Effects of Acoustic Modulation and Mixed Fuel on Flame Synthesis of Carbon Nanomaterials in an Atmospheric Environment
Abstract
:1. Introduction
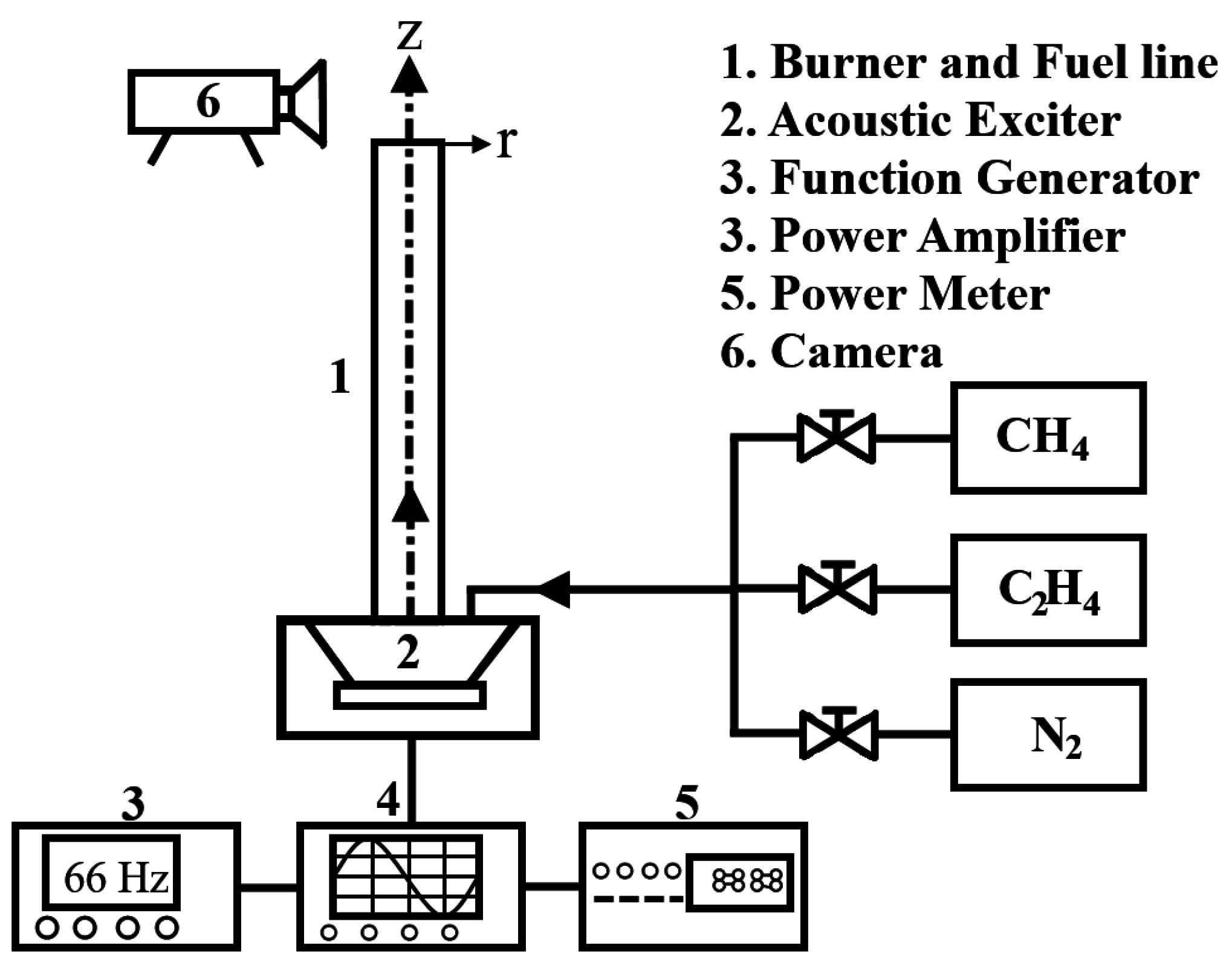
2. Experimental Setup and Method
2.1. Acoustically Modulated Jet Flow Syetem
2.2. Measurement and Sampling Systems
3. Results and Discussion
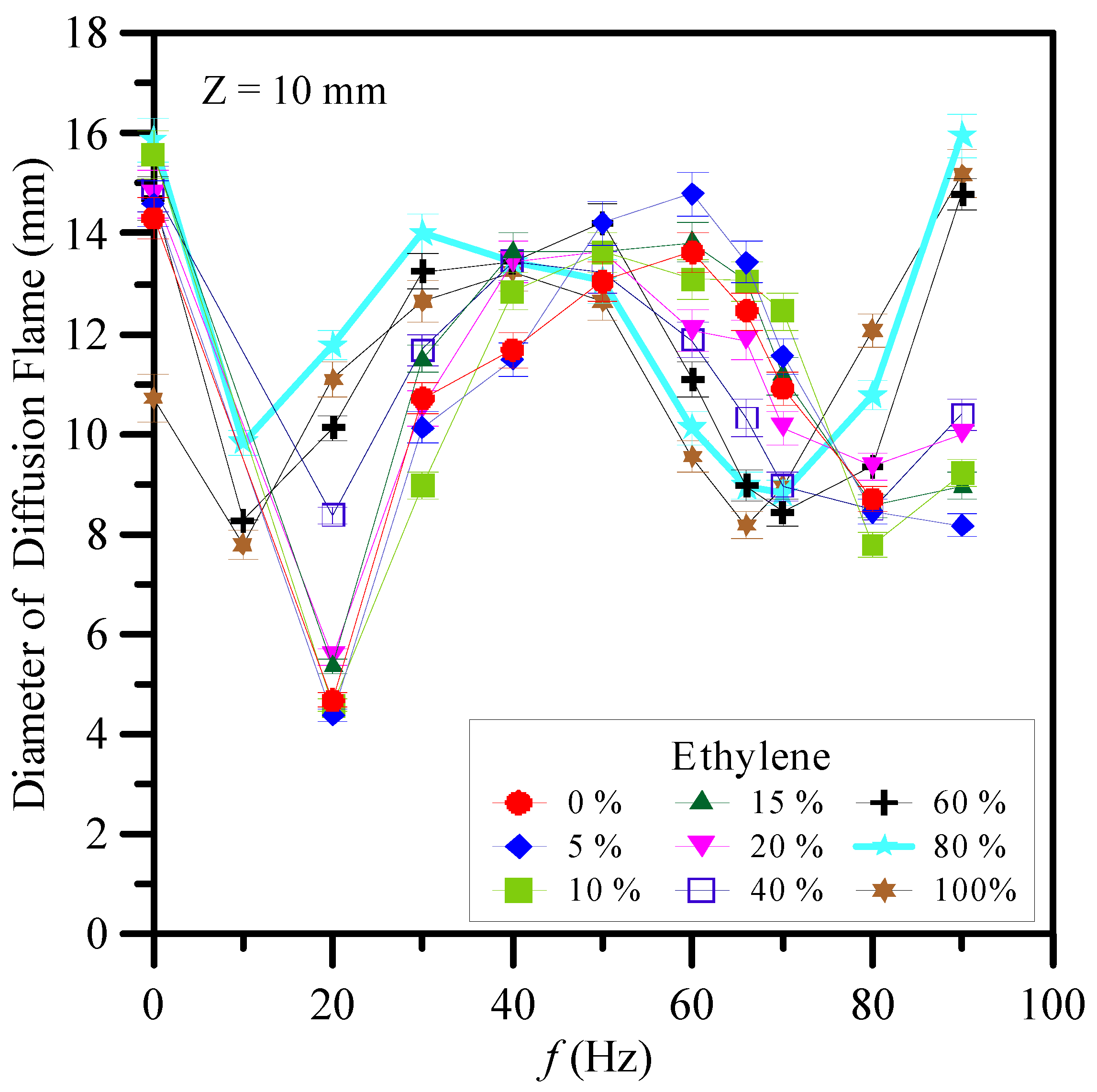
3.1. Flame Structures
3.2. Temperature Measurements
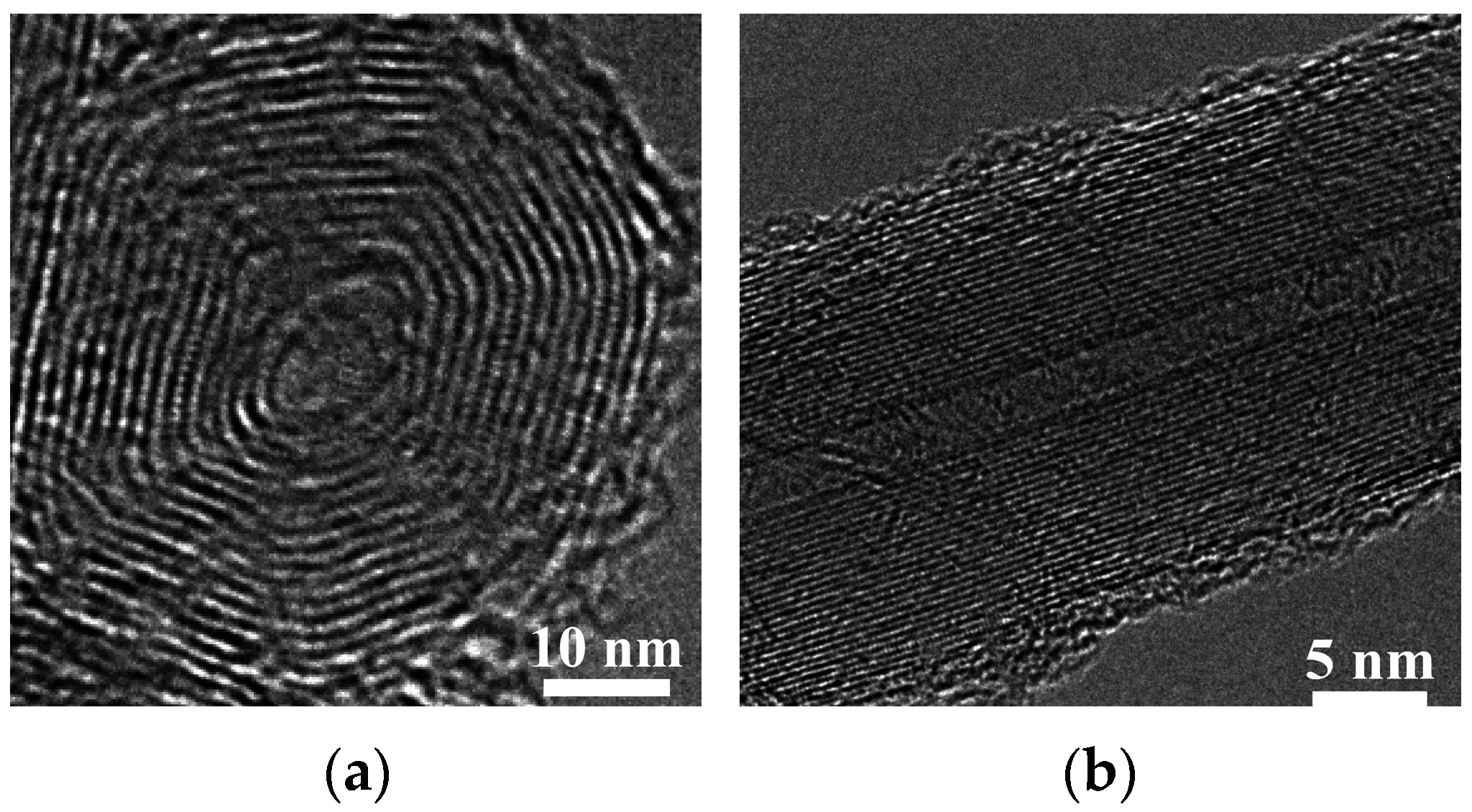
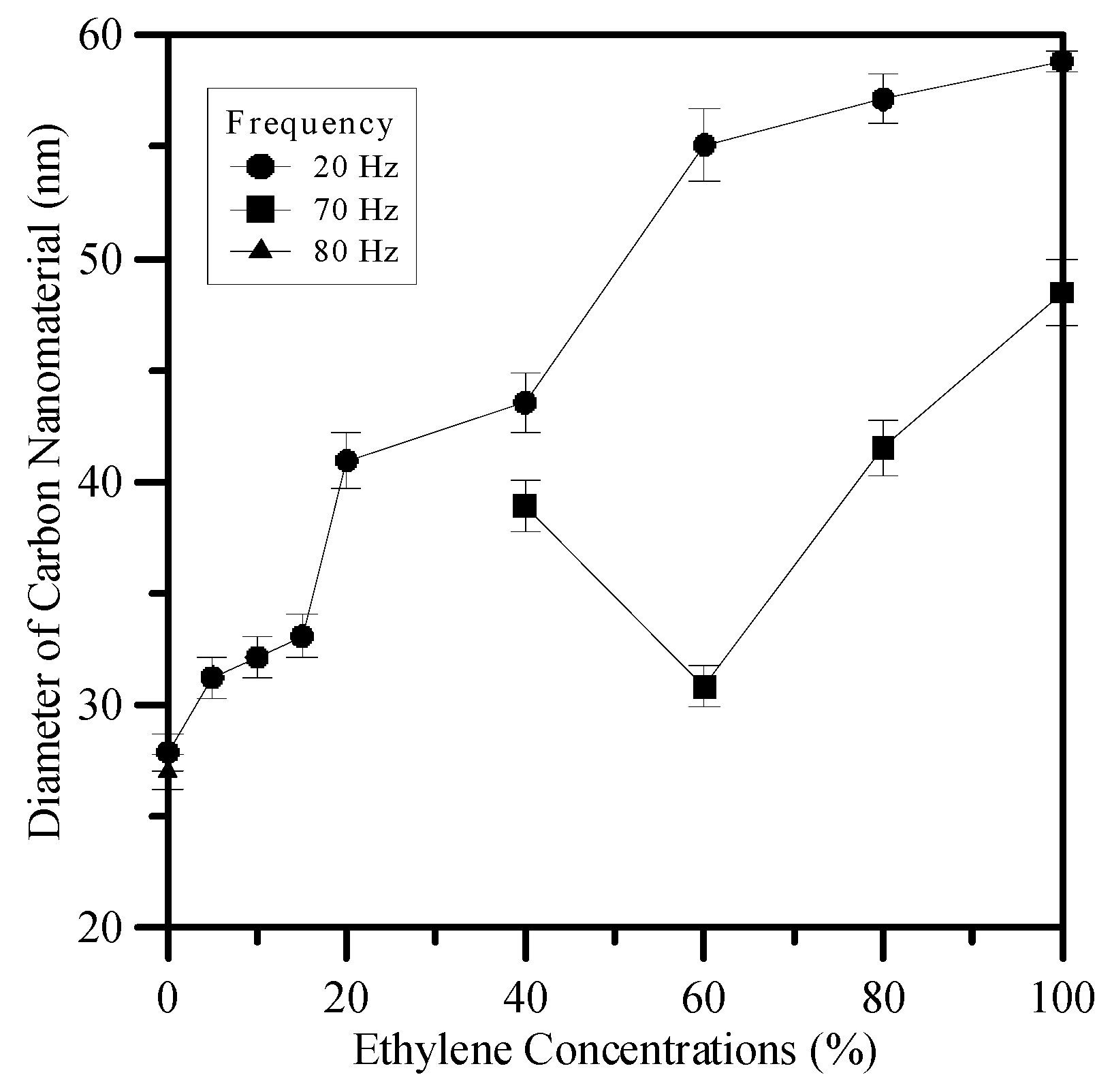
3.3. Flame Synthesis of Carbon Nanomaterials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Kroto, H.W. Carbon onions introduce new flavour to fullerene studies. Nature 1992, 359, 670–671. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. R 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Peled, A. Synthesis of nanoparticles in the gas phase for electronic, optical and magnetic applications—A review. J. Aerosol Sci. 1998, 29, 511–535. [Google Scholar] [CrossRef]
- Nakazawa, S.; Yokomori, T.; Mizomoto, M. Flame synthesis of carbon nanotubes in a wall stagnation flow. Chem. Phys. Lett. 2005, 403, 158–162. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Saveliev, A.V.; Kennedy, L.; Jimenez, W.C. Combustion synthesis of carbon nanotubes and related nanostructures. Prog. Energy Combust. Sci. 2010, 36, 696–727. [Google Scholar] [CrossRef]
- Oulanti, H.; Laurent, F.; Le-Huu, T.; Durand, B.; Donnet, J.B. Growth of carbon nanotubes on carbon fibers using the combustion flame oxy-acetylene method. Carbon 2015, 95, 261–267. [Google Scholar] [CrossRef]
- Hou, S.S.; Huang, W.C. Influence of oxygen concentration, fuel composition, and strain rate on synthesis of carbon nanomaterials. J. Nanopart. Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Ren, Y.; Biswas, P.; Stephen, D.T. Flame aerosol synthesis of nanostructured materials and functional devices: Processing, modeling, and diagnostics. Prog. Energy Combust. Sci. 2016, 55, 1–59. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Ticich, T.M.; Curtis, V.E. Diffusion flame synthesis of single-walled carbon nanotubes. Chem. Phys. Lett. 2000, 323, 217–223. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, H.; Stephen, D.T. Carbon nanotube synthesis on catalytic metal alloys in methane/air counterflow diffusion flames. Proc. Combust. Inst. 2007, 31, 1839–1847. [Google Scholar] [CrossRef]
- Camacho, J.; Choudhuri, A.R. Effects of fuel compositions on the structure and yield of flame synthesized carbon nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2007, 15, 99–111. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Saveliev, A.V.; Kennedy, L.A.; Fridman, A. Formation of carbon nanotubes in counter-flow, oxy-methane diffusion flames without catalyst. Chem. Phys. Lett. 2002, 354, 20–24. [Google Scholar] [CrossRef]
- Saveliev, A.V.; Merchan-Merchan, W.; Kennedy, L.A. Metal catalyzed synthesis of carbon nanostructures in an opposed flow methane oxygen flame. Combust. Flame 2003, 135, 27–33. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Saveliev, A.V.; Kennedy, L.A. High-rate flame synthesis of vertically aligned carbon nanotubes using electric field control. Carbon 2004, 42, 599–608. [Google Scholar] [CrossRef]
- Xu, F.; Liu, X.; Stephen, D.T. Synthesis of carbon nanotubes on metal alloy substrates with voltage bias in methane inverse diffusion flames. Carbon 2006, 44, 570–577. [Google Scholar] [CrossRef]
- Yuan, L.; Saito, K.; Pan, C.; Williams, F.A.; Gordon, A.S. Nanotubes from methane flames. Chem. Phys. Lett. 2001, 340, 237–241. [Google Scholar] [CrossRef]
- Yuan, L.; Li, T.; Saito, K. Synthesis of multiwalled carbon nanotubes using methane/air diffusion flames. Proc. Combust. Inst. 2002, 29, 1087–1092. [Google Scholar] [CrossRef]
- Yuan, L.; Saito, K.; Hu, W.; Chen, Z. Ethylene flame synthesis of well-aligned multi-walled carbon nanotubes. Chem. Phys. Lett. 2001, 346, 23–28. [Google Scholar] [CrossRef]
- Li, T.X.; Zhang, H.G.; Wang, F.J.; Chen, Z.; Saito, K. Synthesis of carbon nanotubes on Ni-alloy and Si-substrates using counterflow methane–air diffusion flames. Proc. Combust. Inst. 2007, 31, 1849–1856. [Google Scholar] [CrossRef]
- Du, X.; Liu, H.Y.; Zhou, C.; Moody, S.; Mai, Y.W. On the flame synthesis of carbon nanotubes grafted onto carbon fibers and the bonding force between them. Carbon 2012, 50, 2347–2350. [Google Scholar] [CrossRef]
- Du, X.; Xu, F.; Liu, H.Y.; Miao, Y.; Guo, W.G.; Mai, Y.W. Improving the electrical conductivity and interface properties of carbon fiber/epoxy composites by low temperature flame growth of carbon nanotubes. RSC Adv. 2016, 6, 48896–48904. [Google Scholar] [CrossRef]
- Dhand, V.; Prasad, J.S.; Rao, M.V.; Bharadwaj, S.; Anjaneyulu, Y.; Jain, P.K. Flame synthesis of carbon nano onions using liquefied petroleum gas without catalyst. Mater. Sci. Eng. C Mater. 2013, 33, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Merchan-Merchan, W.; Richter, H.; Saveliev, A.; Kennedy, L.A. Fullerene formation in atmospheric pressure opposed flow oxy-flames. Proc. Combust. Inst. 2005, 30, 2545–2552. [Google Scholar] [CrossRef]
- Liu, T.C.; Li, Y.Y. Synthesis of carbon nanocapsules and carbon nanotubes by an acetylene flame method. Carbon 2006, 44, 2045–2050. [Google Scholar] [CrossRef]
- Chung, D.H.; Lin, T.H.; Hou, S.S. Flame synthesis of carbon nano-onions enhanced by acoustic modulation. Nanotechnology 2010, 21, 435604–435614. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.H.; Lin, T.H. Nitrogen dilution effect on flame synthesis of carbon nanostructures with acoustic modulation. J. Phys. Chem. C 2011, 115, 16287–16294. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic growth of carbon filament. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Li, T.X.; Kuwana, K.; Saito, K.; Zhang, H.; Chen, Z. Temperature and carbon source effects on methane—Air flame synthesis of CNTs. Proc. Combust. Inst. 2009, 32, 1855–1861. [Google Scholar] [CrossRef]
- Hou, S.S.; Chung, D.H.; Lin, T.H. High-yield synthesis of carbon nano-onions in counterflow diffusion flames. Carbon 2009, 47, 938–947. [Google Scholar] [CrossRef]
- Hu, W.C.; Hou, S.S.; Lin, T.H. Analysis on controlling factors for the synthesis of carbon nanotubes and nano-onions in counterflow diffusion flames. J. Nanosci. Nanotechnol. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Gore, J.P.; Minis, I.; Jang, J.H. Acoustically modulated free jet flames. In Proceedings of the Aerospace Sciences Meeting, Reno, NV, USA, 8–11 January 1990.
- Hou, S.S.; Chen, K.M.; Yang, Z.Y.; Lin, T.H. Enhanced synthesis of carbon nanomaterials using acoustically excited methane diffusion flames. Materials 2015, 8, 4805–4816. [Google Scholar] [CrossRef]
- Kimura, I. Stability of laminar-jet flames. Proc. Combust. Inst. 1965, 10, 295–300. [Google Scholar] [CrossRef]
- Lakshminarasimhan, K.; Clemens, N.T.; Ezekoye, O.A. Characteristics of strongly-forced turbulent jets and non-premixed jet flames. Exp. Fluids 2006, 41, 523–542. [Google Scholar] [CrossRef]


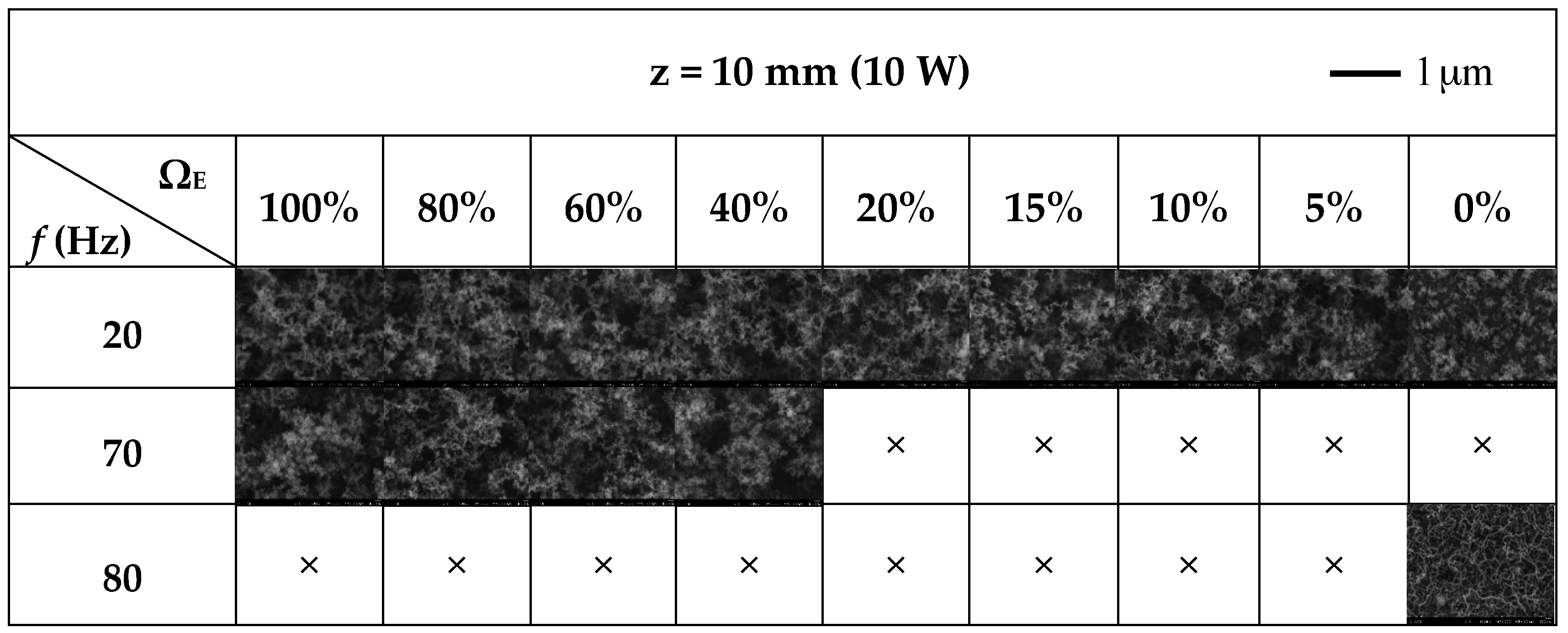
| ΩE | 100% | 80% | 60% | 40% | 20% | 15% | 10% | 5% | 0% | |
|---|---|---|---|---|---|---|---|---|---|---|
| f (Hz) | ||||||||||
| 20 | CNOs | CNOs | CNOs | CNOs | CNOs | CNOs | CNOs | CNOs | CNOs | |
| 70 | CNOs | CNOs | CNOs | CNOs | × | × | × | × | × | |
| 80 | × | × | × | × | × | × | × | × | CNTs | |
 : High-yield CNMs;
: High-yield CNMs;  : Moderate-yield CNMs; ×: No CNMs. CNOs: Carbon nano-onions; CNTs: Carbon nanotubes; CNMs: Carbon nanomaterials.
: Moderate-yield CNMs; ×: No CNMs. CNOs: Carbon nano-onions; CNTs: Carbon nanotubes; CNMs: Carbon nanomaterials.© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.-C.; Sari, S.K.; Hou, S.-S.; Lin, T.-H. Effects of Acoustic Modulation and Mixed Fuel on Flame Synthesis of Carbon Nanomaterials in an Atmospheric Environment. Materials 2016, 9, 939. https://doi.org/10.3390/ma9110939
Hu W-C, Sari SK, Hou S-S, Lin T-H. Effects of Acoustic Modulation and Mixed Fuel on Flame Synthesis of Carbon Nanomaterials in an Atmospheric Environment. Materials. 2016; 9(11):939. https://doi.org/10.3390/ma9110939
Chicago/Turabian StyleHu, Wei-Chieh, Shanti Kartika Sari, Shuhn-Shyurng Hou, and Ta-Hui Lin. 2016. "Effects of Acoustic Modulation and Mixed Fuel on Flame Synthesis of Carbon Nanomaterials in an Atmospheric Environment" Materials 9, no. 11: 939. https://doi.org/10.3390/ma9110939






