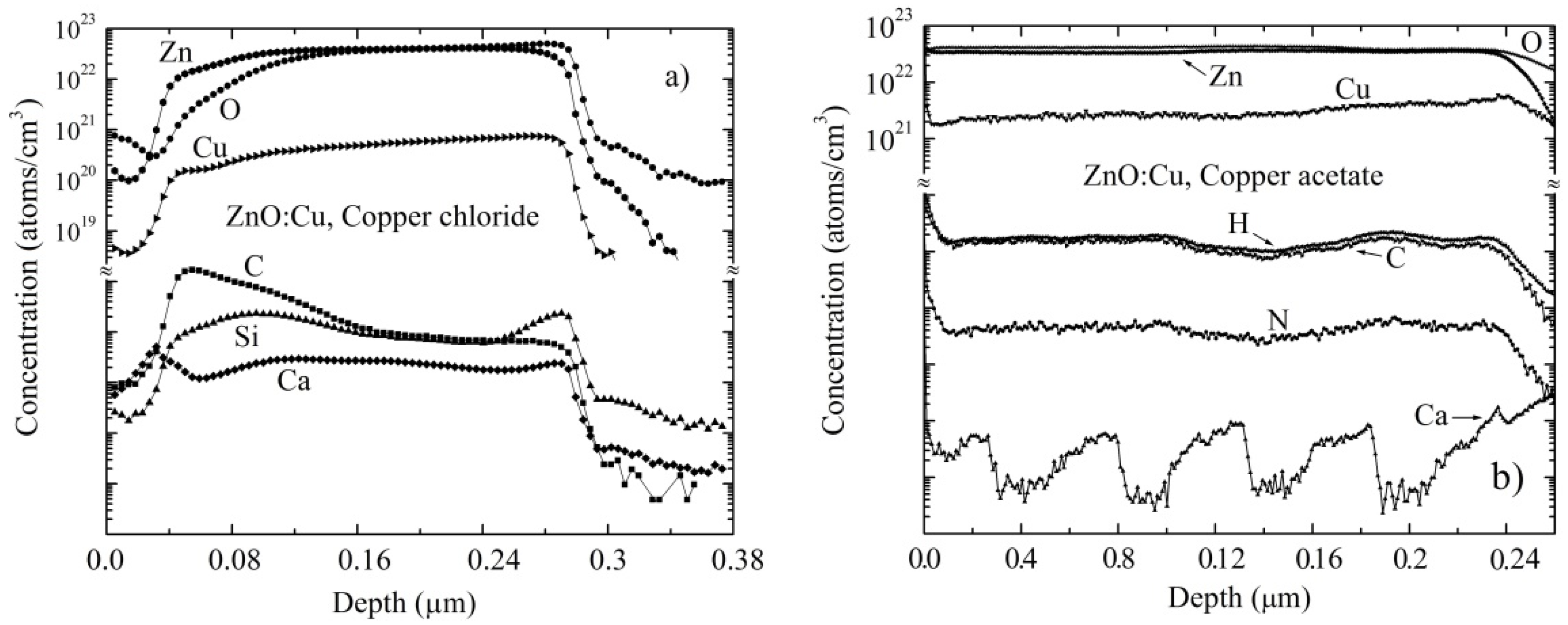
3.4. Sensing Properties

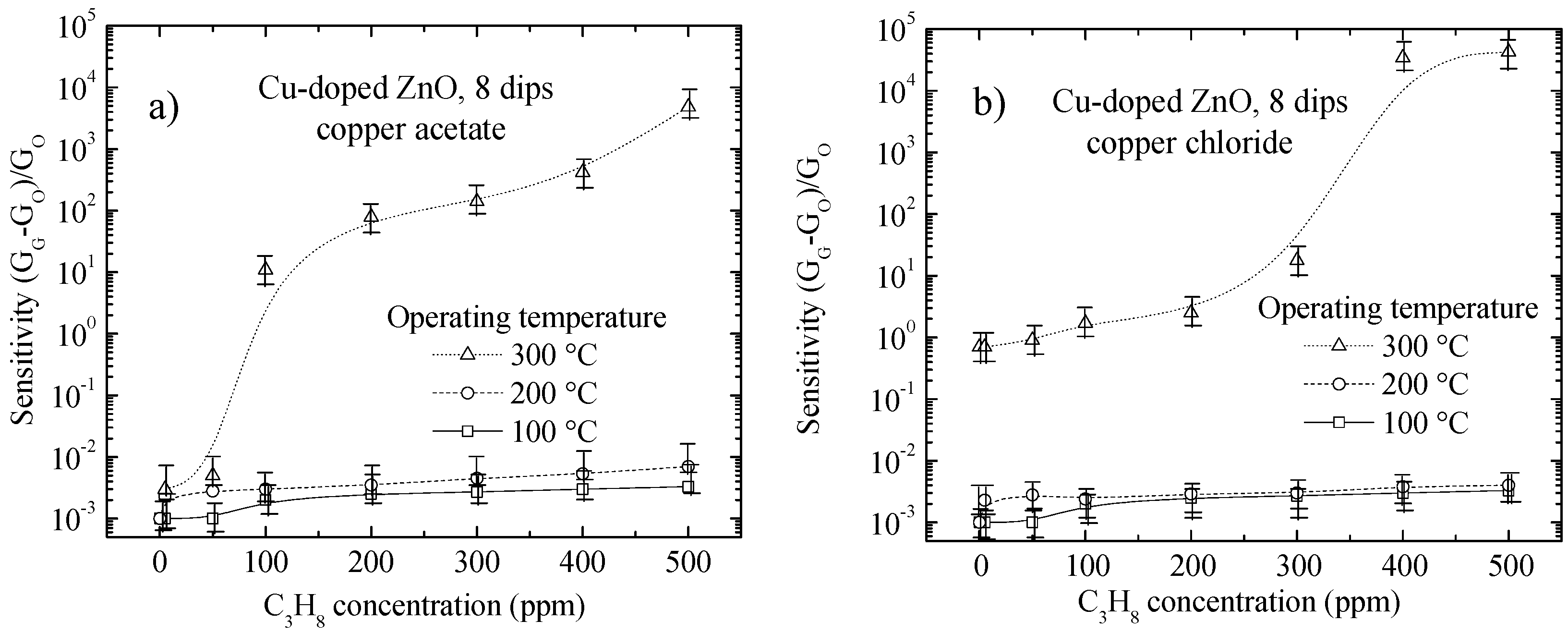
3.4.1. Effect of Surface Morphology (Porosity) on Sensing Properties
| Film | Film Thickness for 6 Dips (nm) | Film Thickness for 8 Dips (nm) |
|---|---|---|
| Cu-doped ZnO (copper chloride) | 300 | 350 |
| Cu-doped ZnO (copper acetate) | 242 | 335 |
| Morphological Parameters for Different Number Dips | Cu-Doped ZnO (Copper Acetate) | Cu-Doped ZnO (Copper Chloride) | ||
|---|---|---|---|---|
| Number of dips | 6 | 8 | 6 | 8 |
| RMS surface roughness | 54.32 | 60.94 | 48.80 | 54.50 |
| Empty volume/grain volume (%) | 54.62 | 46.88 | 58.06 | 48.98 |
3.4.2. Adsorption of Propane Gas over the Cu-Doped ZnO Surface

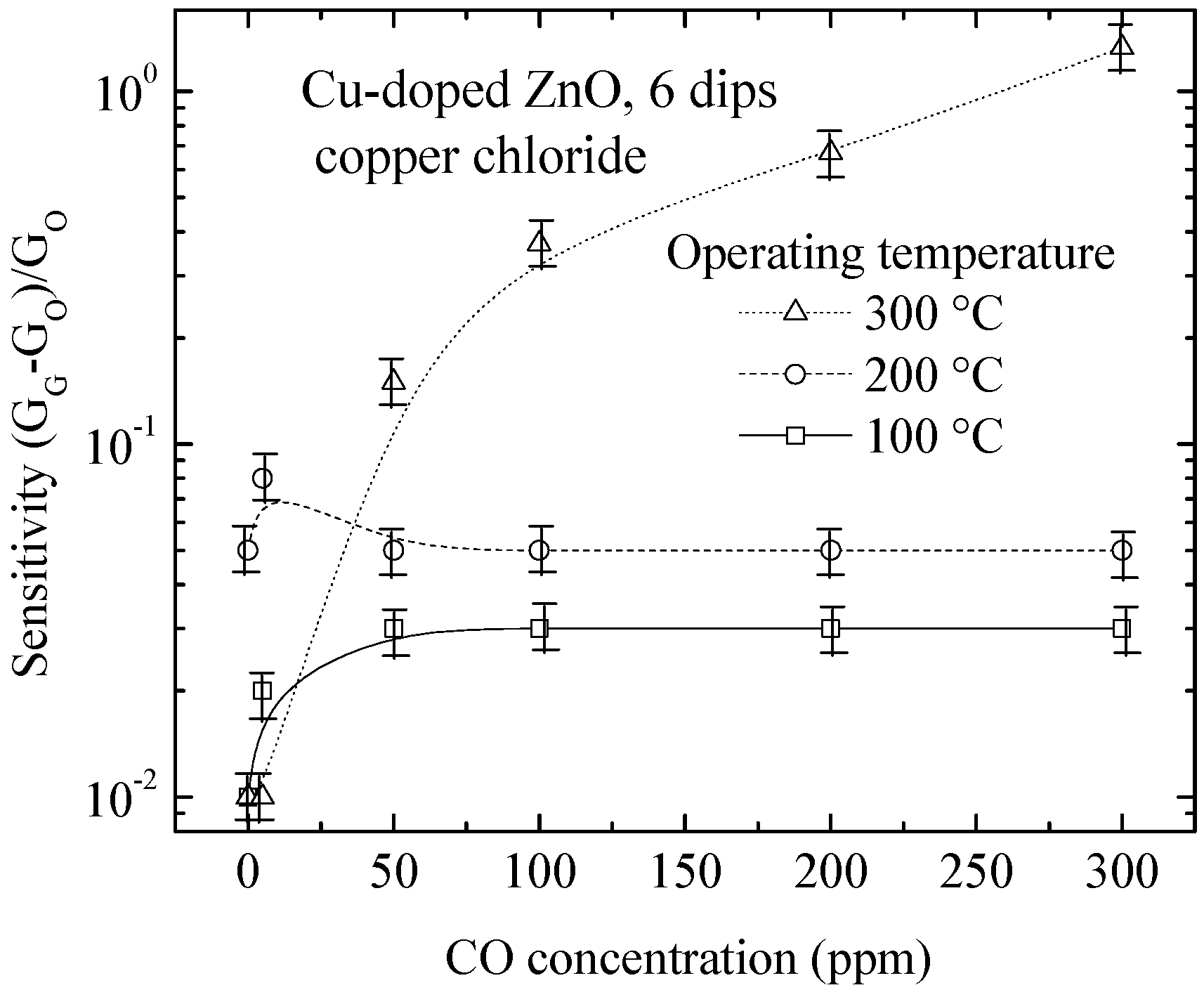
3.4.3. Structural and Compositional Analysis of Cu-Doped ZnO Post-Sensed Films Obtained from Copper Chloride and CO Gas Sensitivity

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Zhao, Y.; Wang, G.; Zhou, H.; Geng, C.; Wu, C.; Xu, J. Enhancing the efficiency of ZnO/Cu2O inorganic nanostructure solar cells simply by CdS quantum dots. Sol. Enery. Mater. Sol. Cells 2014, 130, 387–392. [Google Scholar] [CrossRef]
- Qiu, M.F.; Wei, C.; Guo, W.L.; Zhi, D.L.; Zhe, C.; Chuan, B.X.; Ya, F.T.; Zhi, B.M. Blue/green electroluminescence from a ZnO nanorods/p-GaN heterojunction light emitting diode under different reverse bias. Appl. Surf. Sci. 2014, 293, 225–228. [Google Scholar]
- Zhou, D.Z.; Li, B.; Wang, H.L.; Salik, M.; Wu, H.H.; Hu, Z.F.; Gao, S.; Peng, Y.F.; Yi, L.X.; Zhang, X.Q.; Wang, Y.S. Fabrication and electrical characterization of Li–N dual doped ZnO thin film transistor. Appl. Surf. Sci. 2014, 305, 474–476. [Google Scholar] [CrossRef]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. N-doped ZnO based fast response ultraviolet photoconductive detector. Solid State Electron. 2012, 68, 22–26. [Google Scholar] [CrossRef]
- Mortezaali, A.; Moradi, R. The correlation between the substrate temperature and morphological ZnO nanostructures for H2S gas sensors. Sens. Actuators A 2014, 206, 30–34. [Google Scholar] [CrossRef]
- Kofstad, P. Nonstochiometry, Diffusion, and Electrical Conductivity in Binary Metal Oxides; Wiley: New York, NY, USA, 1972; pp. 8–9. [Google Scholar]
- Moseley, P.T.; Tofield, B.C. Solid State Gas Sensor; Adam Hilger: Bristol, UK, 1987. [Google Scholar]
- Shinde, V.R.; Gujar, T.P.; Lokhande, C.D. Enhanced response of porous ZnO nanobeads towards LPG: Effect of Pd sensitization. Sens. Actuators B Chem. 2007, 123, 701–706. [Google Scholar] [CrossRef]
- Shishiyanu, S.T.; Shishiyanu, T.S.; Lupan, O.I. Sensing characteristics of tin-doped ZnO thin films as NO2 gas sensor. Sens. Actuators B Chem. 2005, 107, 379–386. [Google Scholar] [CrossRef]
- Shin, M.C.; Lay, G.T.; Wei, H.L.; Yen, H.S.; Min, H.H. ZnO:Al thin film gas sensor for detection of ethanol vapor. Sensors 2006, 6, 1420–1427. [Google Scholar]
- Niu, X.; Du, W.; Du, W. Preparation and gas sensing properties of ZnM2O4 (M = Fe, Co, Cr). Sens. Actuators B Chem. 2004, 99, 405–409. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Y.; Zhou, M. Co gas sensing of Pd-doped ZnO nanofibres synthesized by electrospinning method. Mater. Lett. 2010, 64, 2284–2286. [Google Scholar] [CrossRef]
- Yu, P.; Wang, J.; Du, H.; Yao, P.; Hao, Y.; Li, X. Y-doped ZnO nanorods by hydrothermal method and their acetone gas sensitivity. J. Nanomater. 2013, 2013, 1–6. [Google Scholar]
- Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Ono, L.K.; Roldan-Cuenya, B.; Tiginyanu, I.M.; Ursaki, V.V.; Sontea, V.; Schulte, A. Synthesis and characterization of Cu-doped ZnO one-dimensional structures for miniaturized sensor applications with faster response. Sens. Actuators A Phys. 2013, 189, 399–408. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Harsch, A.; Russell, N.V.; Tse, K.F.; Whitam, A.R.; Wilson, A. Nanocrystalline copper doped zinc oxide gas sensors. In Radiation Effects and Defects in Solids: Incorporating Plasma Science and Plasma Technology; Taylor & Francis Group: London, UK, 2006; Volume 137, pp. 51–55. [Google Scholar]
- Paraguay, F.; Yoshida, M.M.; Morales, J.; Solis, J.; Estrada, W. Doping effects on the response of thin film ZnO gas sensor to ethanol vapor. Superf. Vacío 1999, 9, 245–247. [Google Scholar]
- Patil, A.V.; Dighavkar, C.G.; Sonawane, S.K.; Patil, S.J.; Borse, R.Y. Formulation and characterization of Cu doped ZnO thick films as LPG gas sensor. Sens. Transducer J. 2010, 9, 11–20. [Google Scholar]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Facile deposition of ZnO:Cu films: Structural and optical characterization. Mater. Sci. Semicond. Process. 2015, 30, 561–570. [Google Scholar] [CrossRef]
- Hartmann, A.; Puchert, M.K.; Lamb, R.N. Influence of copper dopants on the resistivity of ZnO films. Surf. Interface Anal. 1996, 24, 671–674. [Google Scholar] [CrossRef]
- Puchert, M.K.; Hartmann, A.; Lamb, R.N. Highly resistive sputtered ZnO films implanted with copper. J. Mater. Res. 1996, 11, 2463–2469. [Google Scholar] [CrossRef]
- Shukla, R.K.; Srivastava, A.; Kumar, N.; Pandey, A.; Pandey, M. Optical and sensing properties of Cu doped ZnO nanocrystalline thin films. J. Nanomater. 2015, 1–10. [Google Scholar]
- Furukawa, A.; Ogasawara, N.; Yokozawa, R.; Tokunaga, A. Electron trap level of Cu-doped ZnO. Jpn. J. Appl. Phys. 2008, 47, 8799. [Google Scholar] [CrossRef]
- Qasem, A.D.; Saleem, G.R.; Zain, H.Y.; Mohammed, A.G. Crystalline nanostructured Cu doped ZnO thin films grown at room temperature by pulsed laser deposition technique and their characterization. Appl. Surf. Sci. 2013, 270, 104–108. [Google Scholar]
- Tao, Y.M.; Ma, S.Y.; Chen, H.X.; Meng, J.X.; Hou, L.L.; Jia, Y.F.; Shang, X.R. Effect of the oxygen partial pressure on the microstructure and optical properties of ZnO:Cu films. Vacuum 2011, 85, 744–748. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloy. Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Caglar, M.; Yakuphanoglu, F. Structural and optical properties of copper doped ZnO films derived by sol-gel. Appl. Surf. Sci. 2012, 258, 3039–3044. [Google Scholar] [CrossRef]
- West, C.; Robbins, D.J.; Dean, P.J.; Hays, W. The luminescence of copper in zinc oxide. Phys. B C 1983, 116, 492–499. [Google Scholar] [CrossRef]
- Kröger, F.A.; Vink, H.J. Relations between the concentrations of imperfections in crystalline solids. Solid State Phys. 1956, 3, 307–435. [Google Scholar]
- Shewale, P.S.; Patil, V.B.; Shin, S.W.; Kim, J.H.; Uplane, M.D. H2S gas sensing properties of nanocrystalline Cu-doped ZnO thin films prepared by advanced spray pyrolysis. Sens. Actuators B Chem. 2013, 186, 226–234. [Google Scholar] [CrossRef]
- Dzyaloshinskii, I.E.; Lifshitz, E.M.; Pitaevskii, L.P. General theory of Van Der Waals' forces. Sov. Phys. Uspekhi 1961, 4, 153–176. [Google Scholar] [CrossRef]
- Franke, M.E.; Koplin, T.J.; Simon, U. Metal and metal oxide nanoparticles in chemiresistors: Does the nanoscale matter? Small 2006, 2, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Schierbaum, K.D.; Weimar, U.; Göpel, W.; Kowalkowski, R. Conductance, work function and catalytic activity of SnO2-based gas sensors. Sens. Actuators B Chem. 1991, 3, 205–214. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Masel, R.I. Adsorption I: The binding of molecules to surfaces. In Principles of Adsorption and Reaction on Solid Surfaces; John Wiley & Sons, Inc: New York, NY, USA, 1996. [Google Scholar]
- Gómez-Pozos, H.; Gonzalez, J.L.; Torres, G.A.; de la Luz Olvera, M.; Castañeda, L. Physical characterization and effect of effective surface area on the sensing properties of tin dioxide thin solid films in a propane atmosphere. Sensors 2014, 14, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, C.; Sun, X.; Li, H. Synthesis of mesoporous structured material based on tin oxide. Microporous Mesoporous Mater. 2001, 49, 171–178. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. The role of grain size on the thermal instability of nanostructure metal oxides used in gas sensor applications and approaches for grain-size stabilization. Prog. Cryst. Growth Charact. Mater. 2012, 58, 167–208. [Google Scholar] [CrossRef]
- Korotcenkov, G. The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors. Mater. Sci. Eng. R 2008, 61, 1–39. [Google Scholar] [CrossRef]
- Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Serrini, P.; Briois, V.; Horrillo, M.C.; Traverse, A.; Manes, L. Chemical composition and crystalline structure of SnO2 thin films used as gas sensors. Thin Solid Films 1997, 304, 113–122. [Google Scholar] [CrossRef]
- Musat, V.; Rego, A.M.; Monteiro, R.; Fortunato, E. Microstructure and gas-sensing properties of sol–gel ZnO. Thin Solid Films 2008, 516, 1512–1515. [Google Scholar] [CrossRef]
- Pubchem Open Chemistry Data Base. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/propane (accessed on 26 March 2005).
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall Inc: Upper Saddle River, NJ, USA, 2001; pp. 167–171. [Google Scholar]
- Feldman, L.C.; Mayer, J.W. Fundamentals of Surface and Thin Film Analysis; Prentice Hall Inc: Upper Saddle River, NJ, USA, 1986. [Google Scholar]
- Sze, S.M. Semiconductor Sensors, 1st ed.; Wiley: New York, NY, USA, 1994; pp. 9–10. [Google Scholar]
- McMurdie, H.F.; Morris, M.C.; Evans, E.H.; Paretzkin, B.; Wong-Ng, W.; Ettlinger, L.; Hubbard, C.R. Standard X-Ray Diffraction Powder Patterns from the JCPDS Research Associateship; Cambridge University Press: Cambridge, UK, 1986; Volume 1, pp. 64–77. [Google Scholar]
- Shu, S.Y.; Water, W.; Liaw, J.T. Influence of postdeposition annealing on the properties of ZnO films prepared by RF magnetron sputtering. J. Eur. Ceram. Soc. 2003, 23, 1593–1598. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons, Inc: New York, NY, USA, 2005; pp. 38–55. [Google Scholar]
- Reeber, R.R. Lattice parameters of ZnO from 4.2° to 296°K. J. Appl. Phys. 1970, 41, 5063–5066. [Google Scholar] [CrossRef]
- Caglar, Y. Sol–gel derived nanostructure undoped and cobalt doped ZnO: Structural, optical and electrical studies. J. Alloy. Compd. 2013, 560, 181–188. [Google Scholar] [CrossRef]
- Schuler, T.; Krajewski, T.; Grobelsek, I.; Aegerter, M.A. Influence of structure zone model parameters on the electrical properties of ZnO:Al sol–gel coatings. Thin Solid Films 2006, 502, 67–71. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, Z.; Li, W.; Qiu, J. Preparation of ZnO porous thin films by sol–gel method using PEG template. Mater. Lett. 2005, 59, 3620–3625. [Google Scholar] [CrossRef]
- Gomez, H.; Maldonado, A.; de la L Olvera, M.; Acosta, D.R. Gallium-doped ZnO thin films deposited by chemical spray. Sol. Energy Mater. Sol. Cells 2005, 87, 107–116. [Google Scholar] [CrossRef]
- Gómez, H.; Maldonado, A.; Castanedo-Pérez, R.; Torres-Delgado, G.; de la L Olvera, M. Properties of Al-doped ZnO thin films deposited by a chemical spray process. Mater. Charact. 2007, 58, 708–714. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing, 2nd ed.; Prentice Hall Inc: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Andrew, Z. Physics at Surfaces; Cambridge University Press: Cambridge, UK, 1988; pp. 204–231. [Google Scholar]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Pozos, H.; Arredondo, E.J.L.; Maldonado Álvarez, A.; Biswal, R.; Kudriavtsev, Y.; Pérez, J.V.; Casallas-Moreno, Y.L.; Olvera Amador, M.D.l.L. Cu-Doped ZnO Thin Films Deposited by a Sol-Gel Process Using Two Copper Precursors: Gas-Sensing Performance in a Propane Atmosphere. Materials 2016, 9, 87. https://doi.org/10.3390/ma9020087
Gómez-Pozos H, Arredondo EJL, Maldonado Álvarez A, Biswal R, Kudriavtsev Y, Pérez JV, Casallas-Moreno YL, Olvera Amador MDlL. Cu-Doped ZnO Thin Films Deposited by a Sol-Gel Process Using Two Copper Precursors: Gas-Sensing Performance in a Propane Atmosphere. Materials. 2016; 9(2):87. https://doi.org/10.3390/ma9020087
Chicago/Turabian StyleGómez-Pozos, Heberto, Emma Julia Luna Arredondo, Arturo Maldonado Álvarez, Rajesh Biswal, Yuriy Kudriavtsev, Jaime Vega Pérez, Yenny Lucero Casallas-Moreno, and María De la Luz Olvera Amador. 2016. "Cu-Doped ZnO Thin Films Deposited by a Sol-Gel Process Using Two Copper Precursors: Gas-Sensing Performance in a Propane Atmosphere" Materials 9, no. 2: 87. https://doi.org/10.3390/ma9020087





